Algal Foams Applied in Fixed-Bed Process for Lead(II) Removal Using Recirculation or One-Pass Modes
Abstract
:1. Introduction
2. Results
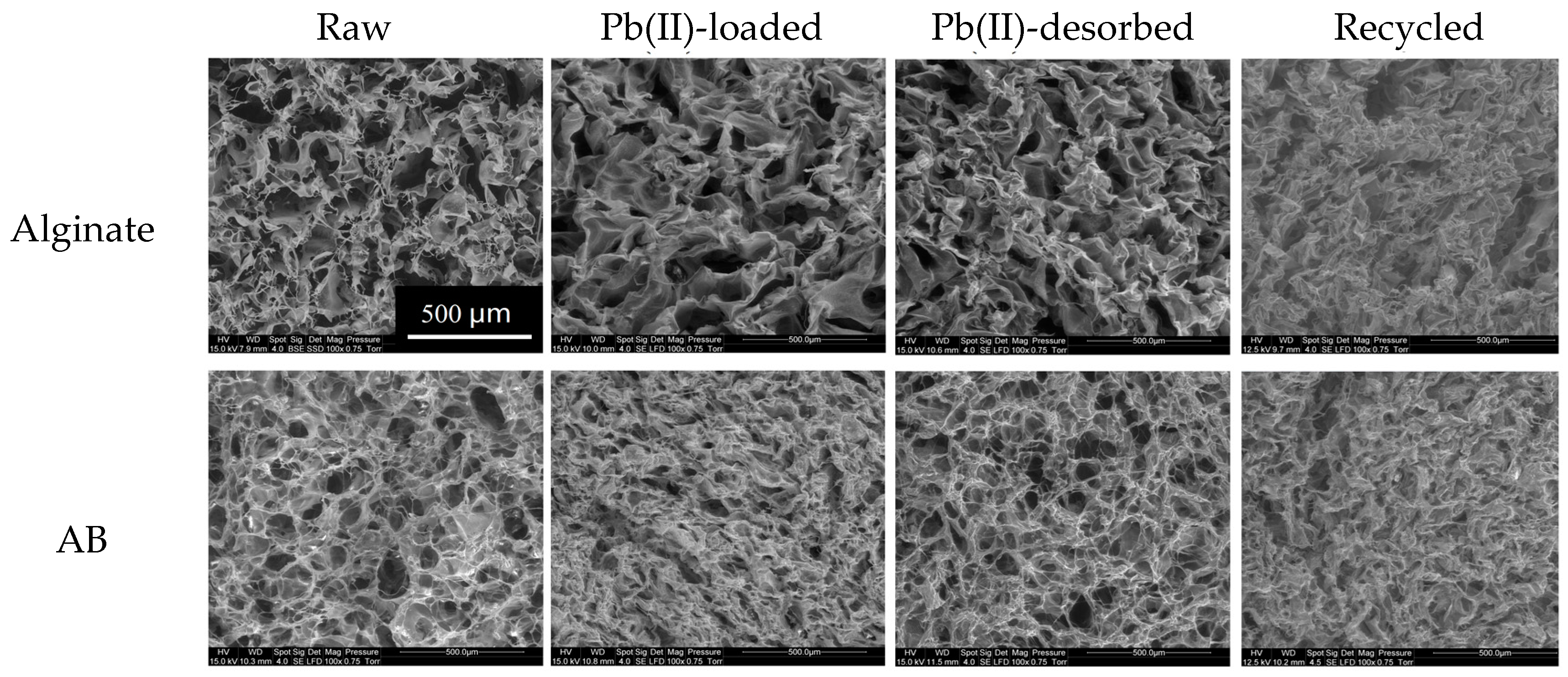
2.1. Characterization
2.2. Recirculation Mode
2.2.1. Sorption Isotherms
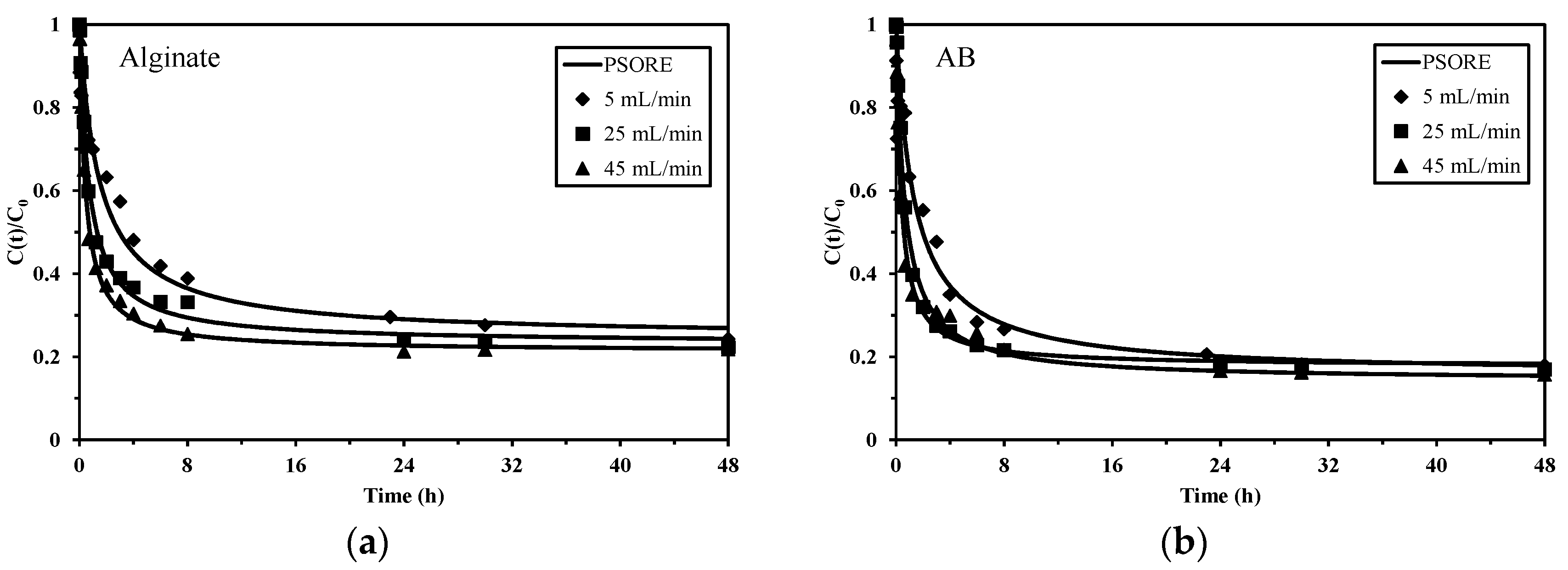
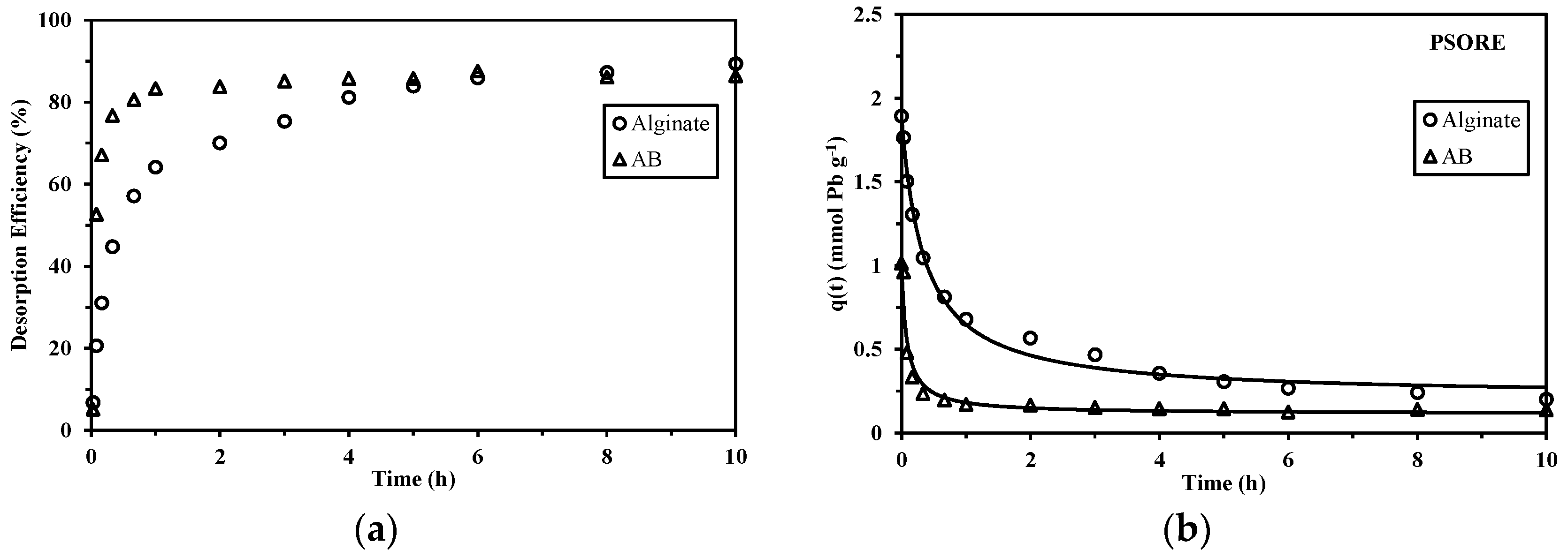
2.2.2. Sorption/Desorption Kinetics
2.2.3. Sorption/Desorption Cycles
2.3. One-Pass Mode
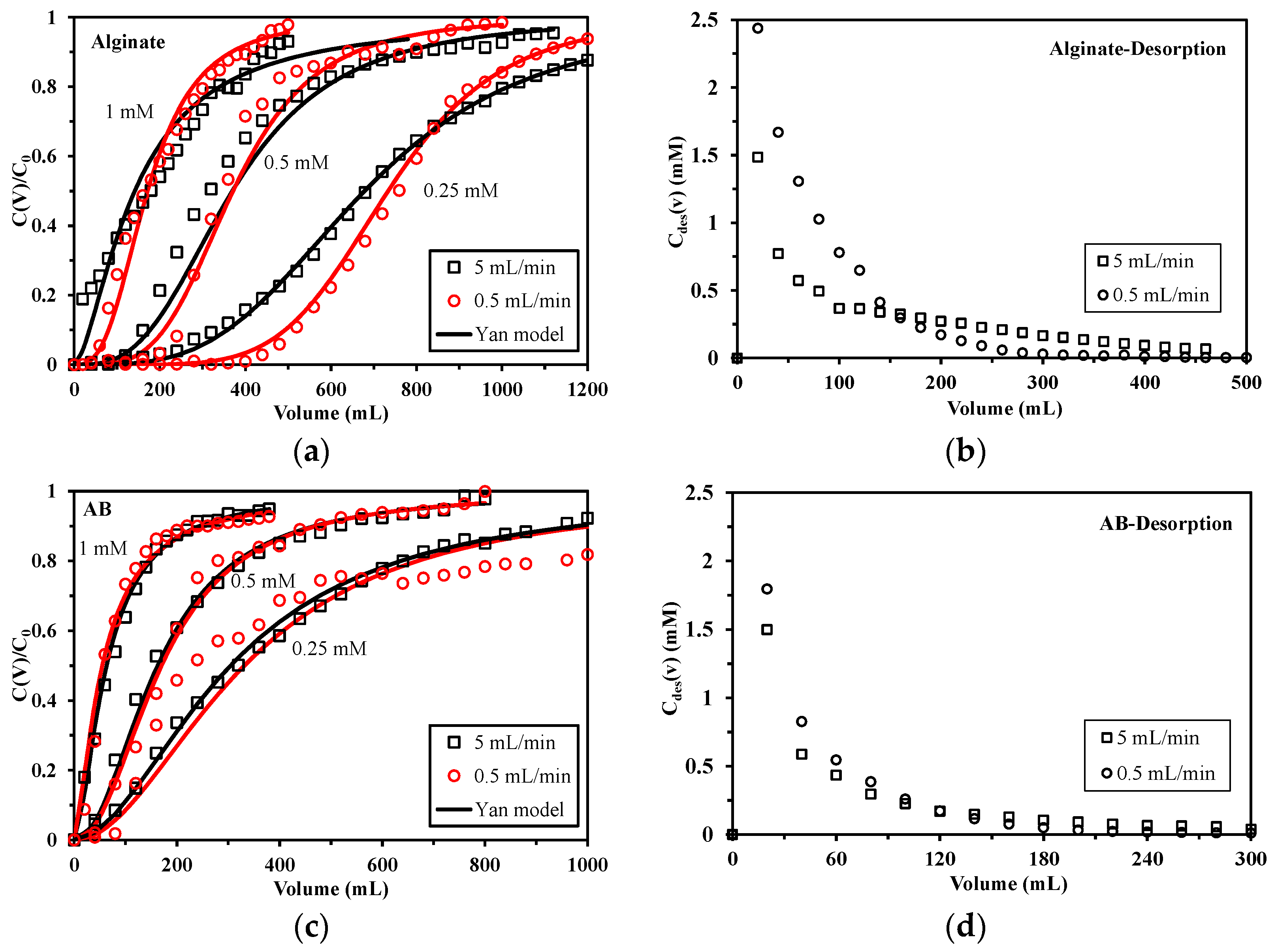
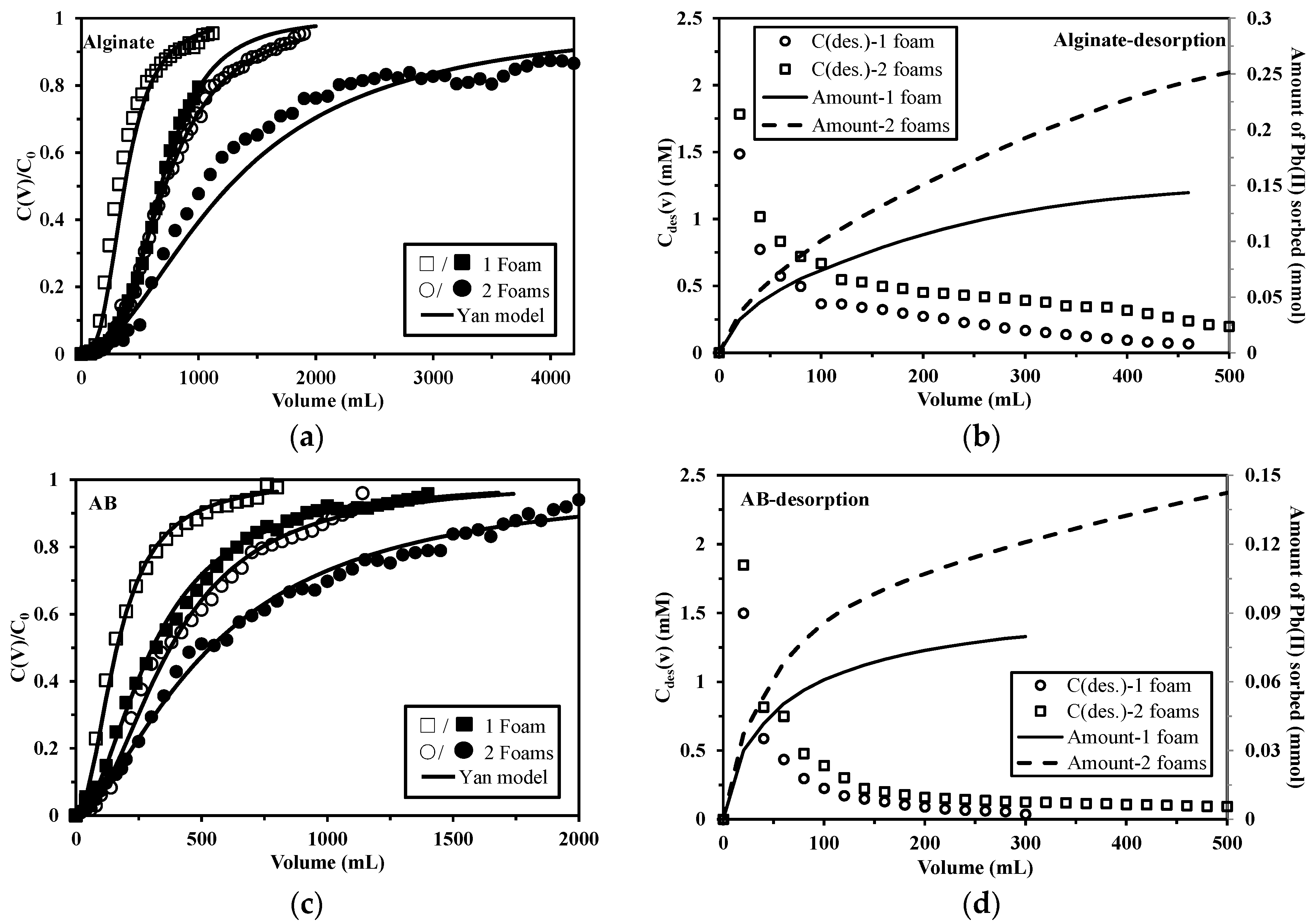
2.3.1. Effect of Velocity on Sorption/Desorption
2.3.2. Effect of Column Depth on Sorption/Desorption
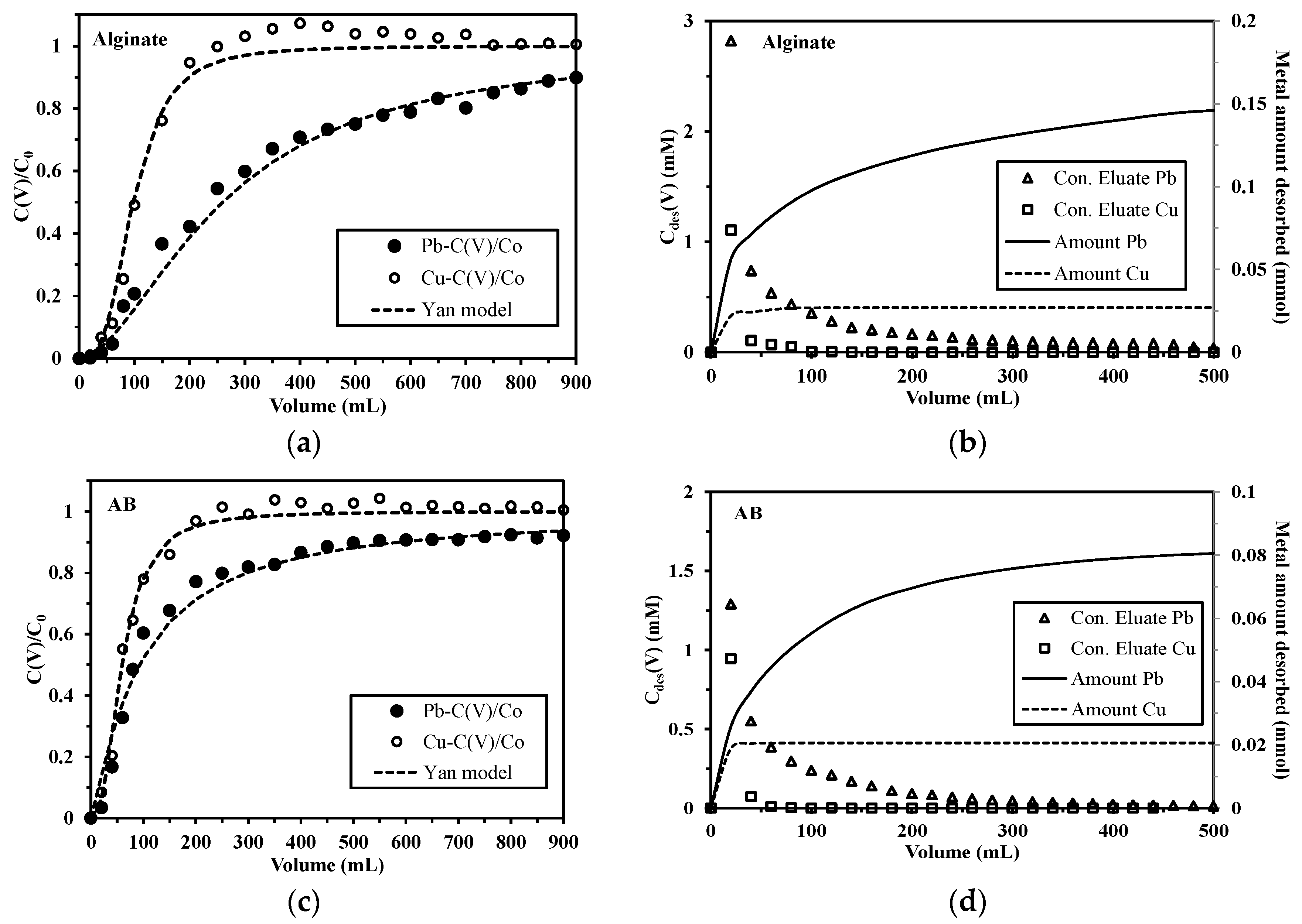
2.3.3. Competitive Sorption in Pb(II)-Cu(II) System
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Macroporous Foams
3.3. Characterization of Materials
3.4. Recirculation Mode
3.4.1. Sorption Isotherms
3.4.2. Sorption Kinetics
3.4.3. Desorption Kinetics
3.4.4. Sorbent Reuse
3.5. Single-Pass Mode
3.6. Modeling of Sorption Isotherms and Uptake Kinetics
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khotimchenko, Y.S.; Khozhaenko, E.V.; Khotimchenko, M.Y.; Kolenchenko, E.A.; Kovalev, V.V. Carrageenans as a new source of drugs with metal binding properties. Mar. Drugs 2010, 8, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Biosorption of Zn (II) from industrial effluents using sugar beet pulp and F. vesiculosus: From laboratory tests to a pilot approach. Sci. Total Environ. 2017, 598, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.V.; Lazar, M.M.; Dragan, E.S. Dual ionic cross-linked alginate/clinoptilolite composite microbeads with improved stability and enhanced sorption properties for methylene blue. React. Funct. Polym. 2017, 116, 31–40. [Google Scholar] [CrossRef]
