Development and Characterization of VEGF165-Chitosan Nanoparticles for the Treatment of Radiation-Induced Skin Injury in Rats
Abstract
:1. Introduction
2. Results and Discussion
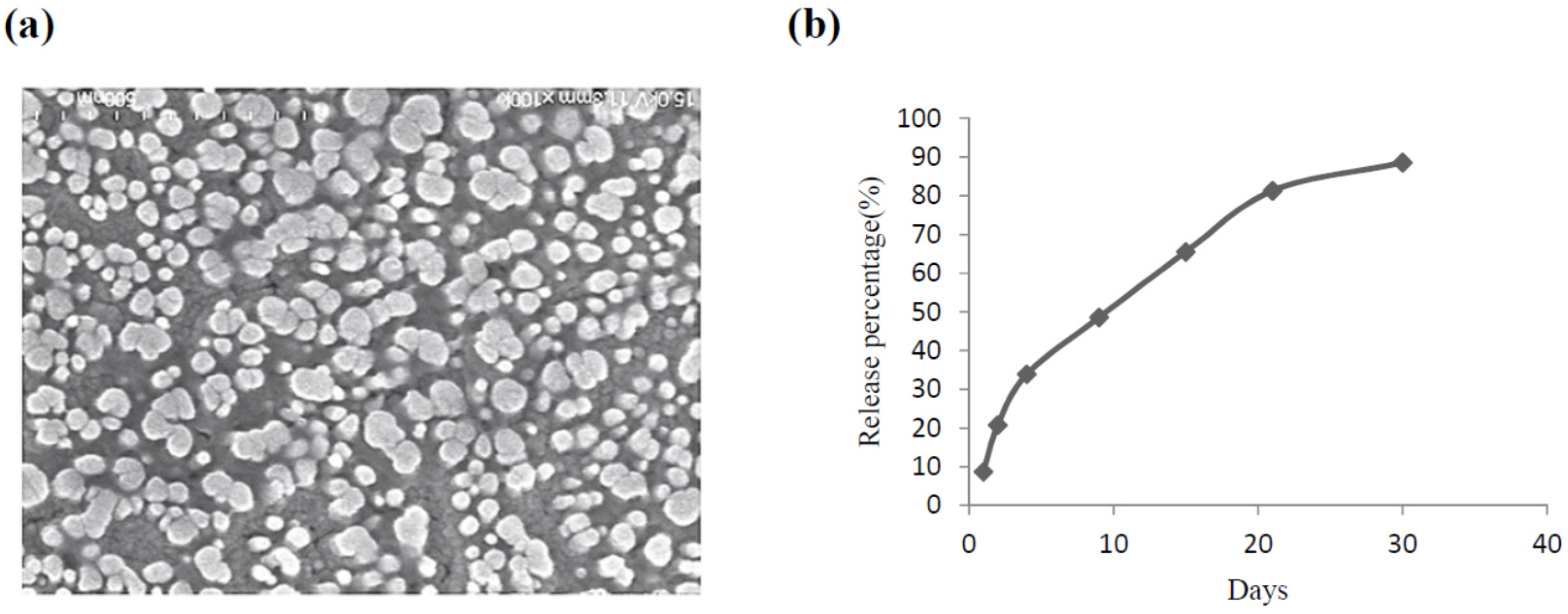
2.1. The Characteristics of CS Nanoparticles Formulated with VEGF
2.2. Establishment of a Radiation-Induced Skin Injury Model
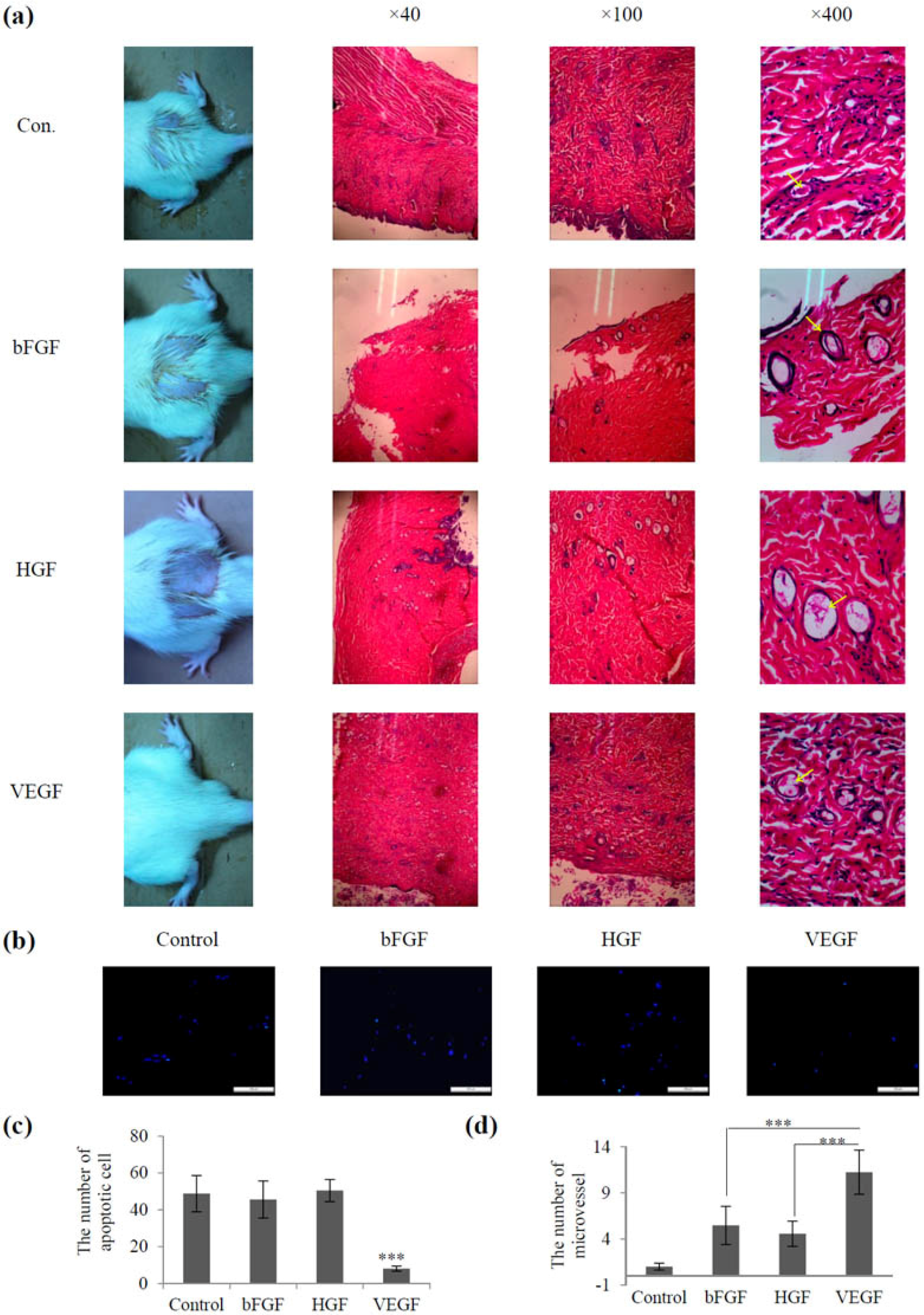
2.3. Screening of Vascular Growth Factor Treatment in Radiation-Induced Skin Injury
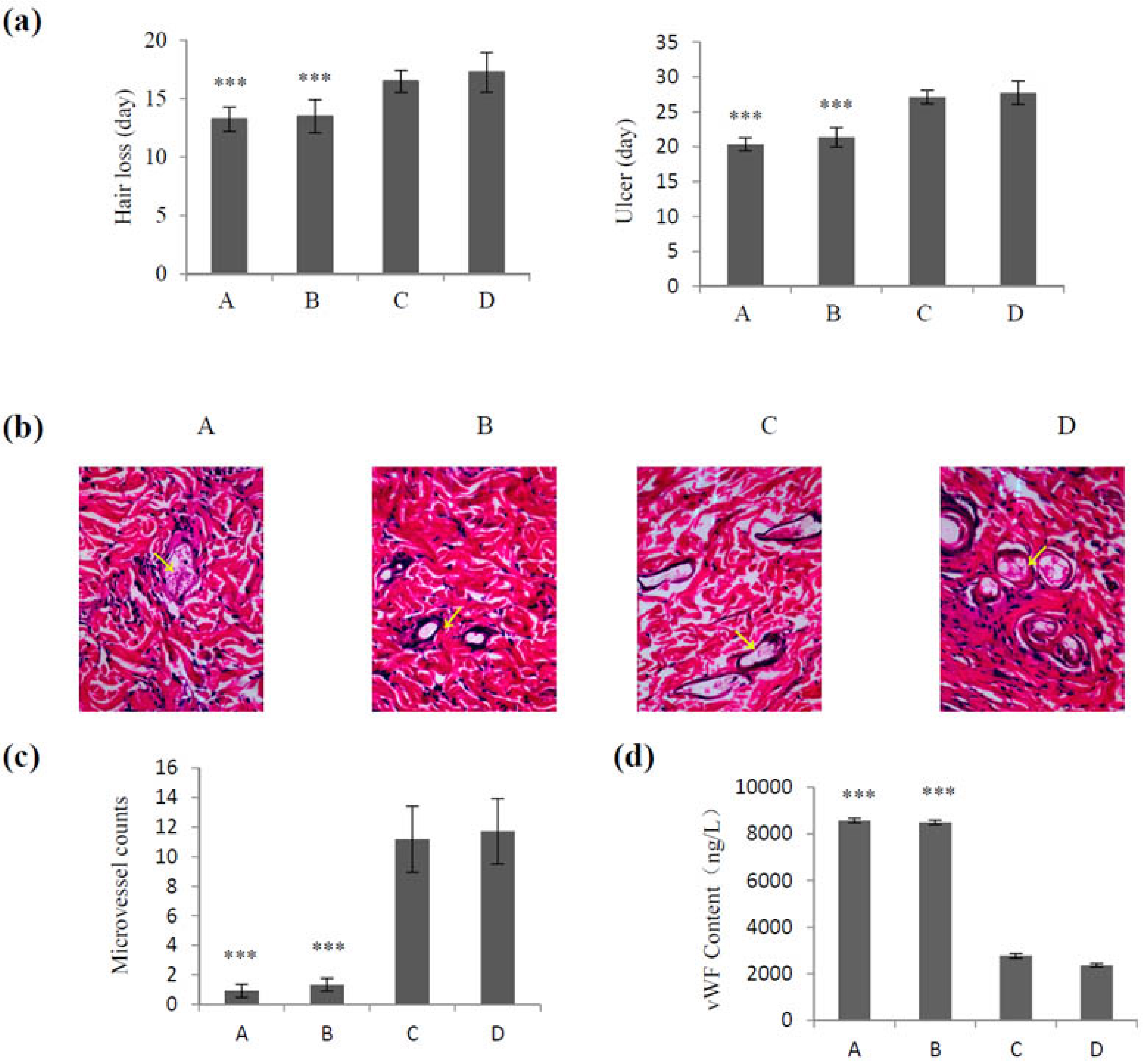
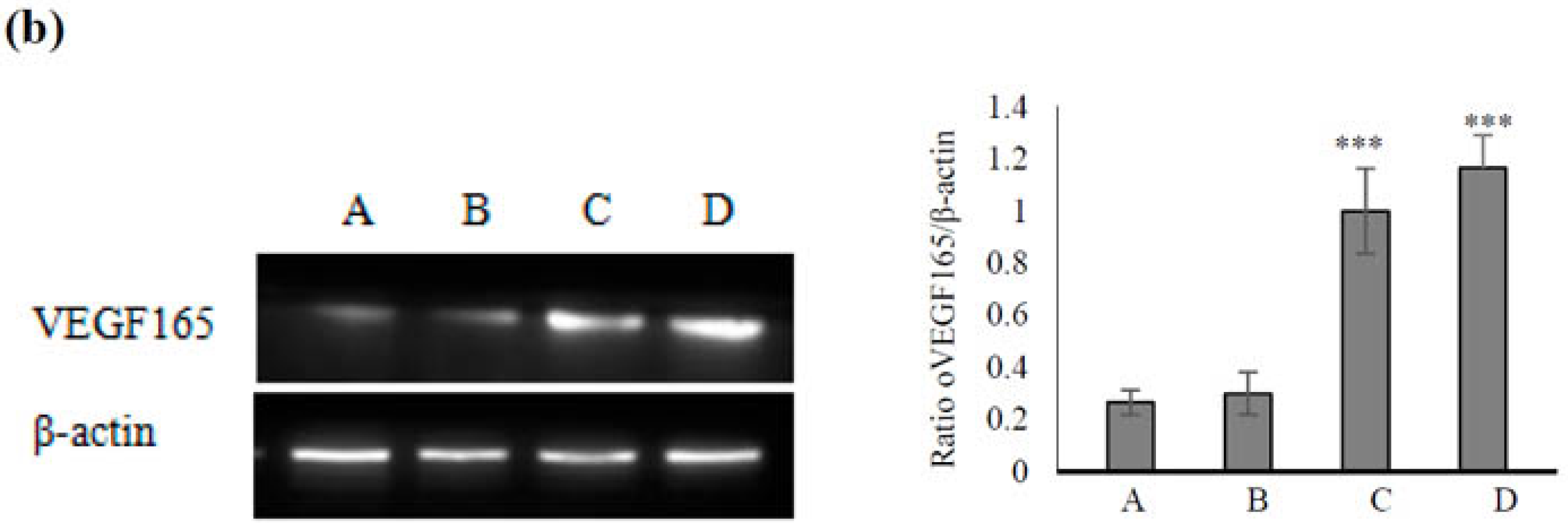
2.4. Effect of VEGF-CS Nanoparticles on Healing of Irradiation-Induced Skin Injury
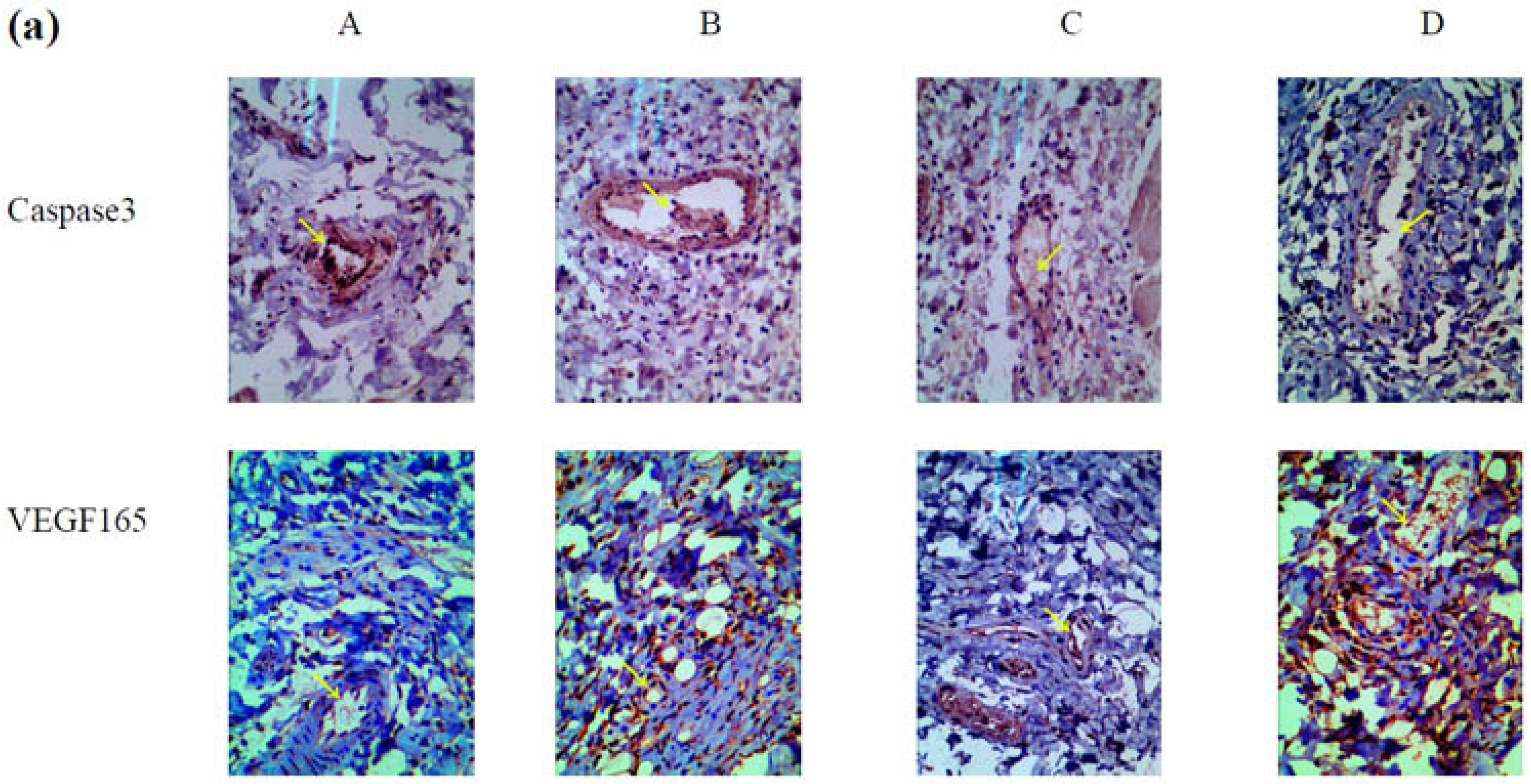
2.5. Mechanism Underlying the Effect of VEGF165-CS on Alleviating Radiation-Induced Skin Injury
3. Experimental Procedures
3.1. Materials
3.2. Methods
3.2.1. The Establishment of a Model
3.2.2. Enzyme-Linked Immunosorbent Assay (ELISA)
3.2.3. Cell Apoptosis Analysis
3.2.4. Screening of Vascular Growth Factors
3.2.5. The Preparation and Characterizations of CS Nanoparticles
3.2.6. Release of VEGF from VEGF-CS Nanoparticles
3.2.7. Application of VEGF-CS Nanoparticles in Vivo
3.2.8. Hematoxylin and Eosin Staining and Immunohistochemistry
3.2.9. Western Blot Determination of VEGF165 Expression
