1. Introduction
Chitin, the β-1,4-linked homopolymer of
N-acetylglucosamine (GlcNAc), is the most abundant polymer in the marine environment, and the second in nature after cellulose. Chitin is the structural basis for exoskeletons of crustaceans and insects, and a component of the fungal cell wall. The global production of chitin is estimated to be 10
11 tons per year, however, chitin does not accumulate as it is hydrolyzed by marine microorganisms [
1,
2]. The hydrolysis is mediated by chitinolytic enzymes and allows the microorganisms to utilize chitin as a carbon and nitrogen source and chitin turnover is important for the biogeochemical C- and N-cycles. Chitin and chitinolytic enzymes are also of biotechnological interest with potential applications in the food, medical and agricultural sectors [
3]. Also, chitin in the form of shellfish waste can be considered as a resource potentially used as a carbon-source in microbial fermentations. Shellfish waste constitutes an environmental problem of increasing magnitude [
4,
5], and the discovery of inexpensive processes, which can degrade chitin into chitooligosaccharides, chitosan and GlcNAc, may address this problem [
6].
As mentioned, the cell wall of fungi contains chitin and some chitinolytic microorganisms can inhibit the growth of fungi by chitin degradation [
7,
8,
9]. Fungal plant diseases are of great concern in agriculture and cause large losses at an estimated 5%–10% of the world’s food production [
10]. Fungal contamination and mycotoxin production is also a problem in the built environment [
11]. Potentially, natural fungicides, such as chitinases, could replace the chemical fungicides in plant biocontrol [
12] and toxic chemicals indoors, and since bacterial chitinases can inhibit fungal growth, they are of particular interest for this purpose [
7,
13].
Chitin is a recalcitrant insoluble polysaccharide and is degraded into soluble oligosaccharides or GlcNAc. Chitinases (EC 3.2.1.14) hydrolyze the β-1,4 glycosidic bonds between the GlcNAc residues to produce chitooligosaccharides. Chitinases are glycosyl hydrolases (GH) and are divided into GH families 18 and 19. Bacterial chitinases usually belong to family 18, although a few belonging to family 19 have been described [
14]. The GH18 and GH19 chitinases differ in sequence similarity, three-dimensional structure and catalytic mechanism. Bacteria often secrete many chitinases, and it is believed that they do so in order to efficiently hydrolyze the different forms of chitin they encounter [
7,
15,
16]. The GH19 chitinases are believed to be the primary enzymes involved in breakdown of fungal chitin, but this is also mediated by other types of chitinases [
7,
17].
Recently, a new extracellular enzyme involved in breakdown of chitin was discovered. Lytic polysaccharide monooxygenases (LPMOs) were first described in 2010 [
18] and are metalloenzymes that oxidize the glycosidic bonds in the crystalline surface of chitin and facilitate access of chitinases. LPMOs were first classified as carbohydrate binding module family 33 and they were believed only to be involved in substrate recognition [
19]. LPMOs are now reclassified in auxiliary activity group 10 (AA10) in the CAZY-database [
20] and their facilitating activity in chitin degradation has become clear [
21]. Another facilitating enzyme, which has so far only been described in some marine bacteria, is chitooligosaccharide deacetylase (COD) [
22]. COD (EC 3.5.1.105) is secreted in low concentrations and produces a signal molecule, GlcNac-GlcN, which acts as an inducer for chitinase production [
23]. Deacetylation by COD is of particular interest for the industrial production of chitosan oligomers. A combination of COD and another chitin deacetylase, NodB, is used for commercial production of defined chitosan oligomers [
24].
The purpose of the present work was to determine the potential for chitin degradation in a collection of marine bacteria. The bacteria were isolated due to their production of antibacterial compounds [
25] and we have recently shown that chitin influences the production of secondary metabolites, such as antibacterial compounds, in some of these bacteria [
26,
27]. We therefore rationalized that they likely would have a high potential for chitin degradation. We envision that these bacteria and their chitinolytic activities will be of interest to the biotech and building industries and in agricultural production. We used in silico genome-wide analysis combined with phenotypic testing to unravel the potential and to exemplify the industrial perspective we cloned and heterologously expressed two chitinases.