- Chen, C.; Tang, Y.; Liu, Y.; Liang, Y.; Zhang, K.; Wang, S.; Liang, Y. Effect of competitive adsorption on zinc removal from aqueous solution and zinc smelting effluent by eucalyptus leaf-based magnetic biosorbent. J. Environ. Sci. Health A 2017, 52, 873–889. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Tang, Y.-K.; Li, K.; Mo, Y.-Y.; Li, H.-F.; Gu, Z.-Q. Combined performance of biochar sorption and magnetic separation processes for treatment of chromium-contained electroplating wastewater. Bioresour. Technol. 2014, 174, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A. Agricultural waste peels as versatile biomass for water purification—A review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Caetano, L.A.; Almeida, A.J.; Gonçalves, L. Effect of experimental parameters on alginate/chitosan microparticles for BCG encapsulation. Mar. Drugs 2016, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Vincent, T.; Roux, J.-C.; Faur, C.; Guibal, E. Pd (II) and Pt (IV) sorption using alginate and algal-based beads. Chem. Eng. J. 2017, 313, 567–579. [Google Scholar] [CrossRef]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Modeling competitive sorption of lead and copper ions onto alginate and greenly prepared algal-based beads. Bioresour. Technol. 2017, 231, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Hong, L.; Wu, S.; Wang, L. Elucidation of interactions between metal ions and Ca alginate-based ion-exchange resin by spectroscopic analysis and modeling simulation. Langmuir 2002, 18, 9413–9421. [Google Scholar] [CrossRef]
- Luo, J.; Wang, L.; Mott, D.; Njoki, P.N.; Kariuki, N.; Zhong, C.-J.; He, T. Ternary alloy nanoparticles with controllable sizes and composition and electrocatalytic activity. J. Mater. Chem. 2006, 16, 1665–1673. [Google Scholar] [CrossRef]
