Dichotocejpins A–C: New Diketopiperazines from a Deep-Sea-Derived Fungus Dichotomomyces cejpii FS110
Abstract
:1. Introduction
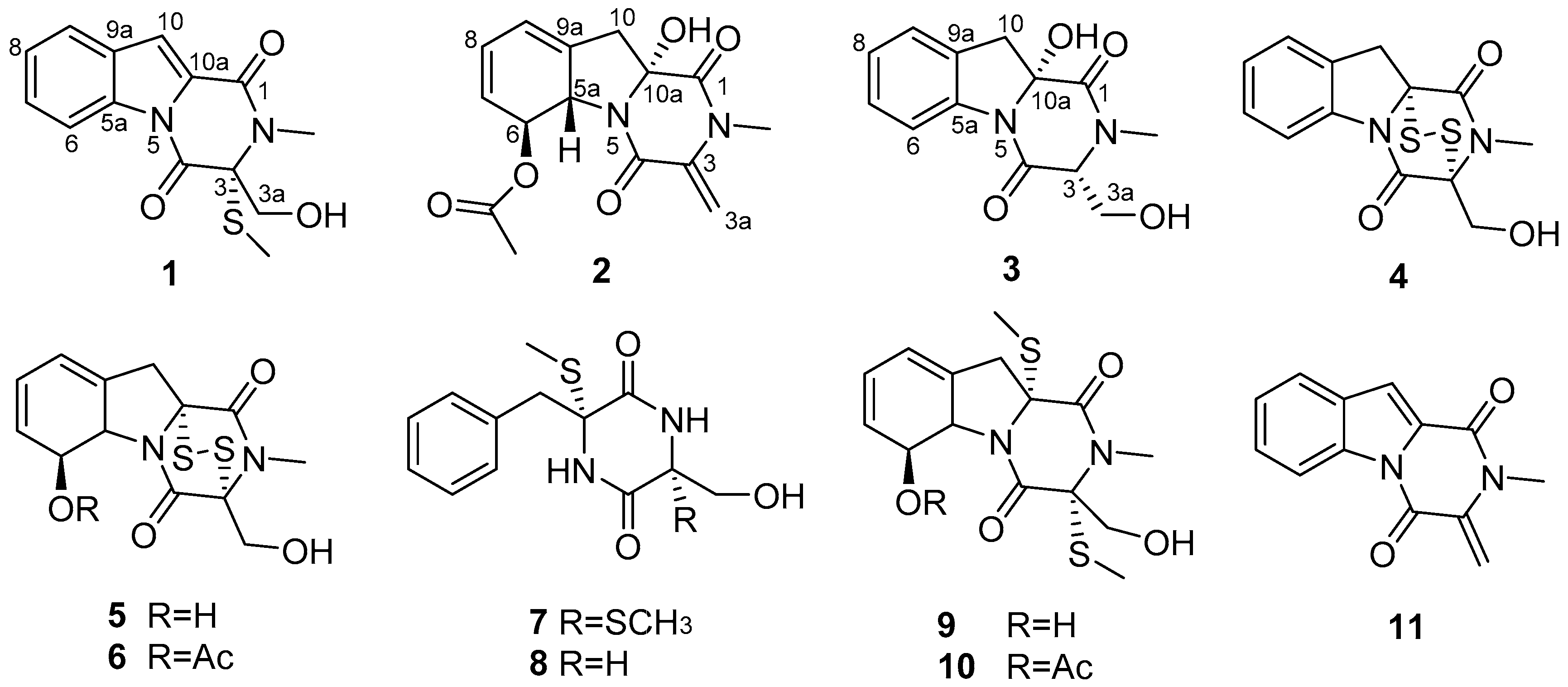
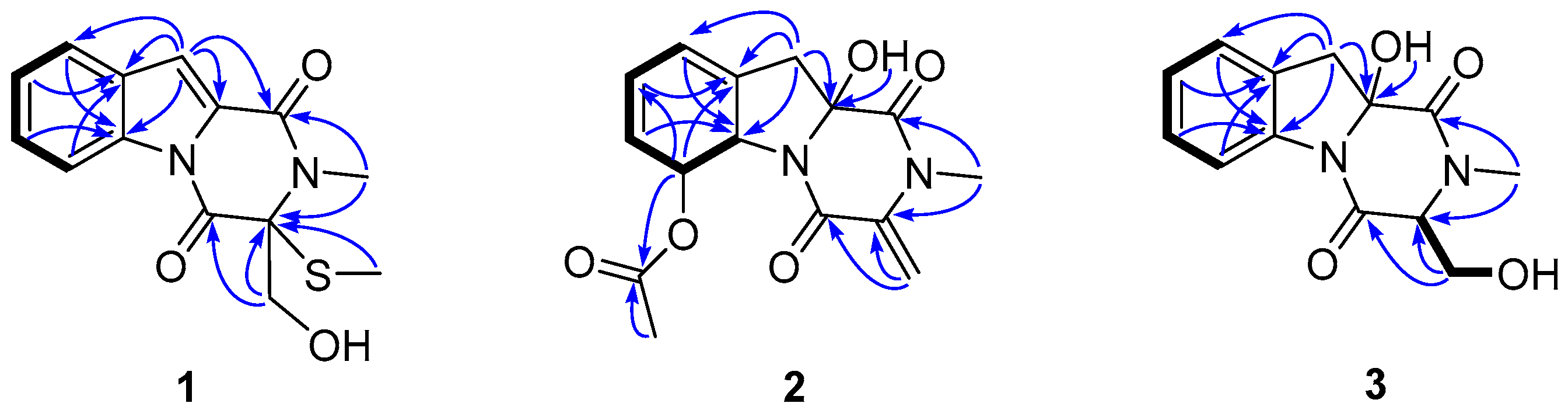
2. Results and Discussion
2.1. Identification of New Compounds
2.2. Cytotoxicity Assay
2.3. α-Glucosidase Inhibitory Activity Assay
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material and Identification
3.3. Fermentation, Extraction, and Isolation
3.4. Quantum-Chemical ECD Calculation
3.5. Crystallographic Data for 2
3.6. Cytotoxicity Assay
3.7. α-Glucosidase Inhibitory Activity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2016, 36, 382–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, K. Barophiles: Deep-sea microorganisms adapted to an extreme environment. Curr. Opin. Microbiol. 1998, 1, 291–295. [Google Scholar] [CrossRef]
- Niu, S.W.; Liu, D.; Hu, X.X.; Proksch, P.; Shao, Z.Z.; Lin, W. Spiromastixones A–O, antibacterial chlorodepsidones from a deep sea-derived Spiromastix sp. fungus. J. Nat. Prod. 2014, 77, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, D.L.; Tao, M.H.; Dan, F.J.; Zhang, W.M. Two new sesquiterpenes from the marine fungus Eutypella scoparia FS26 from the South China Sea. Helv. Chim. Acta 2012, 1, 157–162. [Google Scholar] [CrossRef]
- Sun, L.; Li, D.L.; Tao, M.H.; Chen, Y.C.; Zhang, Q.B.; Dan, F.J.; Zhang, W.M. Two new polyketides from the marine fungus Eutypella scoparia FS26. Nat. Prod. Res. 2012, 27, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, D.L.; Tao, M.H.; Chen, Y.C.; Dan, F.J.; Zhang, W.M. Scopararanes C–G: New oxygenated pimarane diterpenes from the marine sediment-derived fungus Eutypella scoparia FS26. Mar. Drugs 2012, 10, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.W.; Liu, H.X.; Sun, Z.H.; Chen, Y.C.; Tan, Y.Z.; Zhang, W.M. Secondary metabolites from the deep-sea derived fungus Acaromyces ingoldii FS121. Molecules 2016, 21, 371. [Google Scholar] [CrossRef]