3. Discussion
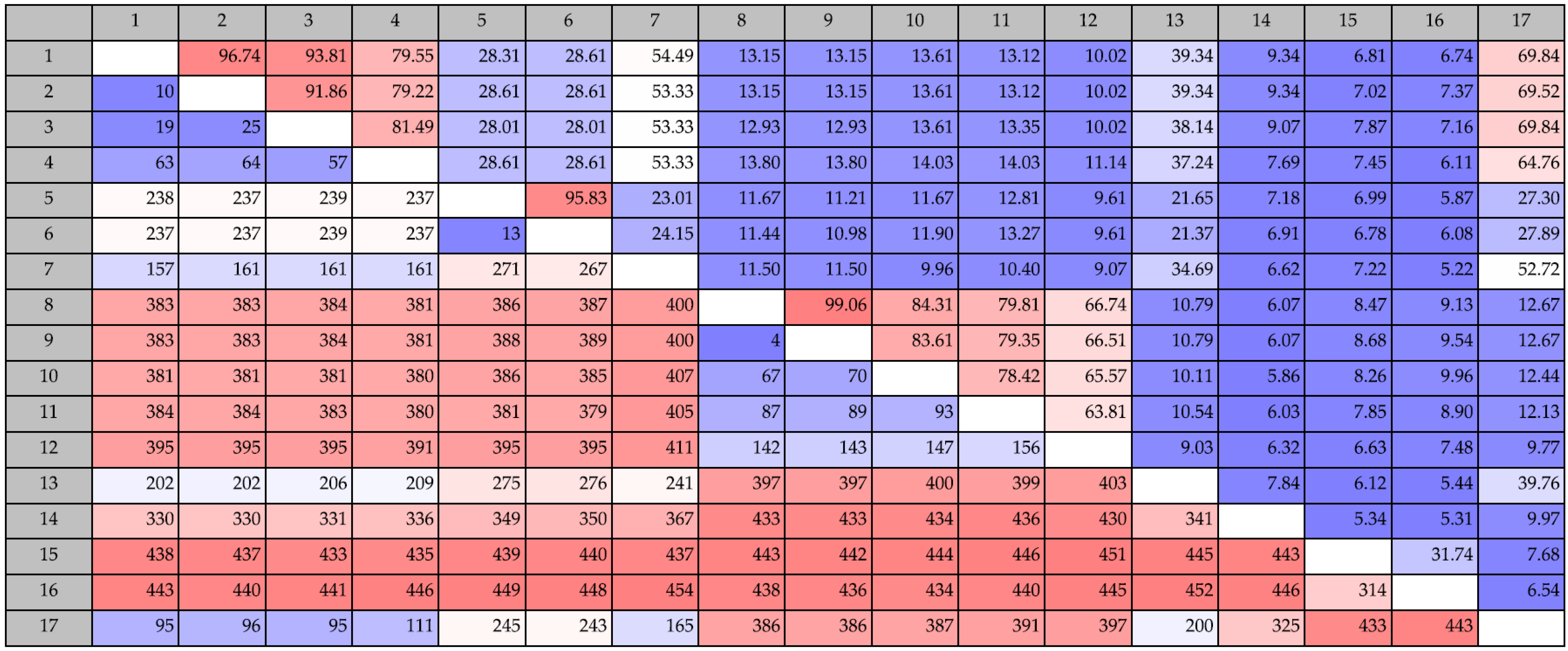
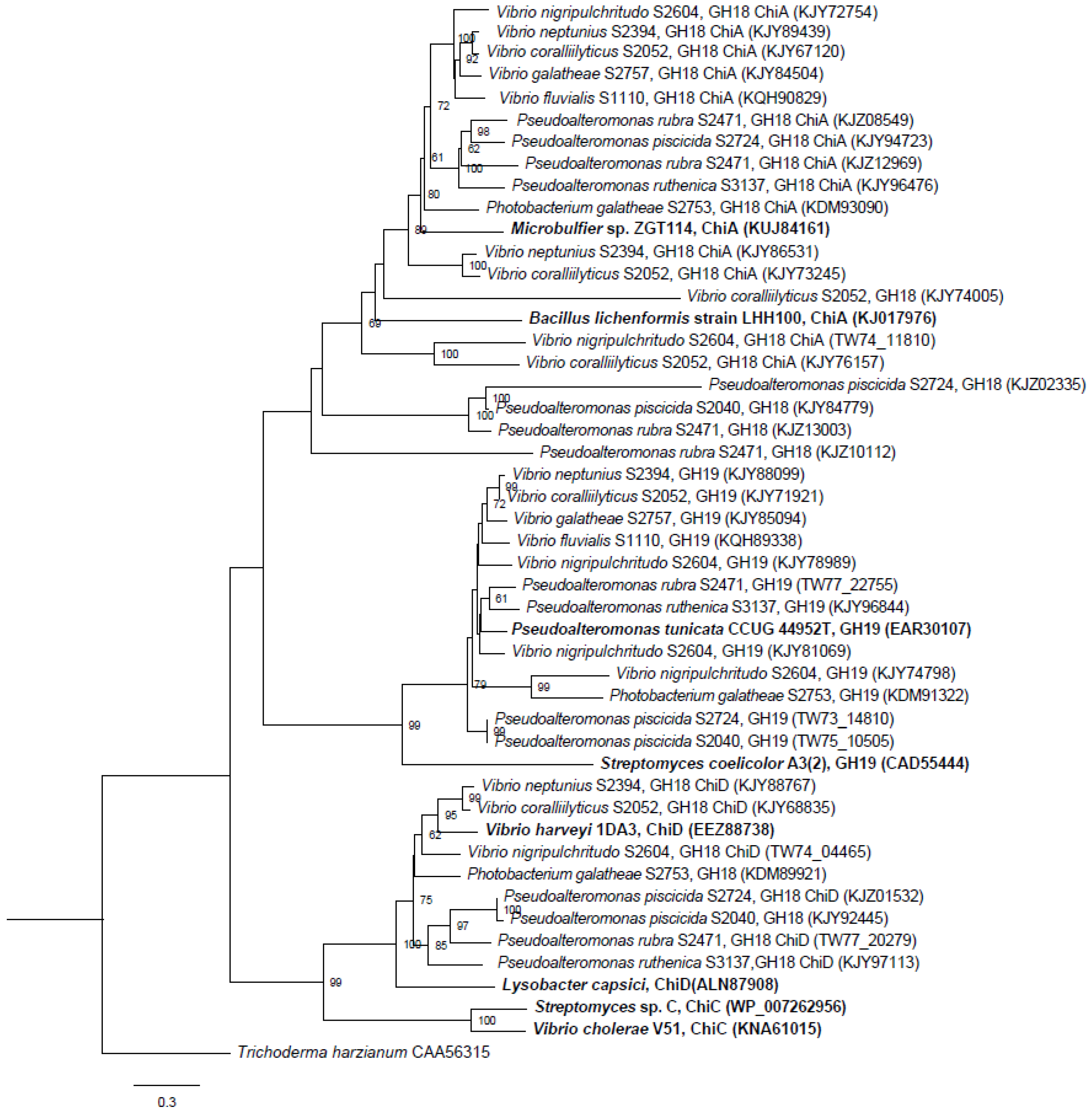
Chitin degradation is an important process in both marine and terrestrial environments. Chitin is also an important resource in different industrial and medical processes, and chitin degrading enzymes or microorganisms are of interest, e.g., as antifungal agents or as bio-insecticides. Hence, there is a growing demand for new enzymes with chitin-modifying properties. We analyzed 11 marine bacteria with antibacterial activity and found a remarkable potential for chitin degradation. In silico, we identified genes involved in the chitin degradation process using an iterative strategy. We found a total of 50 putative chitinase genes of which 11 were considered to be wrongly annotated. The strains contained from two to six putative chitinase genes, and the number of chitinase genes per strain did not correlate with phenotypic chitin degradation ability. The three strains causing the largest clearing zones in chitin agar, S2753, S2724 and S2040, harbored three, three and four chitinase genes, respectively, whereas the poorer chitin degraders, S2050 and S2604, both had six chitinase genes. The chitinases were grouped in different categories of ChiA, ChiD and unclassified belonging to GH18 and GH19 chitinases.
