Anti-Inflammatory Lobane and Prenyleudesmane Diterpenoids from the Soft Coral Lobophytum varium
Abstract
:1. Introduction
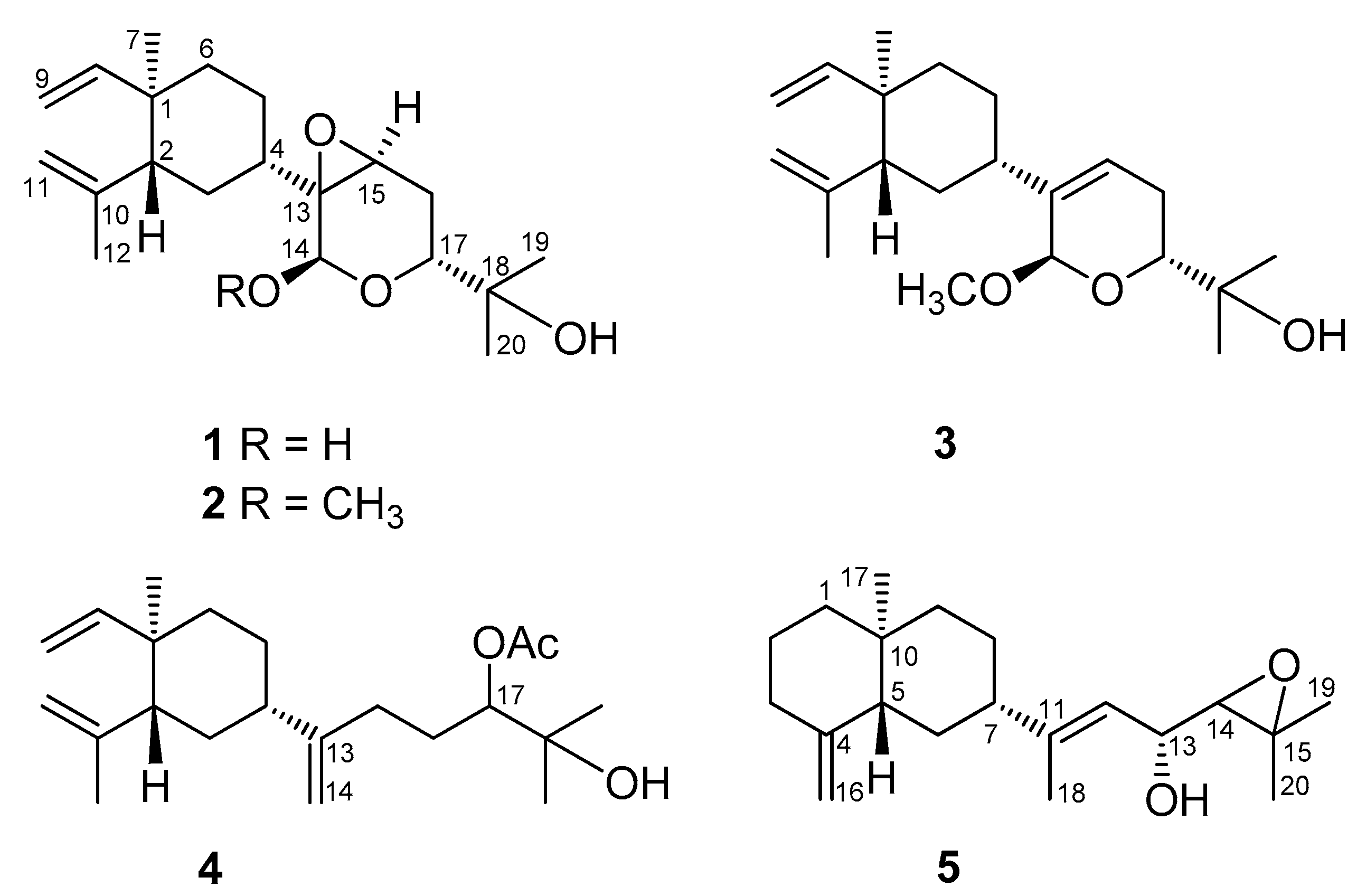
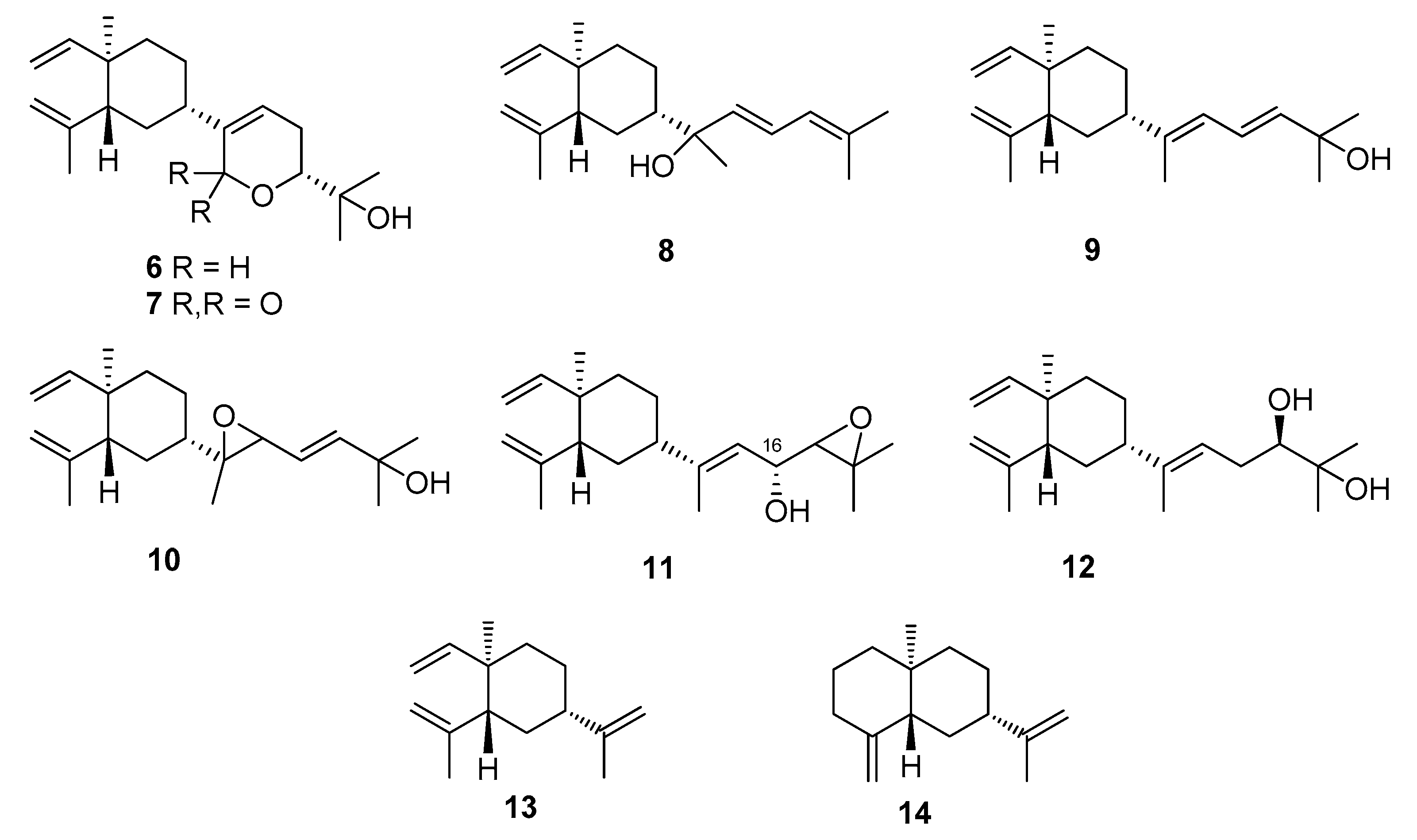
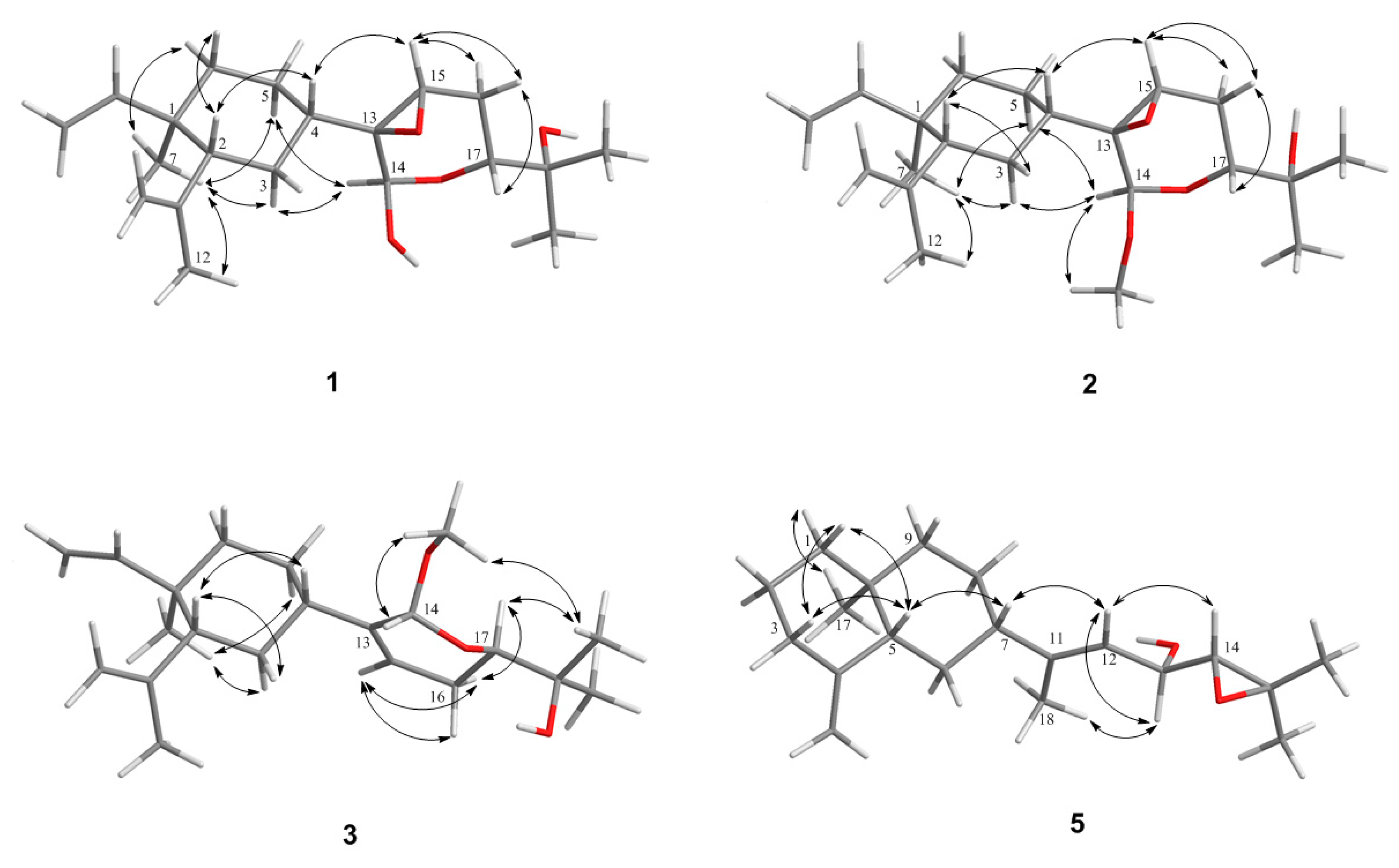
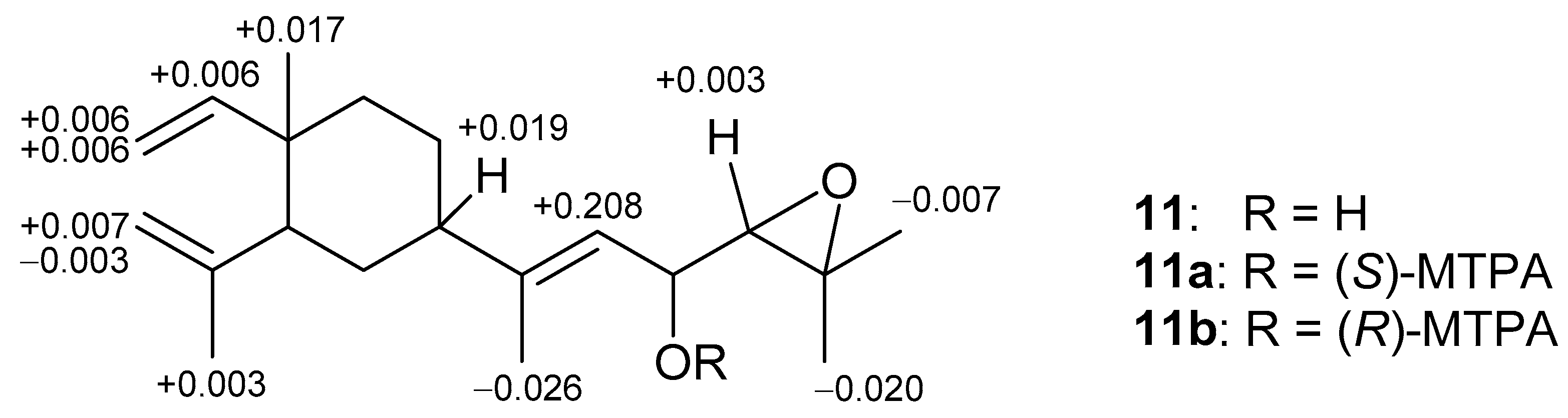
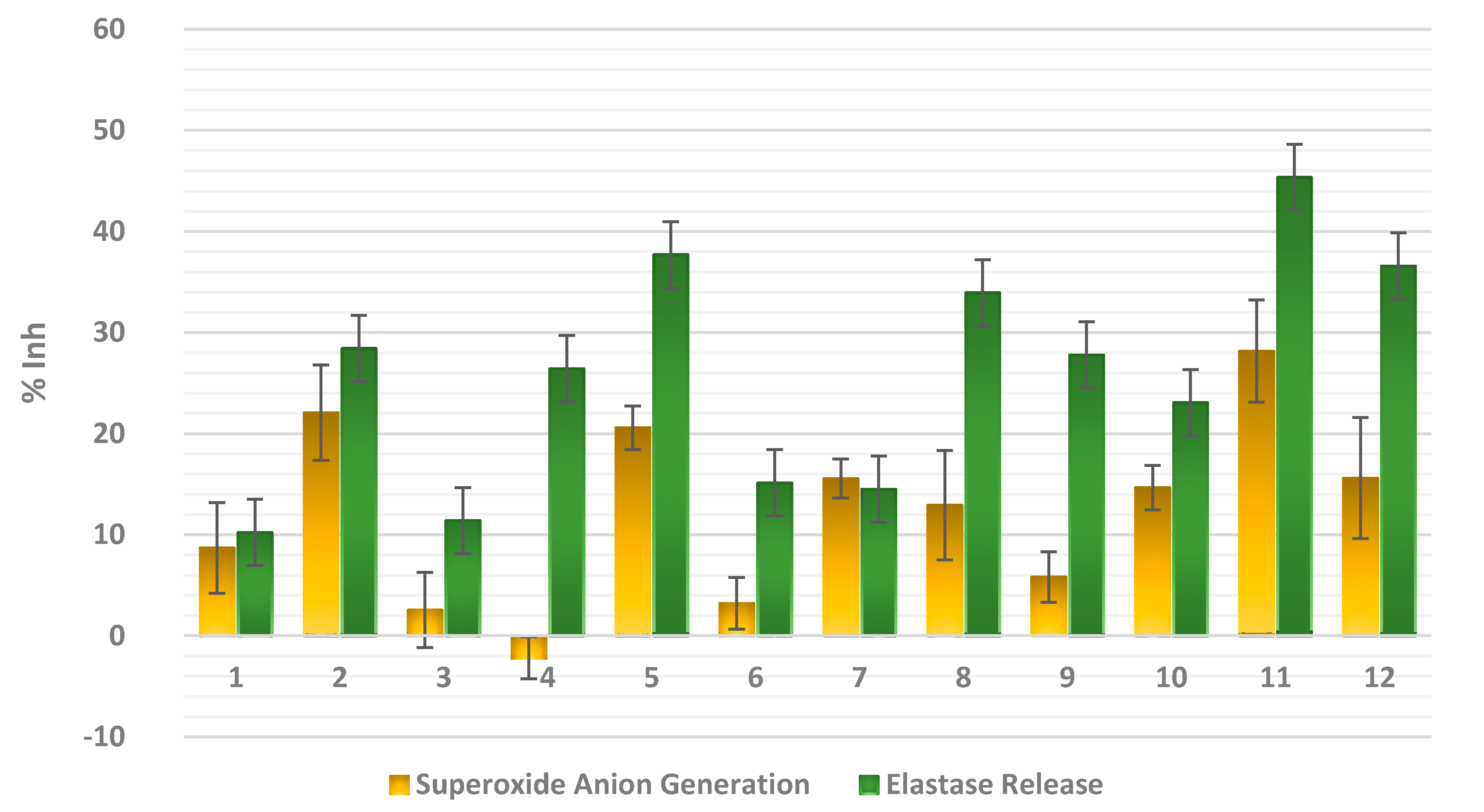
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
3.2. Animal Material
3.3. Extraction and Separation
3.3.1. Lobovarol A (1)
3.3.2. Lobovarol B (2)
3.3.3. Lobovarol C (3)
3.3.4. Lobovarol D (4)
3.3.5. Lobovarol E (5)
3.3.6. Preparation of (S)- and (R)-MTPA Esters of 11
3.4. Cytotoxicity Assay
3.5. In Vitro Anti-Inflammatory Assay
3.5.1. Measurement of Superoxide Anion Generation
3.5.2. Measurement of Elastase Release
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chen, Y.; Chan, W.Y. Marine natural products with anti-inflammatory activity. Appl. Microbiol. Biotechnol. 2016, 100, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.C.; Sung, P.J.; Duh, C.Y.; Chen, B.W.; Sheu, J.H.; Yang, N.S. Anti-inflammatory activities of natural products isolated from soft corals of Taiwan between 2008 and 2012. Mar. Drugs 2013, 11, 4083–4126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yin, J.; Jiang, W.; Ma, M.; Lei, X.; Xiang, Z.; Dong, J.; Huang, K.; Yan, P. Cytotoxic and antibacterial cembranoids from a South China Sea soft coral, Lobophytum sp. Mar. Drugs 2013, 11, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Duh, C.Y. New cytotoxic cembranolides from the soft coral Lobophytum