- Yang, X.L.; Chen, Y.C.; Li, H.H.; Zhang, W.M. Molecular identification of 23 marine fungal strains and their activities against plant pathogenic fungi and cytotoxic activities. Biotechnol. Bull. 2014, 8, 132–137. [Google Scholar]
- Sun, Y.; Takada, K.; Takemoto, Y.; Yoshida, M.; Nogi, Y.; Okada, S.; Matsunaga, S. Gliotoxin analogues from a marine-derived fungus, Penicillium sp., and their cytotoxic and histone methyltransferase inhibitory activities. J. Nat. Prod. 2012, 75, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Mourad, K. Gliotoxin: Uncommon 1H couplings and revised 1H and 13C-NMR assignments. J. Nat. Prod. 1990, 53, 717–719. [Google Scholar]
- Katharine, R.W.; Joseline, R.; Ang, K.H.; Karen, T.; Jennifer, E.C.; James, M.; Phillip, C. Assessing the trypanocidal potential of natural and semi-synthetic diketopiperazines from two deep water marine-derived fungi. Bioorg. Med. Chem. 2010, 18, 2566–2574. [Google Scholar]
- Didier, V.D.; Junji, I.; Kazuro, S.; Hong, Y.; Hideo, T.; Satoshi, O. Inhibition of farnesyl-protein transferase by gliotoxin and acetylgliotoxin. J. Antibiot. 1992, 45, 1802–1805. [Google Scholar]
- Kirby, G.W.; Rao, G.V.; Robins, D.J. New co-metabolites of gliotoxin in Gliocladium virens. J. Chem. Soc. Perkin Trans. 1 1988, 301–304. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Zhu, T.J.; Han, X.X.; Fan, G.T.; Liu, H.B.; Zhu, W.M.; Gu, Q.Q. A new gliotoxin analogue from a marine-derived fungus Aspergillus fumigatus Fres. Nat. Prod. Res. 2009, 23, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Kirby, G.W.; Robins, D.J.; Sefton, M.A.; Talekar, R.R. Biosynthesis of bisdethiobis (methylthio) gliotoxin, a new metabolite of Gliocladium deliquescens. J. Chem. Soc. Perkin Trans. 1 1980, 119–121. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Kalinovskii, A.I.; Pivkin, M.V.; Dmitrenok, P.S.; Kuznetsova, T.A. Alkaloids from the marine isolate of the fungus Aspergillus fumigatus. Chem. Nat. Compd. 2005, 41, 236–238. [Google Scholar] [CrossRef]
