Dual Biological Functions of a Cytoprotective Effect and Apoptosis Induction by Bioavailable Marine Carotenoid Fucoxanthinol through Modulation of the Nrf2 Activation in RAW264.7 Macrophage Cells
Abstract
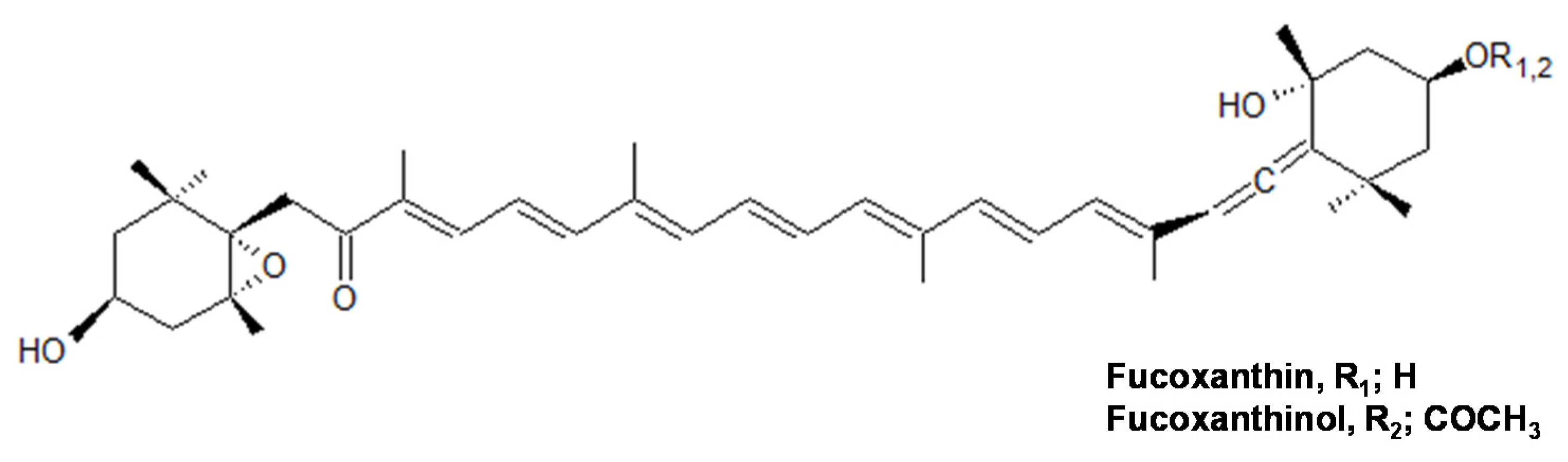
:1. Introduction
2. Results
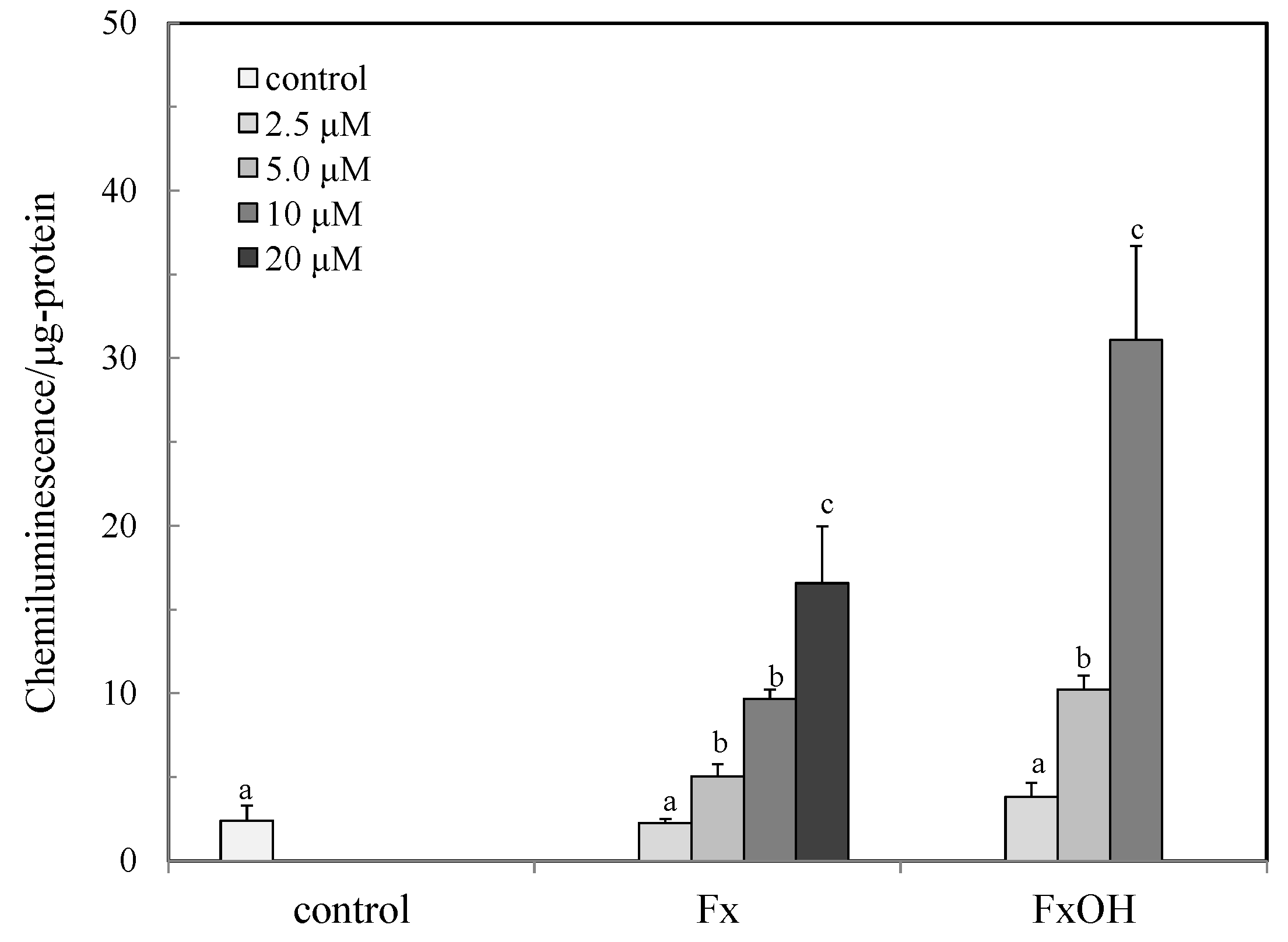
2.1. Antioxidant Capacity by Peroxyl Radical Scavenging
2.2. Cytotoxicity of the Compounds
2.3. Nrf2-ARE Signaling
2.4. Nuclear Nrf2 Expression
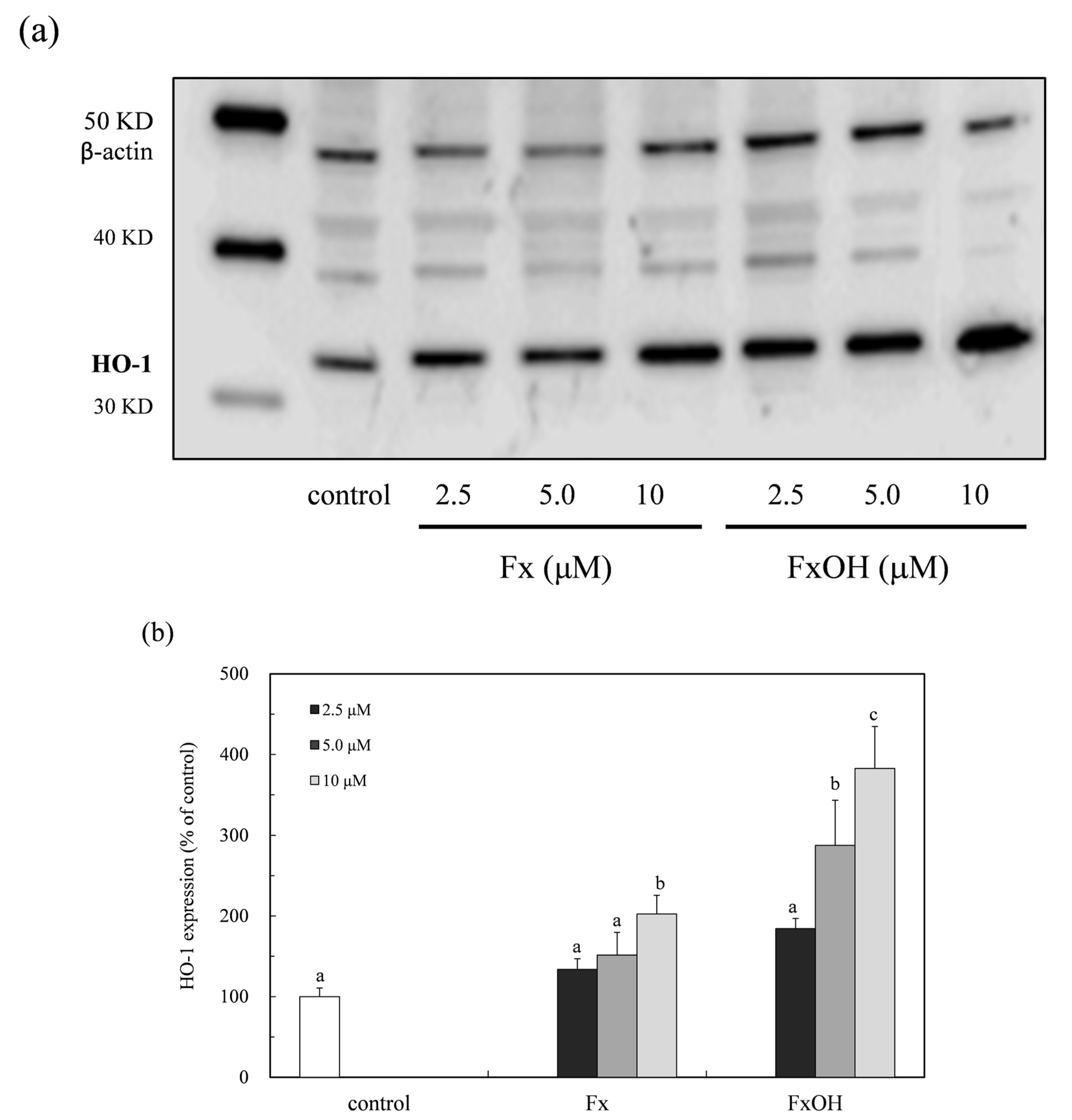
2.5. HO-1 Expression
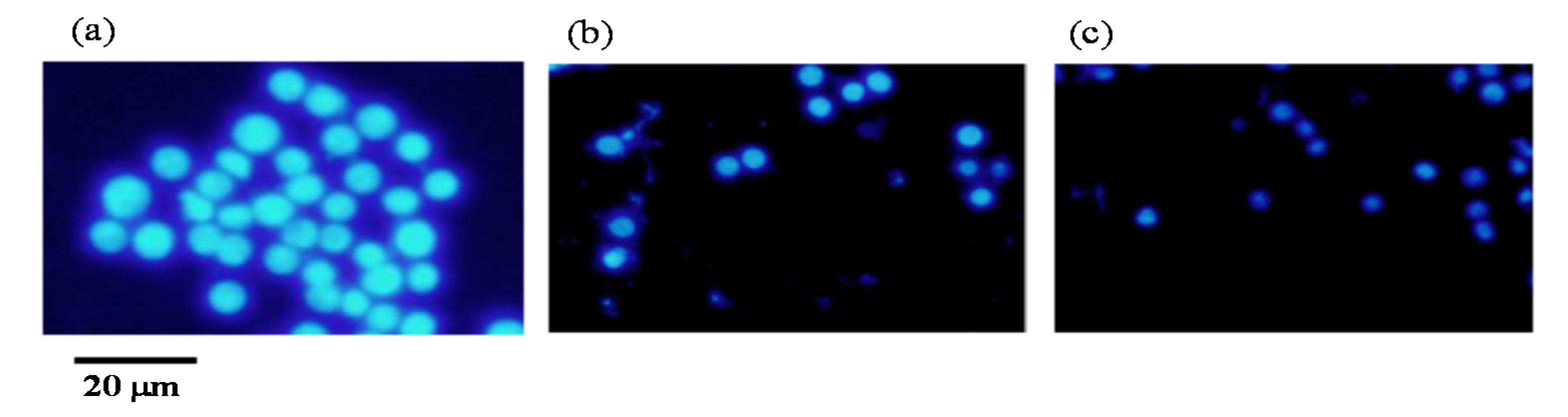
2.6. Apoptotic Cell Induction
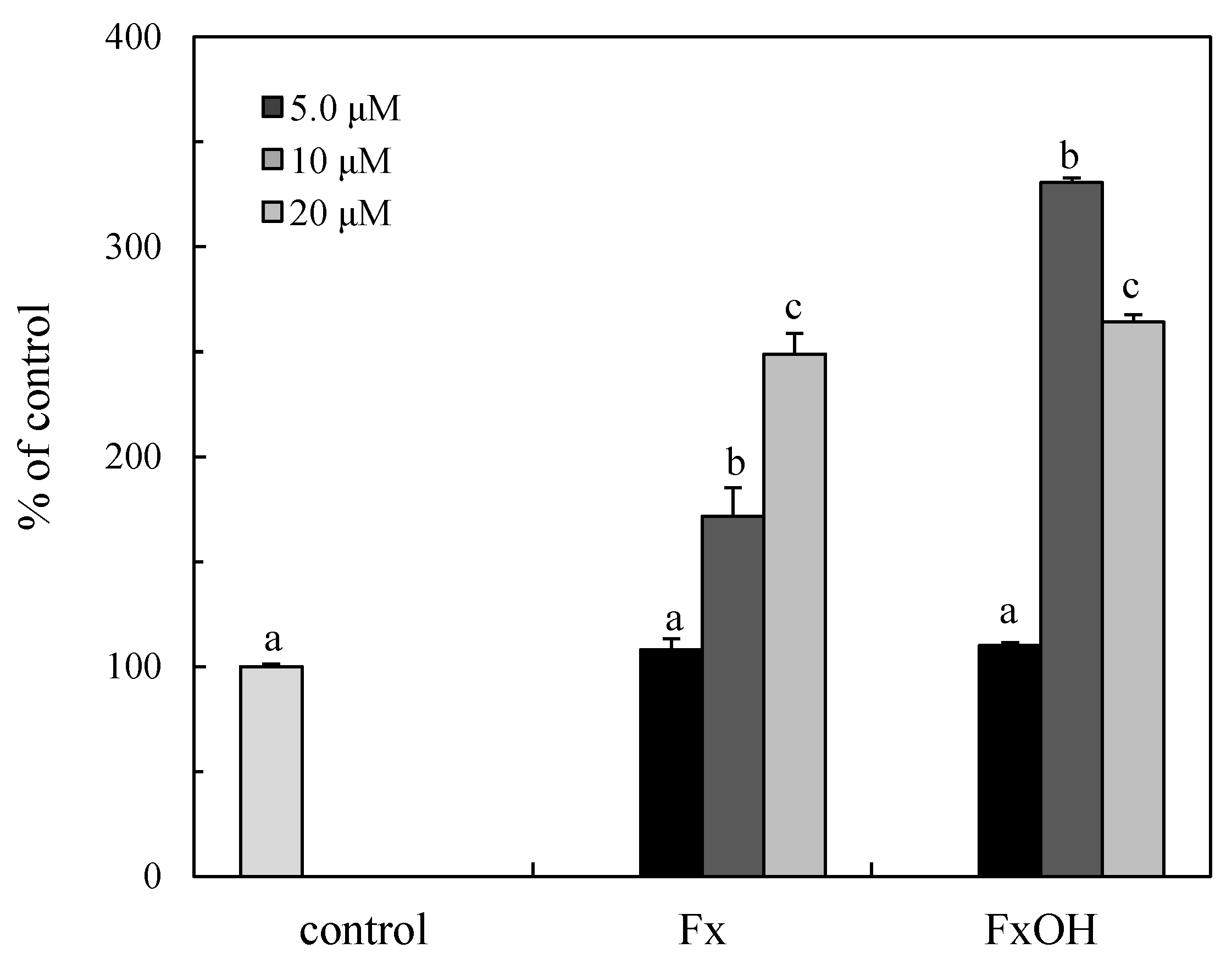
2.7. Caspase Activity
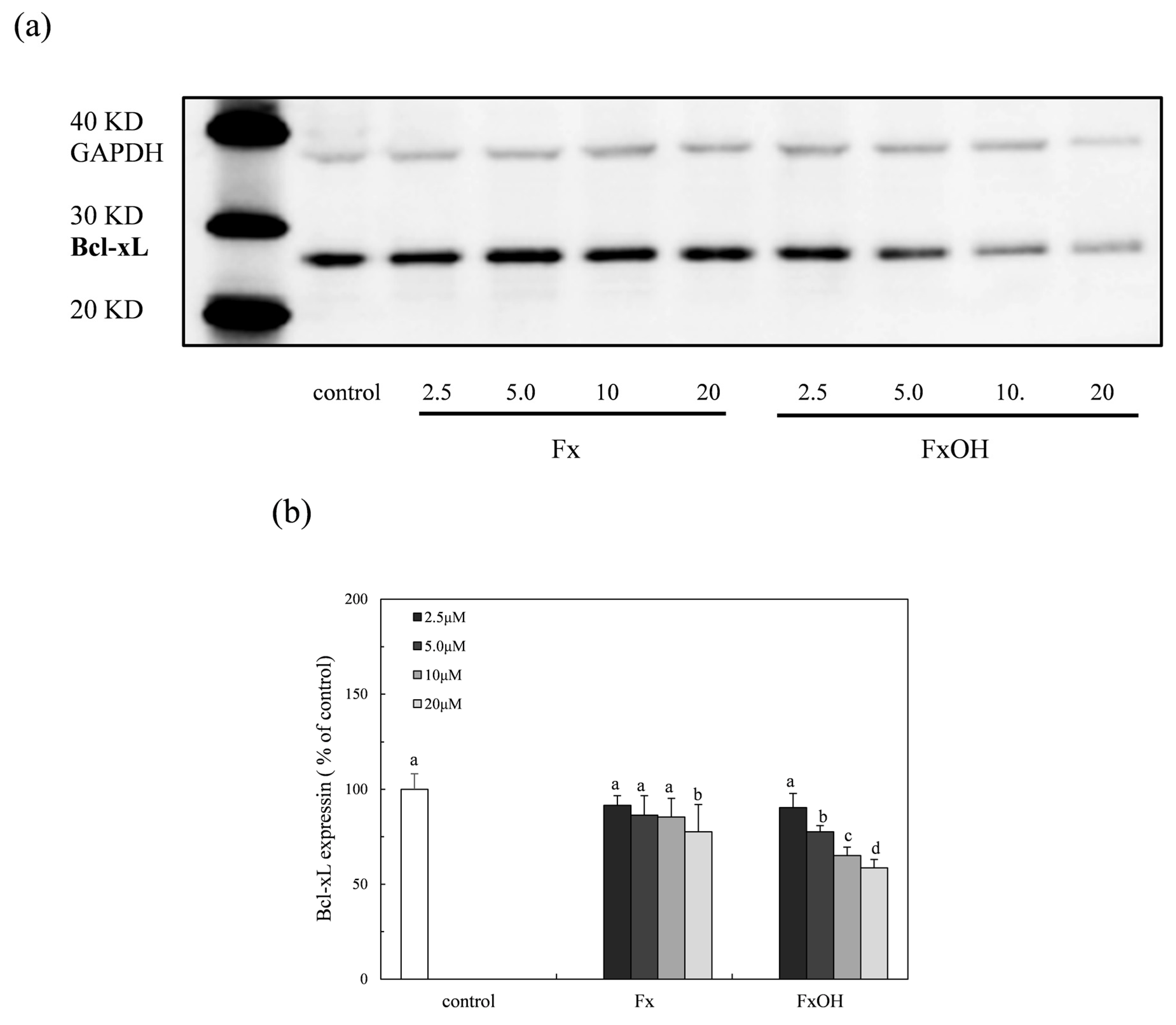
2.8. Bcl-xL Protein Expression
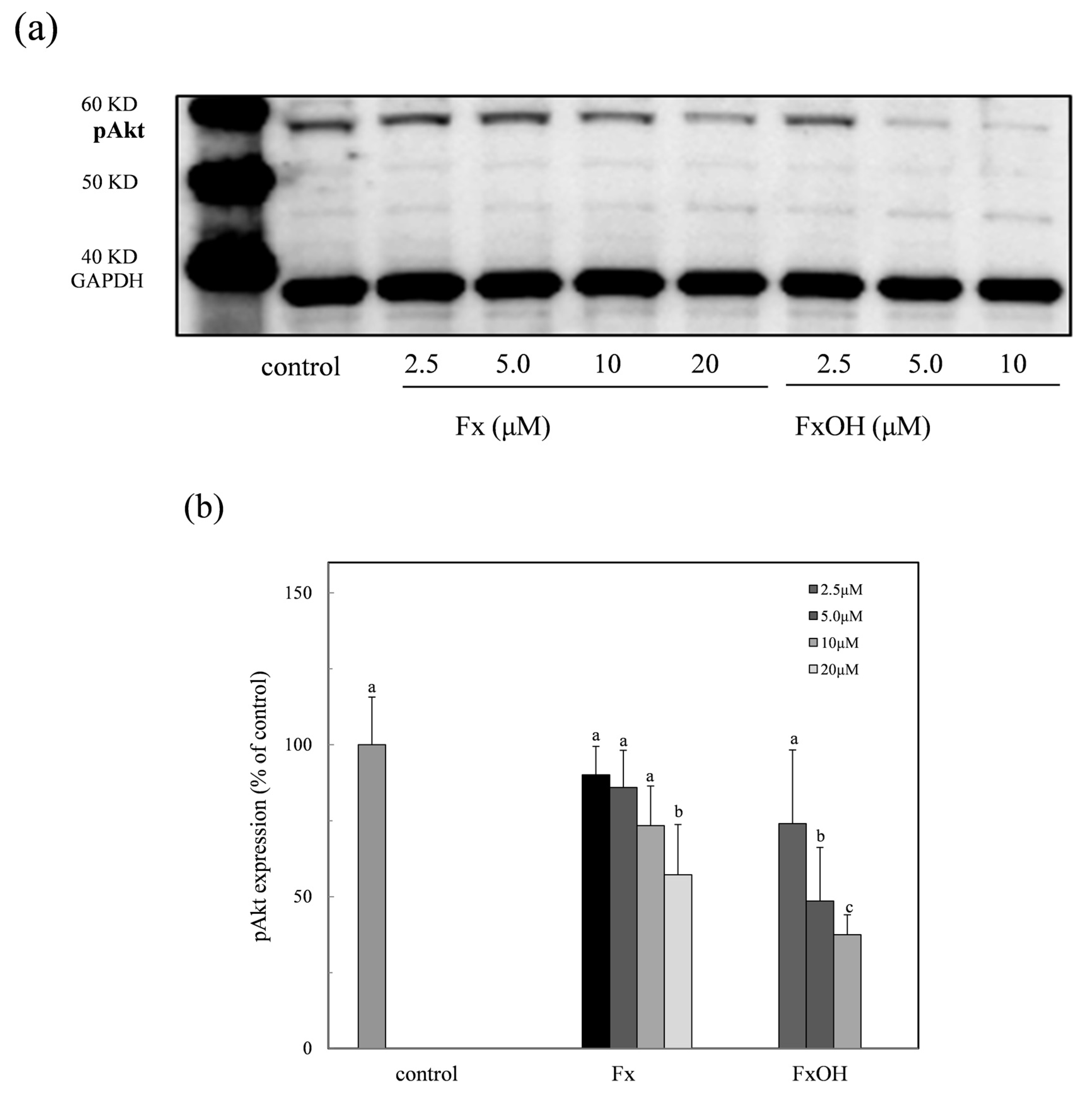
2.9. Phospho-Akt Protein Expression