michaelae. Mar. Drugs 2012, 10, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.Y.; Su, J.H.; Lu, M.C.; Hwang, T.L.; Wang, W.H.; Chen, J.J.; Sheu, J.H.; Kuo, Y.H.; Weng, C.F.; Fang, L.S.; et al. Lobocrassins A-E: New cembrane-type diterpenoids from the soft coral Lobophytum crassum. Mar. Drugs 2011, 9, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.L.; Su, J.H. Tetrahydrofuran cembranoids from the cultured soft coral Lobophytum crassum. Mar. Drugs 2011, 9, 2526–2536. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Lobophytones H-N, biscembranoids from the Chinese soft coral Lobophytum pauciflorum. Chem. Pharm. Bull. 2010, 58, 1591–1595. [Google Scholar] [CrossRef] [PubMed]
- Bonnard, I.; Jhaumeer-Laulloo, S.B.; Bontemps, N.; Banaigs, B.; Aknin, M. New lobane and cembrane diterpenes from two comorian soft corals. Mar. Drugs 2010, 8, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Lobophytones O-T, new biscembranoids and cembranoid from soft coral Lobophytum pauciflorum. Mar. Drugs 2010, 8, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Edrada, R.A.; Proksch, P.; Wray, V.; Witte, L.; van Ofwegen, L. Four new bioactive lobane diterpenes of the soft coral Lobophytum pauciflorum from Mindoro, Philippines. J. Nat. Prod. 1998, 61, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, R.W.; Wells, R.J. Isolation of some novel diterpenes from a soft coral of the Genus Lobophytum. Aust. J. Chem. 1979, 32, 1345–1351. [Google Scholar] [CrossRef]