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, X.J.; Lin, S.; Kang, H.F.; Dai, Z.J.; Bai, M.H.; Ma, X.L.; Ma, X.B.; Liu, M.J.; Liu, X.X.; Wang, B.F. The effect of RHIZOMA COPTIDIS and COPTIS CHINENSIS aqueous extract on radiation-induced skin injury in a rat model. BMC Complement. Altern. Med. 2013, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kolozsvary, A.J.; Jenrow, K.A.; Brown, S.L. Mechanisms of radiation-induced skin injury and implications for future clinical trials. Int. J. Radiat. Biol. 2013, 89, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, R.K.; Meena, R. Emerging targets for radioprotection and radiosensitization in radiotherapy. Tumour Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.; Mack, W.J. Effects of ionizing radiation on cerebral vasculature. World Neurosurg. 2014, 81, 490–491. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Caron, C.; DeGeer, J.; Fournier, P.; Duquette, P.M.; Luangrath, V.; Ishii, H.; Karimzadeh, F.; Lamarche-Vane, N.; Royal, I. CdGAP/ARHGAP31, a Cdc42/Rac1 GTPase regulator, is critical for vascular development and VEGF-mediated angiogenesis. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Gianni-Barrera, R.; Trani, M.; Reginato, S.; Banfi, A. To sprout or to split? VEGF, notch and vascular morphogenesis. Biochem. Soc. Trans. 