Chitinolytic bacteria harbor different chitinases, most likely due to a specificity for different substrates [
16,
30,
31,
32]. Here, all but one bacterial strain contained at least one chitinase belonging to GH18 ChiA, whereas GH18 ChiD and unclassified GH18 chitinases were only present in some strains. This is not surprising, since ChiA-type chitinases are the most dominant chitinases in bacteria and play a key role in chitin degradation [
16,
33,
34]. Also, this was the dominant chitinase group found in un-cultured bacteria [
35].
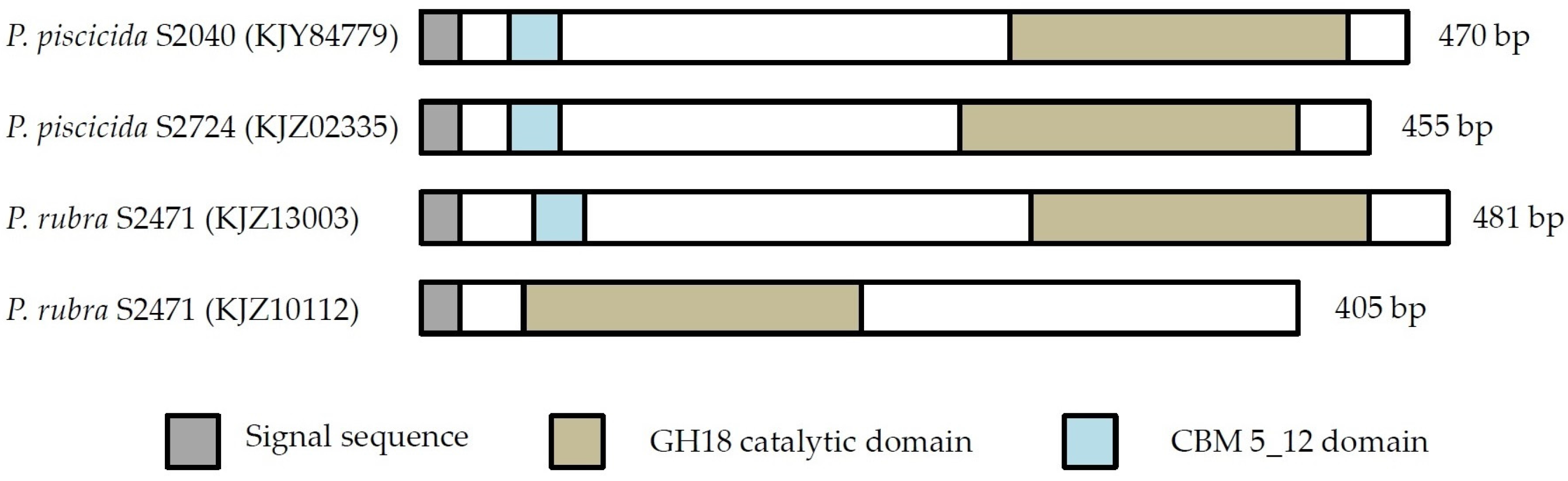
P. piscicida S2040 does not encode for a ChiA-like chitinase, and yet was one of the most potent chitin degraders. The genome of S2040 contained one chitinase of the ChiD-like type, one belonging to GH19 and one unclassified GH18 chitinase, and chitinases of the ChiA-like type are not responsible for the pronounced chitin degradation by this strain. Four chitinases from strains S2724, S2040 and S2471 did not cluster into any existing chitinase subgroups, suggesting they belong to a new subgroup of chitinases. The domain structure provides further evidence that these are indeed chitinases, as they contain the main characteristics of a chitinase (signal peptide, CBM and catalytic domain). However, one chitinase (KJZ10112) does not contain a CBM. This does not disqualify its classification as a chitinase. CBMs are important for the overall performance of the enzyme, but chitinases do not lose function without CBMs, they merely display weaker binding [
36,
37,
38]. These four chitinases were only identified in the
Pseudoalteromonadaceae family, and it is interesting to note that two of the chitinases, KJZ02335 and KJY84779, originate from two of the three most potent chitin degrading strains. These unknown chitinases may play a significant role in chitin degradation by
Pseudoalteromonas species.
All strains had at least one gene encoding chitinases belonging to the GH19 family, which for many years were considered to be unique to higher plants. The first bacterial GH19 chitinase was found in 1996 [
39] and the majority of GH19 chitinases have been found in
Streptomyces species [
14]. However, GH19 chitinases from
Vibrio,
Aeromonas,
Pseudoalteromonas,
Chitiniphilus,
Nocardiopsis and
Burkholderia species have also been described [
8,
40,
41,
42,
43,
44]. The presence of the GH19 chitinase in all 10 chitinolytic strains could indicate that this gene is more widespread in the marine environment than hitherto believed.
Since the discovery of the function of LPMOs as facilitators of chitin degradation, the interest in these particular enzymes has increased. Our in silico analysis revealed that LPMOs are present in nine of the 10 genomes from chitinolytic bacteria. Recombinant LPMOs have not yet been characterized in marine bacteria and this potential should be further explored. These LPMOs could be of interest in enzyme cocktails as shown in a recent study where a LPMO from a
Streptomyces griseus increased the chitin solubilization yields by up to 30-fold when combined with a
Serratia marcescens GH18 chitinase [
45].