- Lakshmana Raju, B.; Subbaraju, G.V.; Bheemasankara Rao, C.; Trimurtulu, G. Two new oxygenated lobanes from a soft coral of Lobophytum species of the Andaman and Nicobar coasts. J. Nat. Prod. 1993, 56, 961–966. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Venkateswara Rao, G.; Raju, K.V.S.; Murthy, M.V.R.K. Two new lobane derivatives from the soft coral Lobophytum pauciflorum of the Havelock Island of the Indian Ocean. Indian J. Chem. 1995, 34B, 1074–1079. [Google Scholar] [CrossRef]
- Rashid, M.A.; Gustafson, K.R.; Boyd, M.R. HIV-inhibitory cembrane derivatives from a Philippines collection of the soft coral Lobophytum species. J. Nat. Prod. 2000, 63, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-H.; Wen, Z.-H.; Wu, Y.-C.; Yeh, H.-C.; Sheu, J.-H. Cytotoxic and Anti-inflammatory Cembranoids from the Soft Coral Lobophytum crassum. J. Nat. Prod. 2008, 71, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.; Mohamed, T.A.; Elshamy, A.I.; Hassanien, A.A.; Abdel-Azim, N.S.; Shreadah, M.A.; Abdelgawad, I.I.; Elkady, E.M.; Pare, P.W. A new steroid from the Red Sea soft coral Lobophytum lobophytum. Nat. Prod. Res. 2016, 30, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.A.; Christie, E.M.; Jaspars, M.; van Ofwegen, L.P. A bioactive secosterol with an unusual A- and B-ring oxygenation pattern isolated from an Indonesian soft coral Lobophytum sp. J. Nat. Prod. 1998, 61, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lin, Y.-C.; Wen, Z.-H.; Su, J.-H.; Sung, P.-J.; Hsu, C.-H.; Kuo, Y.-H.; Chiang, M.Y.; Dai, C.-F.; Sheu, J.-H. Steroid and cembranoids from the Dongsha atoll soft coral Lobophytum sarcophytoides. Tetrahedron 2010, 66, 7129–7135. [Google Scholar] [CrossRef]