2011, 39, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- George, M.L.; Eccles, S.A.; Tutton, M.G.; Abulafi, A.M.; Swift, R.I. Correlation of plasma and serum vascular endothelial growth factor levels with platelet count in colorectal cancer: Clinical evidence of platelet scavenging? Clin. Cancer Res. 2000, 6, 3147–3152. [Google Scholar] [PubMed]
- Geng, H.; Song, H.; Qi, J.; Cui, D. Sustained release of VEGF from PLGA nanoparticles embedded thermo-sensitive hydrogel in full-thickness porcine bladder acellular matrix. Nanoscale Res. Lett. 2011, 6, 312. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, R.; Hwang, M.P.; Van, S.Y.; Do, S.H.; Park, H.; Lee, K.; Kim, S.H.; Yun, K.; Park, K. Osteogenic/angiogenic dual growth factor delivery microcapsules for regeneration of vascularized bone tissue. Adv. Healthc. Mater. 2015, 4, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, A.; Anisha, B.S.; Chennazhi, K.P.; Jayakumar, R. Chitosan-hyaluronic acid/VEGF loaded fibrin nanoparticles composite sponges for enhancing angiogenesis in wounds. Colloids Surf. B Biointerfaces 2015, 127, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xiong, Q.; Xu, G.; Lin, H.; Fang, X.; Cui, D.; Xu, M.; Chen, F.; Geng, H. VEGF-Loaded Nanoparticle-Modified BAMAs Enhance Angiogenesis and Inhibit Graft Shrinkage in Tissue-Engineered Bladder. Ann. Biomed. Eng. 2015, 43, 2577–2586. [Google Scholar] [CrossRef] [PubMed]