COD genes have so far only been identified in
Vibrio species and in
Shewanella woodyi ATCC51908 [
46,
47]. Here, we found six putative COD genes, however, they only had little homology to known CODs. The
Vibrio COD genes were similar to the allantoinase analog PuuE indicating a wrong annotation. Additional evidence for the wrong annotation is the observation that the neighboring genes to the putative
Vibrio CODs are involved in purine degradation. PuuE type allantoinases have high similarity to polysaccharide deacetylases, which is also the observation in this study, where the
Vibrio COD genes had approximately 50% identity to a CDA from a metagenomic sample [
48]. The putative COD genes from the two
Pseudoalteromonas strains were only 23%–27% identical to the metagenomic CDA and the PuuE allantoinase. PuuE proteins can be distinguished from polysaccharide deacetylases by two highly conserved segments, which are only present in PuuE proteins. These conserved segments are present in the
Vibrio genes (data not shown), but not in the
Pseudoalteromonas genes, which provide further evidence for the classification of
Vibrio CODs as PuuE type allantoinases. In contrast to the
Vibrio COD genes, the
Pseudoalteromonas COD genes have signal peptides. Thus, these CODs are likely secreted, and they may therefore potentially act as CDAs. CDAs of bacterial origin are of particular industrial interest, as they catalyze the conversion of chitin to chitosan, a highly coveted polymer [
49]. Irrespective of the real function of the so-called
Pseudoalteromonas COD, the enzymatic deacetylation of polymers has a high industrial potential.
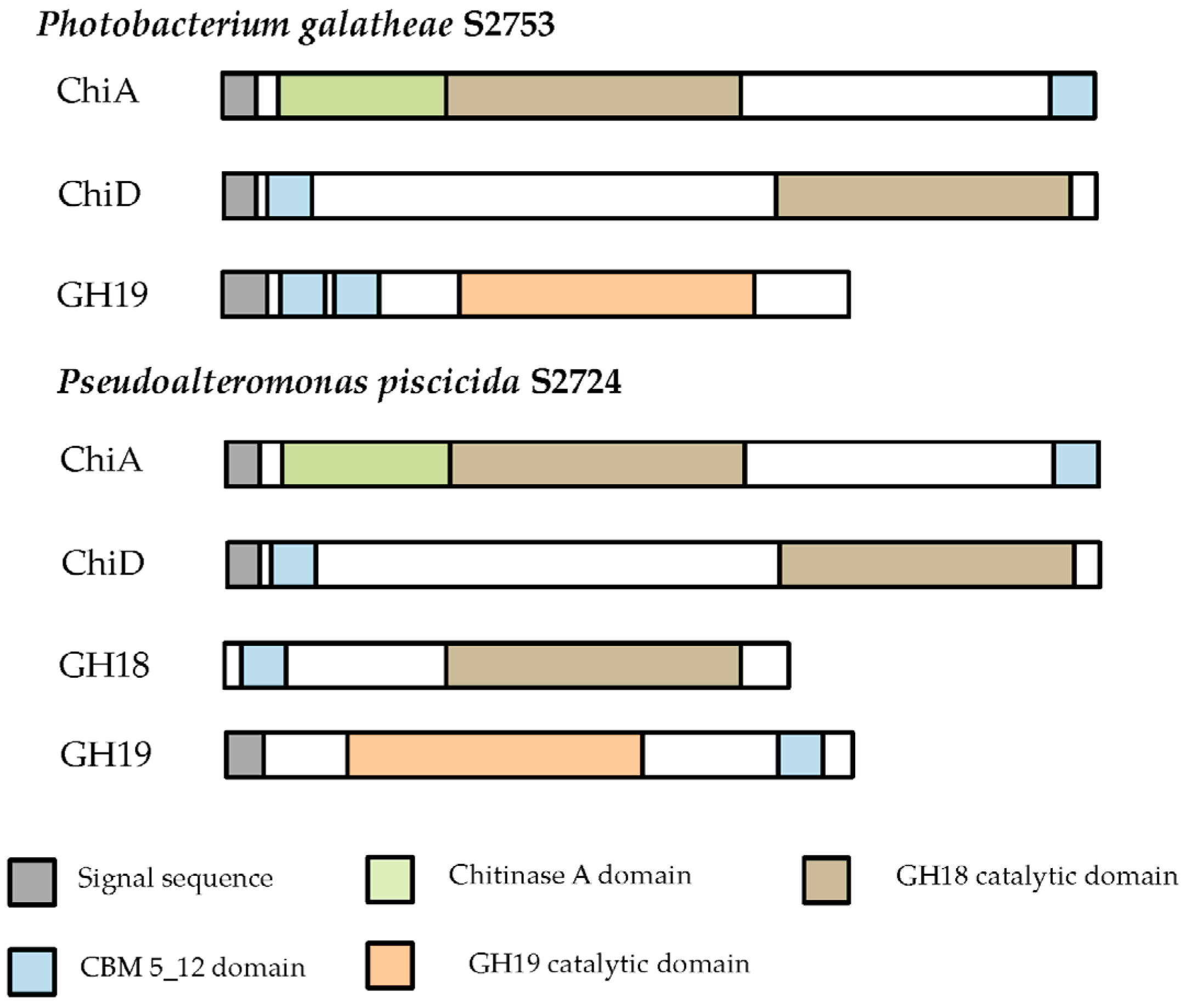
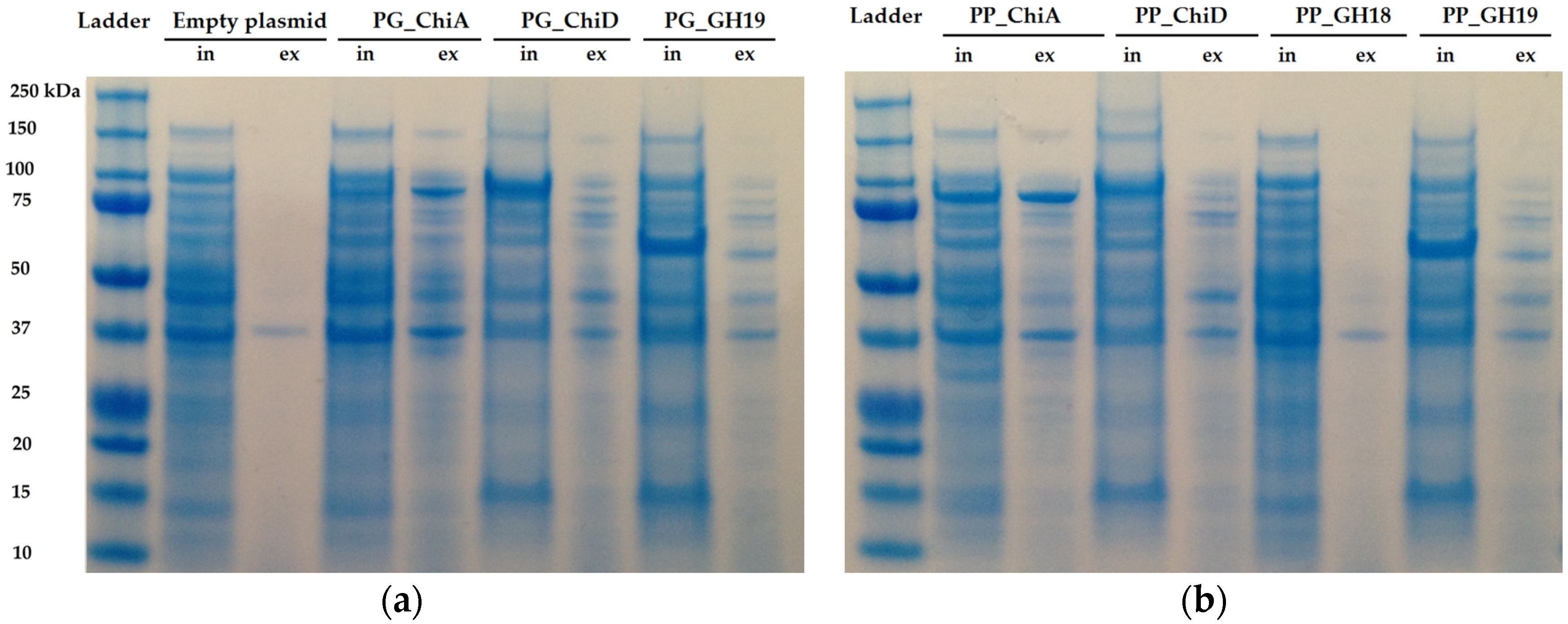
Two of the seven cloned chitinases were secreted whereas the remaining five accumulated inside the cells. The signal peptide of ChiA type chitinases can be recognized by the secretion system in
E. coli, and hence it is not surprising that ChiA is secreted by
E. coli. [
50,
51]. However, cloned ChiA type chitinases are not always secreted [
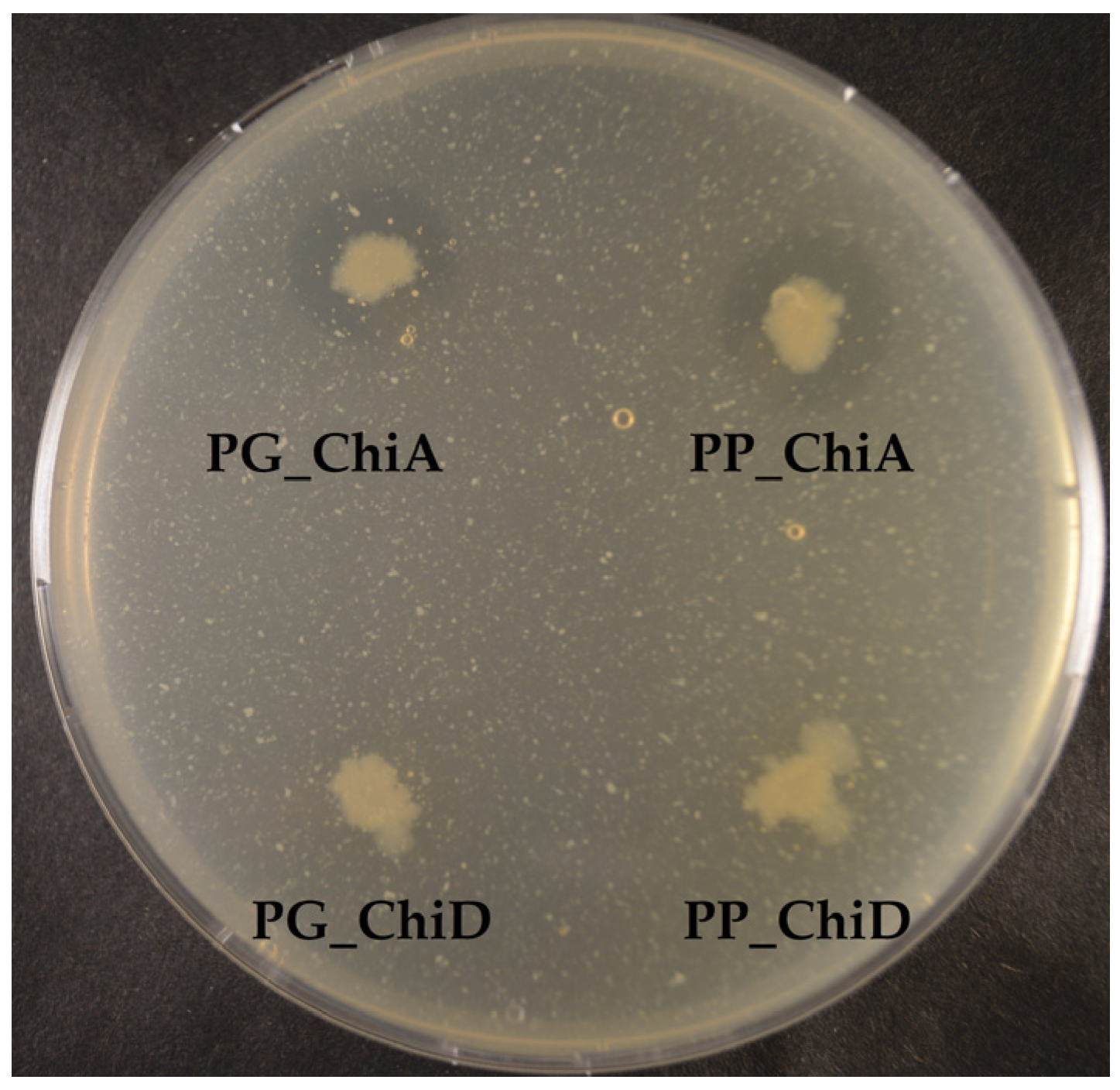
52], so the secretion does not seem to be specific to the chitinase type, but the secretion of the two ChiA-type chitinases from this study allows for an easy purification and exemplifies the potential industrial use of these enzymes. Extracellular and intracellular extracts were tested on different chitinous media, but only the extracts of the two ChiA enzymes degraded colloidal chitin, which may be due to substrate specificity and/or need for synergy between chitinases, as already mentioned.