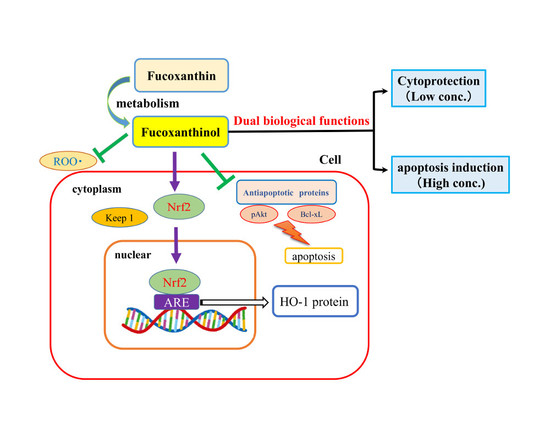
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Peroxyl Radical Scavenging Capacity
4.3. ESR Measurement
4.4. Cell Culture
4.5. Cell Viability
4.6. Reporter Assay of Nrf2-ARE Signaling
4.7. Nuclear Extraction
4.8. Apoptotic Cell Detection
4.9. Caspase 3/7 Activation
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hosokawa, M.; Wanezaki, S.; Miyaguchi, K.; Kurihara, H.; Kohono, H.; Kawabata, J.; Kawabata, J.; Odashima, S.; Takahashi, K. Apoptosis-inducing effect of fucoxanthin on human leukemia cell line HL-60. Food Sci. Technol. Res. 1999, 5, 243–246. [Google Scholar] [CrossRef]
- Hosokawa, H.; Kudo, M.; Maeda, H.; Kohno, H.; Tanaka, T.; Miyashita, K. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARgamma ligand, troglitazone, on colon cancer cells. Biochim. Biophys. Acta 2004, 1675, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Maeda, H.; Miyashita, K.; Tanaka, T.; Miyamoto, S.; Mutoh, M. A marine bio-functional lipid, fucoxanthinol, attenuates human colorectal cancer stem-like cell tumorigenicity and sphere formation. J. Clin. Biochim. Nutr. 2017, 61, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Ishikawa, C.; Katano, H.; Yasumoto, T.; Mori, N. Fucoxanthin and its deacetylated product, fucoxanthinol, induce apoptosis of primary effusion lymphomas. Cancer Lett. 2011, 300, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-N.; Heo, S.-J.; Yoon, W.-J.; Kang, S.-M.; Ahn, T.-H.Y.; Jeon, Y.-J. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-kB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur. J. Pharmacol. 2010, 649, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Taira, J.; Uehara, M. Anti-inflammatory effect of fucoxanthinol as bioavailable marine carotenoid on LPS stimulated RAW264.7 macrophages through iNOS suppression and nitrogen radical-scavenging. World J. Pharm. Sci. 2015, 3, 1747–1754. [Google Scholar]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kong, A.N. Dietary chemopreventive compounds and ARE/EpRE signaling. Free Radic. Biol. Med. 2004, 36, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.-J.; Kundu, J.K.; Na, H.-K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008, 74, 1526–1539. [Google Scholar]
- Xu, C.; Yuan, X.; Pan, Z.; Shen, G.; Kim, J.-H.; Yu, S.; Khor, T.O.; Li, W.; Ma, K.; Ah-Ng, T. Mechanism of action of isothiocyanates: The induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol. Cancer