- Theras, J.E.M.; Kalaivani, D.; Jayaraman, D.; Joseph, V. Growth and spectroscopic, thermodynamic and nonlinear optical studies of L-threonine phthalate crystal. J. Cryst. Growth 2015, 427, 29–35. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Morent, R.; De Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Comparison between XPS-and FTIR-analysis of plasma-treated polypropylene film surfaces. Surf. Interface Anal. 2008, 40, 597–600. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Manns, D.; D’Este, M.; Krause-Jensen, D.; Rasmussen, M.B.; Larsen, M.M.; Alvarado-Morales, M.; Angelidaki, I.; Bruhn, A. Variation in biochemical composition of Saccharina latissima and Laminaria digitata along an estuarine salinity gradient in inner Danish waters. Algal Res. 2016, 13, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Xiaohong, G.; Yang, C.Q. FTIR spectroscopy study of the formation of cyclic anhydride intermediates of polycarboxylic acids catalyzed by sodium hypophosphite. Text. Res. J. 2000, 70, 64–70. [Google Scholar] [CrossRef]
- Khotimchenko, Y.; Khozhaenko, E.; Kovalev, V.; Khotimchenko, M. Cerium binding activity of pectins isolated from the seagrasses Zostera marina and Phyllospadix iwatensis. Mar. Drugs 2012, 10, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Alginate and algal-based beads for the sorption of metal cations: Cu (II) and Pb (II). Int. J. Mol. Sci. 2016, 17, 1453. [Google Scholar] [CrossRef] [PubMed]
- Naidu, G.; Jeong, S.; Johir, M.A.H.; Fane, A.G.; Kandasamy, J.; Vigneswaran, S. Rubidium extraction from seawater brine by an integrated membrane distillation-selective sorption system. Water Res. 2017, 123, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Vincent, C.; Hertz, A.; Vincent, T.; Barré, Y.; Guibal, E. Immobilization of inorganic ion-exchanger into biopolymer foams–application to cesium sorption. Chem. Eng. J. 2014, 236, 202–211. [Google Scholar] [CrossRef]