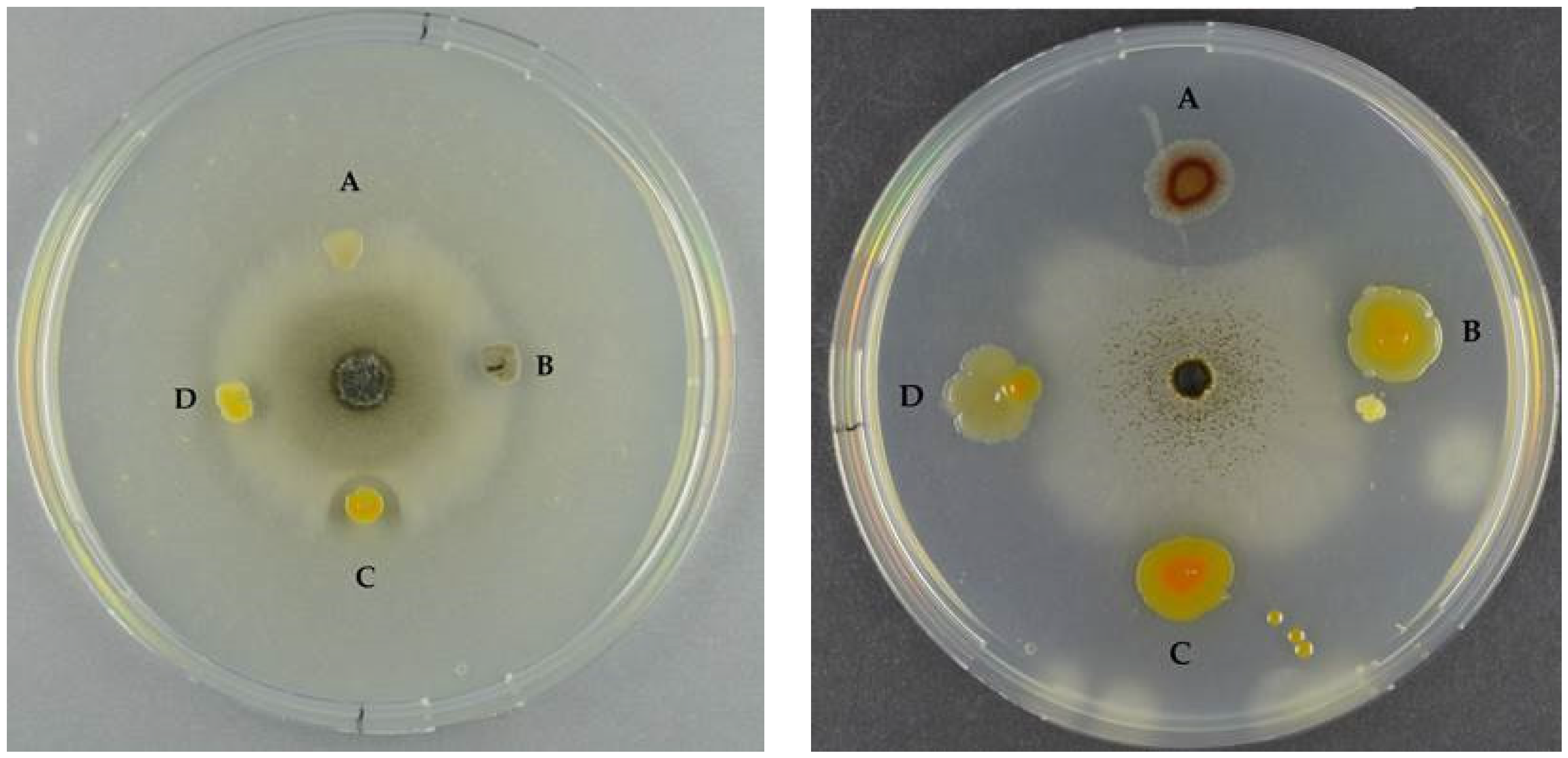
We tested the extracts against seven fungi, covering both indoor contaminants and plant pathogens. GH19 chitinases are thought to be the main antifungal chitinases, but ChiA type chitinases have also displayed antifungal activity, like
StmChiA from
Stenotrophomonas maltophilia which was antifungal against
F. oxysporum [
52]. However, extracts from ChiA clones were not antifungal, nor were any of the other extracts. Since chitinase concentration in the extracts was unknown, an up-concentration could potentially result in a measurable effect.