- Ajay, K.B.; Das, K.G.; Funke, P.T.; Irene, K.; Shukla, O.P.; Khanchandani, K.S.; Suhadolnik, R.J. Biosynthetic studies on gliotoxin using stable isotopes and mass spectral methods. J. Am. Chem. Soc. 1968, 90, 1038–1041. [Google Scholar]
- Liang, W.L.; Le, X.; Li, H.J.; Yang, X.L.; Chen, J.X.; Xu, J.; Liu, H.L.; Wang, L.Y.; Wang, K.T.; Hu, K.C.; et al. Exploring the chemodiversity and biological activities of the secondary metabolites from the marine fungus Neosartorya pseudofischeri. Mar. Drugs 2014, 12, 5657–5676. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09W; Revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Q.; Xia, G.; Huang, H.; Li, H.; Ma, L.; Lu, Y.; He, L.; Xia, X.; She, Z. Polyketides with α-glucosidase inhibitory activity from a mangrove endophytic fungus, Penicillium sp. HN29-3B1. J. Nat. Prod. 2015, 78, 1816–1822. [Google Scholar] [CrossRef] [PubMed]


| Position | 1 a | 2 b | 3 b | |||
|---|---|---|---|---|---|---|
| δH, mult. (J in Hz) | δC, Type | δH, mult. (J in Hz) | δC, Type | δH, mult. (J in Hz) | δC, Type | |
| 1 | 158.0, C | 164.2, C | 165.2, C | |||
| 3 | 76.7, C | 138.4, C | 4.29, t (3.6) | 66.1, CH | ||
| 3a | 4.46, dd (12.0, 7.8) 4.08, dd (12.0, 4.8) | 64.2, CH2 | 5.48, d (1.1) 5.02, d (1.1) | 102.1, CH2 | 3.96, m | 60.5, CH2 |
| 4 | 163.5, C | 157.0, C | 164.6, C | |||
| 5a | 129.1, C | 4.98, d (14.0) | 63.0, CH | 128.8, C | ||
| 6 | 8.44, dt (8.3, 1.0) | 116.9, CH | 5.75, d (14.0) | 75.3, CH | 7.97, d (7.7) | 115.5, CH |
| 7 | 7.47, ddd (8.3, 7.2, 1.0) | 128.3, CH | 5.54, d (9.6) | 127.4, CH | 7.26, t (7.7) | 127.3, CH |
| 8 | 7.26, ddd (8.0, 7.2, 1.0) | 125.9, CH | 5.99, m | 125.4, CH | 7.13, td (7.7, 1.1) | 124.7, CH |
| 9 | 7.42, dt (8.0, 1.0) | 122.8, CH | 5.94, m | 118.5, CH | 7.35, d (7.7) | 125.2, CH |
| 9a | 134.9, C | 135.9, C | 140.0, C | |||
| 10 | 7.30, d (1.0) | 115.1, CH | 2.88, d (15.3) 2.75, d (15.3) | 41.7, CH2 | 3.53, d (17.2) 3.14, d (17.2) | 39.7, CH2 |