Ther. 2006, 5, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Baskaran, V.; Tsuzuki, W.; Nagao, A. Brown algae fucoxanthin is hydrolyzed to fucoxanthinol during absorption by Caco-2 human intestinal cells and mice. J. Nutr. 2002, 132, 946–951. [Google Scholar] [PubMed]
- Motohashi, H.; Yamamoto, M. Nrf2–Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.L.; Yu-Ting, C.; Miao-Lin, H. Fucoxanthin enhances HO-1 and NQO-1 expression in murine hepatic BNL CL.2 cells through activation of the Nrf2/ARE system partially by its pro-oxidant activity. J. Agric. Food Chem. 2011, 59, 11344–11351. [Google Scholar]
- Kotake-Nara, E.; Asai, A.; Nagao, A. Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett. 2005, 220, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Franke, T.F.; Hornik, C.P.; Segev, L.; Shostak, G.A.; Sugimoto, C. PI3K/Akt and apoptosis: Size matters. Oncogene 2003, 22, 8983–8998. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-H.; Chen, Y.-T.; Huang, Y.-W.; Tsai, H.-J.; Kuo, C.-C. 4-Ketopinoresinol, a novel naturally occurring ARE activator, induce the Nrf2/HO-1 axis and protects against oxidative stress-induced cell injury via activation of PI3K/AKT signaling. Free Radic. Biol. Med. 2012, 52, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Samali, A.; Orrenius, S. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 2000, 29, 323–333. [Google Scholar] [CrossRef]
- Mitsuishi, Y.; Taguchi, K.; Kawatani, Y.; Shibata, T.; Nukiwa, T.; Aburatani, A.; Yamamoto, M.; Motohashi, H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 2012, 22, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC)) of plasma and other biological. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Taira, J.; Miyagi, C.; Aniya, Y. Dimerumic acid as an antioxidant from the mold, Monascus anka: The inhibition mechanisms against lipid peroxidation and hemeprotein-mediated oxidation. Biochem Pharmacol. 2002, 63, 1019–1026. [Google Scholar] [CrossRef]
- Taira, J.; Tsuchida, E.; Uehara, M.; Kinjyo, Y.; Roy, P.K.; Ueda, K. Dual biological functions of the apoptotic activity and anti-inflammatory effect by alcyonolide congeners from the Okinawan soft coral, Cespitularia sp. Bioorg. Med. Chem. Lett. 2015, 25, 4496–4499. [Google Scholar]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taira, J.; Sonamoto, M.; Uehara, M. Dual Biological Functions of a Cytoprotective Effect and Apoptosis Induction by Bioavailable Marine Carotenoid Fucoxanthinol through Modulation of the Nrf2 Activation in RAW264.7 Macrophage Cells. Mar. Drugs 2017, 15, 305. https://doi.org/10.3390/md15100305
Taira J, Sonamoto M, Uehara M. Dual Biological Functions of a Cytoprotective Effect and Apoptosis Induction by Bioavailable Marine Carotenoid Fucoxanthinol through Modulation of the Nrf2 Activation in RAW264.7 Macrophage Cells. Marine Drugs. 2017; 15(10):305. https://doi.org/10.3390/md15100305
Chicago/Turabian StyleTaira, Junsei, Miki Sonamoto, and Masatsugu Uehara. 2017. "Dual Biological Functions of a Cytoprotective Effect and Apoptosis Induction by Bioavailable Marine Carotenoid Fucoxanthinol through Modulation of the Nrf2 Activation in RAW264.7 Macrophage Cells" Marine Drugs 15, no. 10: 305. https://doi.org/10.3390/md15100305