- Rama Rao, M.; Venkatesham, U.; Reddy, M.V.R.; Venkateswarlu, Y. An unusual novel C29 steroid from the soft coral Lobophytum crassum. J. Nat. Prod. 1999, 62, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.-H.; Yeh, T.-H. Isolation of a Bioactive Sterol from the Soft Coral Lobophytum Mirabile. J. Chin. Chem. Soc. 1991, 38, 397–399. [Google Scholar] [CrossRef]
- Tursch, B.; Hootele, C.; Kaisin, M.; Losman, D.; Karlsson, R. Chemical studies of marine invertebrates. XVI. Structure and absolute configuration of lobosterol, a novel polyoxygenated sterol from the alcyonacean Lobophytum pauciflorum (coelenterata, octocorallia). Steroids 1976, 27, 137–142. [Google Scholar] [CrossRef]
- Li, L.; Sheng, L.; Wang, C.Y.; Zhou, Y.B.; Huang, H.; Li, X.B.; Li, J.; Mollo, E.; Gavagnin, M.; Guo, Y.W. Diterpenes from the Hainan soft coral Lobophytum cristatum Tixier-Durivault. J. Nat. Prod. 2011, 74, 2089–2094. [Google Scholar] [CrossRef] [PubMed]
- Coll, J.C.; Bowden, B.F.; König, G.M.; Braslau, R.; Price, I.R. Studies of Australian soft corals. XXXX.1 The natural products chemistry of Alcyonacean soft corals with special reference to the genus Lobophytum. Bull. Soc. Chim. Belg. 1986, 95, 815–834. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Chuang, C.T.; Wang, S.K.; Wen, Z.H.; Chiou, S.F.; Hsu, C.H.; Dai, C.F.; Duh, C.Y. Antiviral and anti-inflammatory diterpenoids from the soft coral Sinularia gyrosa. J. Nat. Prod. 2010, 73, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Fenical, W. Fuscosides A-D: Anti-inflammatory diterpenoid glycosides of new structural classes from the caribbean gorgonian Eunicea fusca. J. Org. Chem. 1991, 56, 3153–3158. [Google Scholar] [CrossRef]
- Kerr, R.G.; Brophy, S.; Derksen, D.J. Synthesis and evaluation of anti-inflammatory activity of derivatives of the marine natural products fuscol and eunicol. Bioorg. Med. Chem. Lett. 2014, 24, 4804–4806. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H.; Huang, H.C.; Wu, Y.C.; Lu, C.K.; Dai, C.F.; Sheu, J.H. Glycolipids from the Formosan soft coral Lobophytum crassum. Chem. Pharm. Bull. 2007, 55, 1720–1723. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.L.; Yeh, S.H.; Leu, Y.L.; Chern, C.Y.; Hsu, H.C. Inhibition of superoxide anion and elastase release in human neutrophils by 3′-isopropoxychalcone via a cAMP-dependent pathway. Br. J. Pharmacol. 2006, 148, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.N.; Tai, C.J.; Hwang, T.L.; Sheu, J.H. Krempfielins J-M, new eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Mar. Drugs 2013, 11, 2741–2750. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Ahmed, A.F.; Su, J.H.; Sung, P.J.; Hwang, T.L.; Chiang, P.L.; Dai, C.F.; Liaw, C.C.; Sheu, J.H. Bioactive new withanolides from the cultured soft coral Sinularia brassica. Bioorg. Med. Chem. Lett. 2017, 27, 3267–3271. [Google Scholar] [CrossRef] [PubMed]