- Al Rubeaan, K.; Rafiullah, M.; Jayavanth, S. Oral insulin delivery systems using chitosan-based formulation: A review. Expert Opin. Drug Deliv. 2016, 13, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Lauzon, M.A.; Daviau, A.; Marcos, B.; Faucheux, N. Nanoparticle-mediated growth factor delivery systems: A new way to treat Alzheimer’s disease. J. Control. Release 2015, 206, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Guo, L.; Gu, X.; Zhang, B.; Hu, X.; Zhang, J.; Chen, J.; Wang, Y.; Chen, C.; Gao, B.; et al. Prevention of colorectal cancer liver metastasis by exploiting liver immunity via chitosan-TPP/nanoparticles formulated with IL-12. Biomaterials 2012, 33, 3909–3918. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Yao, H.H.; Qin, Z.H. Strategies for short hairpin RNA delivery in cancer gene therapy. Expert Opin. Biol. Ther. 2009, 9, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Losordo, D.W.; Vale, P.R.; Symes, J.F.; Dunnington, C.H.; Esakof, D.D.; Maysky, M.; Ashare, A.B.; Lathi, K.; Isner, J.M. Gene therapy for myocardial angiogenesis: Initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation 1998, 98, 2800–2804. [Google Scholar] [CrossRef] [PubMed]