- Seki, H.; Suzuki, A. Adsorption of lead ions on composite biopolymer adsorbent. Ind. Eng. Chem. Res. 1996, 35, 1378–1382. [Google Scholar] [CrossRef]
- Ren, H.; Gao, Z.; Wu, D.; Jiang, J.; Sun, Y.; Luo, C. Efficient Pb (II) removal using sodium alginate–carboxymethyl cellulose gel beads: Preparation, characterization, and adsorption mechanism. Carbohydr. Polym. 2016, 137, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Asthana, A.; Singh, A.K.; Prasad, S.; Susan, M.A.B.H. Novel glycine-functionalized magnetic nanoparticles entrapped calcium alginate beads for effective removal of lead. Microchem. J. 2017, 130, 168–178. [Google Scholar] [CrossRef]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Price, N.T.; Pinilla, M.Á.G.; O’Shea, K.E. Effective removal of phosphate from aqueous solution using humic acid coated magnetite nanoparticles. Water Res. 2017, 123, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Plazinski, W. Sorption of lead, copper, and cadmium by calcium alginate. Metal binding stoichiometry and the pH effect. Environ. Sci. Pollut. Res. 2012, 19, 3516–3524. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, K.; Devi, B.M.; Latha, S.; Gomathi, T.; Sudha, P.; Venkatesan, J.; Anil, S. Batch adsorption and desorption studies on the removal of lead (II) from aqueous solution using nanochitosan/sodium alginate/microcrystalline cellulose beads. Int. J. Biol. Macromol. 2017, 104, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.D.C.; Khorramabadi, G.S.; Khataee, A.; Jorfi, S. Silica nanopowders/alginate composite for adsorption of lead (II) ions in aqueous solutions. J. Taiwan Inst. Chem. Eng. 2014, 45, 973–980. [Google Scholar] [CrossRef]
- Yan, Y.; An, Q.; Xiao, Z.; Zheng, W.; Zhai, S. Flexible core-shell/bead-like alginate@ PEI with exceptional adsorption capacity, recycling performance toward batch and column sorption of Cr (VI). Chem. Eng. J. 2017, 313, 475–486. [Google Scholar] [CrossRef]