| 10a | 127.4, C | 88.7, C | 88.0, C | |||
| N-CH3 | 3.32, s | 28.2, CH3 | 3.11, s | 29.6, CH3 | 2.98, s | 32.2, CH3 |
| S-CH3 | 1.90, s | 12.2, CH3 | ||||
| OH-3a | 3.39, t (7.1) | 6.53, brs | ||||
| OH-10a | 7.14, s | 6.88, brs | ||||
| OAc | 2.02, s | 169.9, C 21.3, CH3 | ||||
| Compounds | IC50 (μM) a | ||||
|---|---|---|---|---|---|
| SF-268 | MCF-7 | NCI-H460 | HepG-2 | α-glucosidase | |
| 1 | 35.7 ± 2.1 | 29.5 ± 2.3 | >100 | 28.9 ± 3.0 | 138 ± 6.7 |
| 2 | >100 | >100 | >100 | >100 | >500 |
| 3 | >100 | >100 | >100 | >100 | >500 |
| 4 | 1.35 ± 0.05 | 0.68 ± 0.02 | 1.27 ± 0.04 | 1.52 ± 0.03 | >500 |
| 5 | 0.24 ± 0.10 | 0.08 ± 0.0 | 0.24 ± 0.01 | 0.21 ± 0.01 | >500 |
| 6 | 0.25 ± 0.03 | 0.22 ± 0.04 | 0.32 ± 0.02 | 0.49 ± 0.07 | >500 |
| 7 | >100 | >100 | >100 | >100 | >500 |
| 8 | >100 | >100 | >100 | >100 | >500 |
| 9 | >100 | >100 | >100 | >100 | >500 |
| 10 | 34.0 ± 3.6 | 3.1 ± 0.10 | 5.4 ± 0.60 | 7.0 ± 0.17 | >500 |
| 11 | 3.3 ± 0.28 | 4.67 ± 1.4 | 12.3 ± 0.24 | 2.29 ± 0.30 | >500 |
| positive control | 2.37 ± 0.35 b | 3.09 ± 0.27 b | 2.43 ± 0.15 b | 1.39 ± 0.18 b | 463 ± 35 c |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Z.; Sun, Z.-H.; Liu, Z.; Chen, Y.-C.; Liu, H.-X.; Li, H.-H.; Zhang, W.-M. Dichotocejpins A–C: New Diketopiperazines from a Deep-Sea-Derived Fungus Dichotomomyces cejpii FS110. Mar. Drugs 2016, 14, 164. https://doi.org/10.3390/md14090164
Fan Z, Sun Z-H, Liu Z, Chen Y-C, Liu H-X, Li H-H, Zhang W-M. Dichotocejpins A–C: New Diketopiperazines from a Deep-Sea-Derived Fungus Dichotomomyces cejpii FS110. Marine Drugs. 2016; 14(9):164. https://doi.org/10.3390/md14090164
Chicago/Turabian StyleFan, Zhen, Zhang-Hua Sun, Zhong Liu, Yu-Chan Chen, Hong-Xin Liu, Hao-Hua Li, and Wei-Min Zhang. 2016. "Dichotocejpins A–C: New Diketopiperazines from a Deep-Sea-Derived Fungus Dichotomomyces cejpii FS110" Marine Drugs 14, no. 9: 164. https://doi.org/10.3390/md14090164