All 11 bacterial strains, including the non-chitin degrader, were antifungal when tested as live cultures, however, with different fungal targets. Allowing pre-growth of the potential producer prior to fungal inoculation increased the antifungal activity of some of the bacteria, which is in agreement with the study by Giubergia and co-workers [
27], in which bioactivity of a collection of
Vibrionaceae increased 3-fold when the producer was allowed a 2-day pre-growth period.
P. piscicida S2040 displayed pronounced antifungal effect towards all fungi, independently of the time of spotting, and hence would serve as a candidate for a broad-range antifungal bio-pesticide.
In summary, three of the ten strains were of interest due to their remarkable chitin degrading abilities and their antifungal activities. These strains could have potential in biodegradation of chitin-waste, and in biocontrol of unwanted fungal growth in agriculture and the building industry.
4. Materials and Methods
4.1. Strains and Plasmids
The bacterial strains used in this study (
Table 4).
The bacteria were isolated during the Galathea 3 expedition [
25] and they have been whole-genome sequenced [
28]. Genomes were assembled using CLC Genomics Workbench 7 (CLC bio, Aarhus, Denmark) and contig-based draft genomes were obtained. Gene annotation was performed using Rapid Annotation using Subsystem Technology (RAST), [
53,
54,
55]. The genomes are available at the National Center for Biotechnology Information (NCBI) [
56]. To compare annotations, the genomes were also downloaded from NCBI containing the NCBI-annotated genes.
Escherichia coli Top10 was used for cloning and propagation of plasmids, and
E. coli BL21 (DE3) was used for expression of chitinase genes from
P.
galatheae S2753 and
P. piscicida S2724. The cloning and expression vector was pBAD_Myc_HisA. Plasmids were isolated using the QIAprep
® Spin Miniprep kit (Qiagen, 27106, Hilden, Germany), and genomic DNA was extracted using the NucleoSpin
® Tissue kit (Machery-Nagel, 740952, Düren, Germany). Strains and plasmids used for cloning can be seen in
Table 5.
4.2. Preparation of Colloidal Chitin
Colloidal chitin was prepared from shrimp shell chitin (Sigma, C7170, Deisenhofen, Germany) or crab shell (Sigma, C9752, Deisenhofen, Germany) chitin as follows: 10 g chitin was hydrolyzed in 400 mL ice-cold 37% HCl for 6 h at 4 °C with stirring. The solution was transferred to 4 L cold dH2O over night for settlement of chitin. The solution was neutralized using NaOH and adjusted to pH 7. Colloidal chitin was collected by centrifugation at 4000× g for 5 min and resuspended in dH2O for a final concentration of 2%. The chitin solution was autoclaved at 121 °C for 15 min.
4.3. Chitinase and Chitosanase Activity Screening
The strains were tested for chitinase and chitosanase activity on plates containing different chitinous substrates. The basic media consisted of 2% Sea Salt (Sigma, S9883, Deisenhofen, Germany), 1,5% agar, 0.3% casamino acids and was supplemented with either 0.2% colloidal chitin from shrimp or crab, 0.2% crystalline shrimp chitin or 0.2% shrimp chitosan (Sigma, 50494, Deisenhofen, Germany) or crab chitosan (Sigma, 48165, Deisenhofen, Germany). Plates were spotted with one single colony from a streaked culture from freeze-stock on marine agar plates (Difco 2216). The plates were incubated at 4, 15, 25 and 35 °C for 11 days for colloidal chitin and chitosan and 35 days for crystalline chitin. The natural turbidity of the media allows for visual evaluation of chitin/chitosan degradation appearing as a clearing zone around the spotted bacteria. A qualitative grading of chitinase activity (clearing zone) was given, determined from the edge of the bacterial colony to the edge of the clearing zone, where a zone of 0–6.99 mm was graded one plus, +, and >7 mm was graded two plusses, ++.
4.4. In Silico Analysis
An annotation based search for chosen genes involved in chitin degradation was conducted using CLC Main Workbench 7 and included genes putatively encoding chitinases, chitin sensors (ChiS or CdsS), COD and LPMOs. 50 chitinase-encoding genes were found using RAST. The complete genes were translated to protein, and divided into GH18 and GH19 families by analysis using the Pfam protein family database [
57,
58] and further divided into subfamilies by a protein blast in the non-redundant database in NCBI [
59]. Since chitinases are extracellular proteins they were checked for signal peptides using SignalP 4.1 [
60]. 11 RAST-annotated chitinases were eliminated from the analysis, as they did not contain either GH18 or GH19 domains, and two others were deleted as they did not contain signal peptides or CBMs. All included and eliminated chitinases can be seen in
Supplementary Materials Table S1. Since the COD proteins showed little homology to other known CODs, they were blasted against the non-redundant protein database to find proteins of high sequence similarity. The COD encoding genes were compared to already known CODs, chitin deacetylases and an allantoinase analog by pairwise comparison in CLC.
4.5. Phylogenetic Analysis of Chitinases
A phylogenetic tree was created consisting of the identified chitinase genes from the ten chitinolytic strains. To indicate correct groupings and potentially group the unidentified chitinase genes, known chitinase genes belonging to the GH18 subfamilies A, C and D and GH19 chitinases from the NCBI database were included. The phylogenetic tree was created from a multiple alignment of the translated chitinase proteins with a neighbor-joining construction method and a bootstrap analysis with 1000 replicates was included. The tree was visualized using FigTree [
61]. A fungal chitinase from
Trichoderma harzianum (accession number CAA56315) was used as root.