- Escudero, C.; Fiol, N.; Villaescusa, I.; Bollinger, J.-C. Arsenic removal by a waste metal (hydr) oxide entrapped into calcium alginate beads. J. Hazard. Mater. 2009, 164, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Fagundes-Klen, M.R.; Veit, M.T.; Borba, C.E.; Bergamasco, R.; de Lima Vaz, L.G.; Da Silva, E.A. Copper biosorption by biomass of marine alga: Study of equilibrium and kinetics in batch system and adsorption/desorption cycles in fixed bed column. Water Air Soil Pollut. 2010, 213, 15–26. [Google Scholar] [CrossRef]
- Abdolali, A.; Ngo, H.H.; Guo, W.; Zhou, J.L.; Zhang, J.; Liang, S.; Chang, S.W.; Nguyen, D.D.; Liu, Y. Application of a breakthrough biosorbent for removing heavy metals from synthetic and real wastewaters in a lab-scale continuous fixed-bed column. Bioresour. Technol. 2017, 229, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Q.; Wang, X. Formation of cyclic anhydride intermediates and esterification of cotton cellulose by multifunctional carboxylic acids: An infrared spectroscopy study. Text. Res. J. 1996, 66, 595–603. [Google Scholar] [CrossRef]
- Muhamad, H.; Doan, H.; Lohi, A. Batch and continuous fixed-bed column biosorption of Cd2+ and Cu2+. Chem. Eng. J. 2010, 158, 369–377. [Google Scholar] [CrossRef]
- Thomas, H.C. Heterogeneous ion exchange in a flowing system. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Yan, G.; Viraraghavan, T.; Chen, M. A new model for heavy metal removal in a biosorption column. Adsorpt. Sci. Technol. 2001, 19, 25–43. [Google Scholar] [CrossRef]
- Shahbazi, A.; Younesi, H.; Badiei, A. Functionalized SBA-15 mesoporous silica by melamine-based dendrimer amines for adsorptive characteristics of Pb (II), Cu (II) and Cd (II) heavy metal ions in batch and fixed bed column. Chem. Eng. J. 2011, 168, 505–518. [Google Scholar] [CrossRef]