- Kusumi, T.; Hamada, T.; Ishitsuka, M.; Ohtani, I.; Kakisawa, H. Elucidation of the relative and absolute stereochemistry of lobatriene, a marine diterpene, by a modified Mosher method. J. Org. Chem. 1992, 57, 1033–1035. [Google Scholar] [CrossRef]
- Govindam, S.V.; Yoshioka, Y.; Kanamoto, A.; Fujiwara, T.; Okamoto, T.; Ojika, M. Cyclolobatriene, a novel prenylated germacrene diterpene, from the soft coral Lobophytum pauciflorum. Bioorg. Med. Chem. 2012, 20, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Kusumi, T.; Ishitsuka, M.O.; Kakisawa, H. Structures and absolute configurations of new lobane diterpenoids from the Okinawan soft coral Sinularia flexibilis. Chem. Lett. 1992, 21, 33–36. [Google Scholar] [CrossRef]
- Gopichand, Y.; Schmitz, F.J. Marine natural products: Fuscol, a new elemene-type diterpene alcohol from the gorgonian Eunicea fusca. Tetrahedron Lett. 1978, 39, 3641–3644. [Google Scholar] [CrossRef]
- Babu, U.V.; Bhandari, S.P.S.; Garg, H.S. Diterpenes from the soft coral Lobophytum pauciflorum of Indian coast. Indian J. Chem. 1998, 37B, 576–578. [Google Scholar]
- Corey, E.J.; Roberts, B.E.; Dixon, B.R. Enantioselective total synthesis of β-elemene and fuscol based on enantiocontrolled Ireland-Claisen rearrangement. J. Am. Chem. Soc. 1995, 117, 193–196. [Google Scholar] [CrossRef]
- CambridgeSoft. CS Chem3D 9.0 (Molecular Modeling and Analysis Standard); CambridgeSoft Inc.: Cambridge, MA, USA, 2004; Available online: http://www.cambridgesoft.com/support/DesktopSupport/ Documentation/Manuals/files/chem3d_9_english.pdf (accessed on 28 September 2017).