4.6. Construction of Plasmids for Chitinase Expression
Expression plasmids for the seven chitinase genes from
P. galatheae S2753 and
P. piscicida S2724 were constructed via USER cloning, as preciously described [
62], using pBAD-Myc_HisA as the vector plasmid. Plasmid and chitinase genes were amplified by PCR using PfuX7 polymerase [
63] and primers (
Table 6) using following settings: initial denaturation at 98 °C for 2 min, 30 cycles of 98 °C for 20 s, 57 °C for 20 s, 72 °C for 1:30 min and a final extension at 72 °C of 2:30 min. For PG_GH19, PP_GH18 and PP_GH19 extension time was reduced to 50 s and final extension time reduced to 1:45 min. For plasmid DNA, extension time was prolonged to 2:30 min and final extension prolonged to 4:30 min. Each PCR reaction (50 μL) consisted of 5 μL Pfu buffer (200 mM Tris-HCl pH 8.8, 100 mM KCl, 60 mM (NH
4)
2SO
4, 20 mM MgSO
4, 1 mg/mL BSA (in nuclease-free water) and 1% Triton X-100), 5 μL dNTPs (2 mM), 1.2 μL MgCl
2 (50 mM), 5 μL forward primer (5 μM), 5 μL reverse primer (5 μM), 1 μL PfuX7 polymerase and 0.5–1 μL DNA.
In short, the chitinase containing plasmids were constructed in a reaction of 10 μL, consisting of 100 ng of each purified PCR product, 1 μL T4 DNA ligase buffer (New England BioLabs, #B0202S, Ipswich, MA, USA ) and 1 μL USER™ enzyme (New England BioLabs, #M5505S, Ipswich, MA, USA). The reaction was incubated at 37 °C for 15 min, followed by 26 °C for 15 min and 10 °C for 10 min. 3 μL USER reaction was mixed with 40 μL chemically competent E. coli Top10. The mixture was incubated on ice for 30 min, followed by a 60 s heat shock at 42 °C, and 2 min incubation on ice. Cells were recovered in 1 mL LB for 1 h and subsequently harvested at 1600× g for 2 min. The pellet was plated on LB agar containing 100 μg/mL ampicillin and incubated at 37 °C overnight, and the following day colonies were grown in LB media with antibiotics and stored as glycerol stocks. Plasmids were purified and confirmed by sequencing (Macrogen, Amsterdam, The Netherlands).
4.7. Protein Expression and SDS-Page Analysis
Protein expression was initiated by transformation of the purified plasmids into electrocompetent
E. coli BL21 (DE3) cells. Transformants were incubated at 37 °C overnight and one colony was used to incubate 10 mL LB media supplemented with 100 μg/mL ampicillin and incubated at 37 °C, 250 rpm, overnight. ON culture was used to incubate fresh LB media supplemented with ampicillin. At OD
600 between 0.5 and 0.8 the cultures were induced with arabinose (total concentration 0.02%) and the cultures were further incubated at 37 °C for 4 h. 2 mL cell suspension was centrifuged for 1 min at 12,000×
g. The supernatant was separated from the pellet, and the pellet was resuspended in 1 mL lysis buffer (50 mM potassium phosphate, pH 7.4, 500 mM sodium acetate, 0.1 mM EDTA and 20% glycerol). The lysis mixtures were kept on ice and lysed by sonication (Soniprep, amplitude 10 μm) for 2 × 30 s, and hereafter centrifuged for 1 min at 12,000×
g. For SDS-page, protein concentration was estimated using the method of Bradford [
64] with BSA as standard. Protein concentration of extracellular extracts ranged from 0.1 to 0.3 mg/mL and intracellular were diluted to a total concentration of 0.5 mg/mL. Fifty μL of protein extract were incubated with 10 μL loading dye (300 mM Tris HCl, pH 6.8, 0.01% bromophenol blue, 15%
v/
v glycerol and 6% SDS) at 95 °C for 5 min and 20 μL of each solution was loaded on a precast 4%–12% Bis-Tris gel (NuPAGE™ Novex™, Thermo Scientific, NP0321, Waltham, MA, USA). The gel was run for 1.5 h at 90 V and stained with Coomassie Brilliant Blue G-250. To identify the proteins on the gel, the sizes were estimated using the compute PI webtool [
65].
4.8. Antifungal Activity
Seven different fungi were used for antifungal activity testing of both wildtype bacteria as well as the cloned chitinase enzyme extracts. The following fungi were chosen to cover indoor mold and plant-pathogenic fungi: Penicillium chrysogenum (IBT 33843), Stachybotrys chartarum (IBT 7709), Chaetomium globosum (IBT 7029), Neosartorya hiratsukae (IBT 28630), Aspergillus niger (IBT 32191), Fusarium oxysporum (IBT 41964) and Botrytis cinerea (IBT 41856) and were from the IBT Culture Collection at Department of Biotechnology and Biomedicine, Technical University of Denmark. The antifungal activity of wildtype bacterial strains was tested by adding a 20 μL spore suspension to a puncture well on the center of a MA plate. Due to different growth rates, the fungi were allowed to grow for 4–8 days, after which colony mass of each wildtype bacteria was spotted approximately 2 mm from the edge of the fungal colony. The plates were left for 4 days and antifungal activity was observed. We also tested the antifungal activity of the wild-type strains in a setting where the bacteria were spotted 2 days prior to the inoculating the fungi. The bacteria were spotted 2 cm from center of the plates. In both the above settings, plates were checked again after approximately 14 days to see if the bacteria retained their inhibitive effect. Antifungal effect was graded qualitatively, where one plus (+) describes antifungal effect which did not retain the effect after 14 days, and two pluses (++) describes the antifungal effect which was retained after 14 days.
For the chitinase extracts, the fungi were inoculated as described above and after 3–4 days of growth, holes were punched 2 mm from the edge of the fungal colony and 50 μL of each enzyme solution, prepared as in
Section 4.7, was added to the wells. Plates were incubated for 2–4 days and checked for antifungal activity. All experiments were conducted at room temperature.