- Hassler, S.N.; Johnson, K.M.; Hulsebosch, C.E. Reactive oxygen species and lipid peroxidation inhibitors reduce mechanical sensitivity in a chronic neuropathic pain model of spinal cord injury in rats. J. Neurochem. 2014, 131, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, Z.M. Structure of von Willebrand factor and its function in platelet adhesion and thrombus formation. Best Pract. Res. Clin. Haematol. 2001, 14, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Blann, A. von Willebrand factor: A marker of endothelial dysfunction in vascular disorders? Cardiovasc. Res. 1997, 34, 255–265. [Google Scholar] [CrossRef]
- Yoon, C.S.; Jung, H.S.; Kwon, M.J.; Lee, S.H.; Kim, C.W.; Kim, M.K.; Lee, M.; Park, J.H. Sonoporation of the minicircle-VEGF(165) for wound healing of diabetic mice. Pharm. Res. 2009, 26, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liao, T.; Wang, H.; Deng, W.; Yu, D. Transplantation of bone marrow stromal cells overexpressing human vascular endothelial growth factor 165 enhances tissue repair in a rat model of radiation-induced injury. Chin. Med. J. 2014, 127, 1093–1099. [Google Scholar] [PubMed]
- Tang, J.J.; Meng, Q.Y.; Cai, Z.X.; Li, X.Q. Transplantation of VEGFl65-overexpressing vascular endothelial progenitor cells relieves endothelial injury after deep vein thrombectomy. Thromb. Res. 2016, 137, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpourdounighi, N.; Behfar, A.; Ezabadi, A.; Zolfagharian, H.; Heydari, M. Preparation