- Charifson, P.S.; Bowen, J.P.; Wyrick, S.D.; Hoffman, A.J.; Cory, M.; McPhail, A.T.; Mailman, R.B. Conformational analysis and molecular modeling of 1-phenyl-, 4-phenyl-, and 1-benzyl-1,2,3,4-tetrahydroisoquinolines as D1 dopamine receptor ligands. J. Med. Chem. 1989, 32, 2050–2058. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.J.; Sattler, I.; Moyna, G.; Scott, A.I.; Bell, A.A.; Vinson, S.B. Diversity in cyclic sesquiterpene production by Gossypium hirsutum. Phytochemistry 1995, 40, 1633–1636. [Google Scholar] [CrossRef]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-Field FT NMR application of Mosher’s Method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Randazzo, A.; Bifulco, G.; Giannini, C.; Bucci, M.; Debitus, C.; Cirino, G.; Gomez-Paloma, L. Halipeptins A and B: Two novel potent anti-inflammatory cyclic depsipeptides from the Vanuatu marine sponge Haliclona species. J. Am. Chem. Soc. 2001, 123, 10870–10876. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Ahmed, A.F.; Su, J.H.; Chao, C.H.; Wu, Y.C.; Chiang, M.Y.; Sheu, J.H. Crassolides A-F, cembranoids with a trans-fused lactone from the soft coral Sarcophyton crassocaule. J. Nat. Prod. 2006, 69, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parandoosh, Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef]
- Yang, S.C.; Chung, P.J.; Ho, C.M.; Kuo, C.Y.; Hung, M.F.; Huang, Y.T.; Chang, W.Y.; Chang, Y.W.; Chan, K.H.; Hwang, T.L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.P.; Hsieh, P.W.; Chang, Y.J.; Chung, P.J.; Kuo, L.M.; Hwang, T.L. 2-(2-Fluorobenzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef] [PubMed]


| # | 1 a | 2 b | 3 a | 4 a | 5 b |
|---|---|---|---|---|---|
| 1 | - | - | - | - | 1.46 m; 1.28 m |
| 2 | 1.94 br dd (9.2, 6.0) c | 1.95 dd (6.0, 6.0, 3.0) | 2.02 dd (12.4, 4.0) | 2.00 m | 1.62, 2H, m |
| 3 | 1.56, 2H, m | 1.59 m; 1.49 m | 1.61 m; 1.54 m | 1.54, 2H, m | 2.31 d (13.0); 2.01 m |
| 4 | 1.56 m | 1.56 m | 2.00 m | 1.89 m | |
| 5 | 1.64 m; 1.36 m | 1.63 m; 1.33 m | 1.64 m; 1.49 m | 1.61 m; 1.41 m | 1.83 d (12.0) |
| 6 | 1.38–1.50, 2H, m | 1.46, 2H, m | 1.48, 2H, m | 1.46, 2H, m | 1.54 m; 1.34 m |
| 7 | 0.99, 3H, s | 0.98 3H, s | 1.00, 3H, s | 1.00, 3H, s | 1.97 m |
| 8 | 5.78 dd (17.6, 10.4) | 5.79 dd (18.0, 10.5) | 5.82 dd (17.6, 10.4) | 5.82 dd (18.0, 10.8) | 1.53 m, 1.28 m |
| 9 | 4.90 d (17.6); 4.90 d (10.4) | 4.90 d (16); 4.89 d (13.5) | 4.92 d (17.6); 4.91 d (10.4) | 4.92 d (16.4); 4.90 d (11.6) | 1.52, m; 1.28 m |
| 11 | 4.84 br s; 4.60 br s | 4.83 s; 4.58 s | 4.82 dd (1.6, 1.6); 4.58 br d (1.6) | 4.82 s; 4.59 s | - |
| 12 | 1.70 3H, s | 1.70 3H, s | 1.71 3H, s | 1.71, 3H, s | 5.33 d (8.5) |
| 13 | 4.25 dd (8.5, 8.0) | ||||
| 14 | 5.30 s | 4.91 s | 4.88 s | 4.82 s; 4.74 s | 2.82 d (8.0) |
| 15 | 3.49 dd (2.0, 2.0) | 3.30 br s | 5.73 br d (6.0) | 2.03, 2H, m | - |
| 16 | 2.04 ddd (14.8, 2.4, 2.4); 1.82 ddd (14.8, 11.2, 2.0) | 2.05 ddd (14.0, 2.5, 2.5); 1.84 ddd (14.0, 12.0, 2.5) | 2.22 m; 1.99 m | 1.75, 2H, m | 4.72 s; 4.43 s |