- Wang, S.; Hamza, M.F.; Vincent, T.; Faur, C.; Guibal, E. Praseodymium sorption on Laminaria digitata algal beads and foams. J. Colloid Interface Sci. 2017, 504, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Akhigbe, L.; Ouki, S.; Saroj, D. Disinfection and removal performance for Escherichia coli and heavy metals by silver-modified zeolite in a fixed bed column. Chem. Eng. J. 2016, 295, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Bohart, G.; Adams, E. Some aspects of the behavior of charcoal with respect to chlorine. J. Am. Chem. Soc. 1920, 42, 523–544. [Google Scholar] [CrossRef]
- Guibal, E.; Lorenzelli, R.; Vincent, T.; Cloirec, P.L. Application of silica gel to metal ion sorption: Static and dynamic removal of uranyl ions. Environ. Technol. 1995, 16, 101–114. [Google Scholar] [CrossRef]
- Han, R.; Ding, D.; Xu, Y.; Zou, W.; Wang, Y.; Li, Y.; Zou, L. Use of rice husk for the adsorption of congo red from aqueous solution in column mode. Bioresour. Technol. 2008, 99, 2938–2946. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-D.; Yu, J.-X.; Wang, F.; Tang, J.-Q.; Zhang, Y.-F.; Xu, Y.-L.; Chi, R.-A. Selective adsorption and recycle of Cu2+ from aqueous solution by modified sugarcane bagasse under dynamic condition. Environ. Sci. Pollut. Res. 2017, 24, 9202–9209. [Google Scholar] [CrossRef] [PubMed]
- Escudero, C.; Poch, J.; Villaescusa, I. Modelling of breakthrough curves of single and binary mixtures of Cu (II), Cd (II), Ni (II) and Pb (II) sorption onto grape stalks waste. Chem. Eng. J. 2013, 217, 129–138. [Google Scholar] [CrossRef]
- Chassary, P.; Vincent, T.; Marcano, J.S.; Macaskie, L.E.; Guibal, E. Palladium and platinum recovery from bicomponent mixtures using chitosan derivatives. Hydrometallurgy 2005, 76, 131–147. [Google Scholar] [CrossRef]