of chitosan nanoparticles containing Naja naja oxiana snake venom. Nanomedicine 2010, 6, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Vimal, S.; Taju, G.; Nambi, K.S.N.; Majeed, S.A.; Babu, V.S.; Ravi, M.; Hameed, A.S.S. Synthesis and characterization of CS/TPP nanoparticles for oral delivery of gene in fish. Aquaculture 2012, 358, 14–22. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; Li, S.; Wang, S.; Li, X.; Zhu, M.; Huang, S.; Sun, L.; Zhang, Y.; Liu, Y.; Wang, S. Development and Characterization of VEGF165-Chitosan Nanoparticles for the Treatment of Radiation-Induced Skin Injury in Rats. Mar. Drugs 2016, 14, 182. https://doi.org/10.3390/md14100182
Yu D, Li S, Wang S, Li X, Zhu M, Huang S, Sun L, Zhang Y, Liu Y, Wang S. Development and Characterization of VEGF165-Chitosan Nanoparticles for the Treatment of Radiation-Induced Skin Injury in Rats. Marine Drugs. 2016; 14(10):182. https://doi.org/10.3390/md14100182
Chicago/Turabian StyleYu, Daojiang, Shan Li, Shuai Wang, Xiujie Li, Minsheng Zhu, Shai Huang, Li Sun, Yongsheng Zhang, Yanli Liu, and Shouli Wang. 2016. "Development and Characterization of VEGF165-Chitosan Nanoparticles for the Treatment of Radiation-Induced Skin Injury in Rats" Marine Drugs 14, no. 10: 182. https://doi.org/10.3390/md14100182