| 17 | 3.45 dd (11.2, 3.0) | 3.58 dd (12, 2.5) | 3.67 dd (11.6, 3.6) | 4.84 dd (10.4, 2.8) | 0.73, 3H, s |
| 18 | - | - | - | - | 1.72, 3H, s |
| 19 | 1.13, 3H, s | 1.14, 3H, s | 1.18, 3H, s | 1.21, 3H, s | 1.33, 3H, s |
| 20 | 1.22, 3H, s | 1.25, 3H, s | 1.29, 3H, s | 1.22, 3H, s | 1.32, 3H, s |
| OMe | - | 3.47, 3H, s | 3.46 3H, s | - | - |
| OAc | - | - | - | 2.13, 3H, s | - |
| # | 1 a | 2 b | 3 a | 4 a | 5 b |
|---|---|---|---|---|---|
| 1 | 39.7 (C) | 39.7 (C) | 39.7 (C) | 39.8 (C) | 41.9 (CH2) |
| 2 | 52.1 (CH) c | 52.0 (CH) | 52.8 (CH) | 52.8 (CH) | 23.4 (CH2) |
| 3 | 28.7 (CH2) | 28.9 (CH2) | 34.1 (CH2) | 33.3 (CH2) | 36.9 (CH2) |
| 4 | 41.9 (CH) | 41.8 (CH) | 40.5 (CH) | 44.4 (CH) | 150.9 (C) |
| 5 | 22.8 (CH2) | 22.9 (CH2) | 26.4 (CH2) | 27.2 (CH2) | 49.9(CH) |
| 6 | 39.1 (CH2) | 39.2 (CH2) | 39.8 (CH2) | 40.0 (CH2) | 29.2 (CH2) |
| 7 | 16.5 (CH3) | 16.5 (CH3) | 16.6 (CH3) | 16.6 (CH3) | 47.6 (CH) |
| 8 | 149.6 (CH) | 149.8 (CH) | 150.2 (CH) | 150.2 (CH) | 26.6 (CH2) |
| 9 | 110.2 (CH2) | 110.1 (CH2) | 109.9 (CH2) | 109.9 (CH2) | 41.0 (CH2) |
| 10 | 147.1 (C) | 147.2 (C) | 147.5 (C) | 147.6 (C) | 36.0 (C) |
| 11 | 112.5 (CH2) | 112.4 (CH2) | 112.2 (CH2) | 112.1 (CH2) | 146.1 (C) |
| 12 | 24.9 (CH3) | 24.7 (CH3) | 24.7 (CH3) | 24.8 (CH3) | 120.8 (CH) |
| 13 | 64.2 (C) | 61.7 (C) | 140.6 (C) | 153.6 (C) | 67.9 (CH) |
| 14 | 89.5 (CH) | 97.7 (CH) | 97.8 (CH) | 107.5 (CH2) | 67.5 (CH) |
| 15 | 59.1 (CH) | 55.3 (CH) | 121.1 (CH) | 31.4 (CH2) | 59.8 (C) |
| 16 | 24.8 (CH2) | 25.2 (CH2) | 24.8 (CH2) | 28.1 (CH2) | 105.4 (CH2) |
| 17 | 68.3 (CH) | 69.5 (CH) | 72.1 (CH) | 79.7 (CH) | 16.4 (CH) |
| 18 | 71.1 (C) | 71.4 (C) | 71.5 (C) | 72.5 (C) | 15.5 (CH3) |
| 19 | 24.0 (CH3) | 24.5 (CH3) | 24.4 (CH3) | 24.9 (CH3) | 24.9 (CH3) |
| 20 | 26.5 (CH3) | 27.0 (CH3) | 26.7 (CH3) | 26.8 (CH3) | 19.6 (CH3) |
| 14-OMe | 55.6 (CH3) | 55.4 (CH3) | |||
| 17-OAc | 21.1 (CH3) | ||||
| 171.3 (C) |
| Compound | 1 (β-Epoxide) | 1a (α-Epoxide) |
|---|---|---|
| Dihedral angle of C(3)-C(4)-C(13)-O | −80° | −150° |
| Minimum energy conformer (Kcal/mol) | 75.78 | 80.92 |
| Calculated distances | ||
| H(4)-H(15) | 2.45 Å | 2.45 Å |
| H(14)-H(3α) | 2.54 Å | 3.73 Å |
| H(14)-H(5α) | 2.30 Å | 2.51 Å |
| H(14)-H(5β) | 3.35 Å | 2.79 Å |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.F.; Teng, W.-T.; Huang, C.-Y.; Dai, C.-F.; Hwang, T.-L.; Sheu, J.-H. Anti-Inflammatory Lobane and Prenyleudesmane Diterpenoids from the Soft Coral Lobophytum varium. Mar. Drugs 2017, 15, 300. https://doi.org/10.3390/md15100300
Ahmed AF, Teng W-T, Huang C-Y, Dai C-F, Hwang T-L, Sheu J-H. Anti-Inflammatory Lobane and Prenyleudesmane Diterpenoids from the Soft Coral Lobophytum varium. Marine Drugs. 2017; 15(10):300. https://doi.org/10.3390/md15100300
Chicago/Turabian StyleAhmed, Atallah F., Wan-Ting Teng, Chiung-Yao Huang, Chang-Feng Dai, Tsong-Long Hwang, and Jyh-Horng Sheu. 2017. "Anti-Inflammatory Lobane and Prenyleudesmane Diterpenoids from the Soft Coral Lobophytum varium" Marine Drugs 15, no. 10: 300. https://doi.org/10.3390/md15100300