- An, B.; Lee, H.; Lee, S.; Lee, S.-H.; Choi, J.-W. Determining the selectivity of divalent metal cations for the carboxyl group of alginate hydrogel beads during competitive sorption. J. Hazard. Mater. 2015, 298, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hu, J.; Wang, J. Competitive adsorption of Pb (II), Cu (II) and Zn (II) onto xanthate-modified magnetic chitosan. J. Hazard. Mater. 2012, 221, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bai, R.; San Ly, Q. Selective removal of copper and lead ions by diethylenetriamine-functionalized adsorbent: Behaviors and mechanisms. Water Res. 2008, 42, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, D.; Pagano, M.; Tiravanti, G.; Passino, R. Lead removal and recovery from battery wastewaters by natural zeolite clinoptilolite. Solvent Extr. Ion Exch. 1999, 17, 677–694. [Google Scholar] [CrossRef]
- Noeline, B.; Manohar, D.; Anirudhan, T. Kinetic and equilibrium modelling of lead (II) sorption from water and wastewater by polymerized banana stem in a batch reactor. Sep. Purif. Technol. 2005, 45, 131–140. [Google Scholar] [CrossRef]
- McHugh, D.J. Production and Utilization of Products from Commercial Seaweeds. Chapter 2—Production, Properties and Uses of Alginates; FAO: Rome, Italy, 1987; pp. 58–115. [Google Scholar]
- Bertagnolli, C.; Espindola, A.P.D.M.; Kleinuebing, S.J.; Tasic, L.; Carlos da Silva, M.G. Sargassum filipendula alginate from Brazil: Seasonal influence and characteristics. Carbohydr. Polym. 2014, 111, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Agulhon, P.; Robitzer, M.; David, L.; Quignard, F. Structural regime identification in ionotropic alginate gels: Influence of the cation nature and alginate structure. Biomacromolecules 2012, 13, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Vincent, T.; Roux, J.-C.; Faur, C.; Guibal, E. Innovative conditioning of algal-based sorbents: Macro-porous discs for palladium sorption. Chem. Eng. J. 2017, 325, 521–532. [Google Scholar] [CrossRef]

| Model | Parameter | Alginate | AB |
|---|---|---|---|
| Experiment | qeq,exp (mmol Pb g−1) | 1.88 | 1.03 |
| PFORE | qeq,calc (mmol Pb g−1) | 1.76 | 0.93 |
| k1 × 103 (min−1) | 28.25 | 34.09 | |
| R2 | 0.979 | 0.972 | |
| PSORE | qeq,calc (mmol Pb g−1) | 1.89 | 1.00 |
| k2 × 103 (g mmol−1 min−1) | 20.09 | 44.21 | |
| R2 | 0.996 | 0.989 |
| Velocity (mL min−1) | C0 (mmol Pb g−1) | tb (min) | ts (min) | Vb (mL) | Vs (mL) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Alginate | AB | Alginate | AB | Alginate | AB | Alginate | AB | ||
| 0.5 | 0.25 | 880 | 160 | 2480 | 2400 | 440 | 80 | 1240 | 1200 |
| 0.5 | 400 | 80 | 1760 | 1440 | 200 | 40 | 880 | 720 | |
| 1 | 120 | <40 | 920 | 760 | 60 | <20 | 460 | 380 | |
| 5 | 0.25 | 48 | 8 | 320 | 280 | 240 | 40 | 1600 | 1400 |
| 0.5 | 24 | 8 | 216 | 144 | 120 | 40 | 1080 | 720 | |
| 1 | <4 | <4 | 100 | 88 | <20 | <20 | 500 | 440 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Algal Foams Applied in Fixed-Bed Process for Lead(II) Removal Using Recirculation or One-Pass Modes. Mar. Drugs 2017, 15, 315. https://doi.org/10.3390/md15100315
Wang S, Vincent T, Faur C, Guibal E. Algal Foams Applied in Fixed-Bed Process for Lead(II) Removal Using Recirculation or One-Pass Modes. Marine Drugs. 2017; 15(10):315. https://doi.org/10.3390/md15100315
Chicago/Turabian StyleWang, Shengye, Thierry Vincent, Catherine Faur, and Eric Guibal. 2017. "Algal Foams Applied in Fixed-Bed Process for Lead(II) Removal Using Recirculation or One-Pass Modes" Marine Drugs 15, no. 10: 315. https://doi.org/10.3390/md15100315




