Release Behavior and Antibacterial Activity of Chitosan/Alginate Blends with Aloe vera and Silver Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
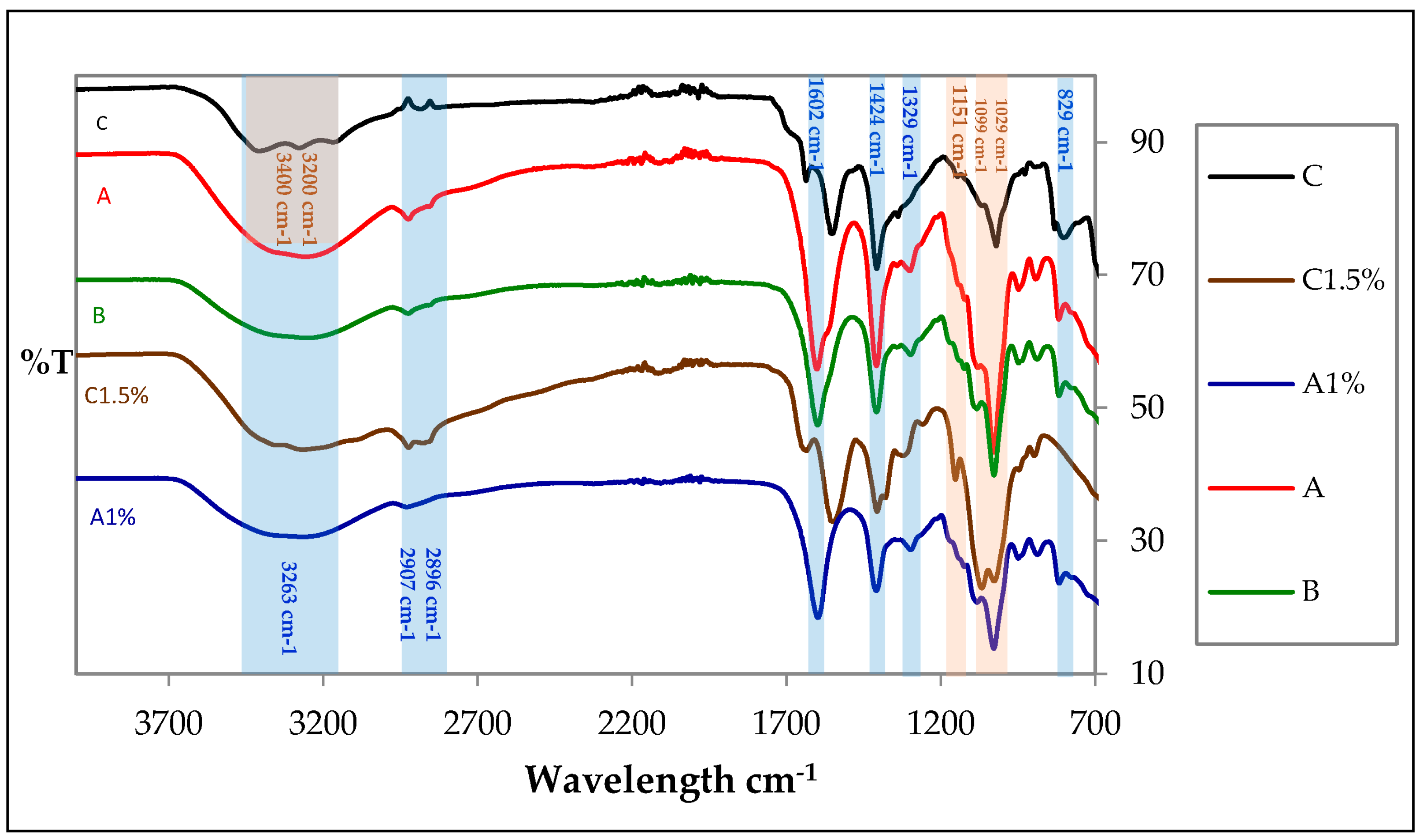
2.1. ATR-FTIR Spectroscopy
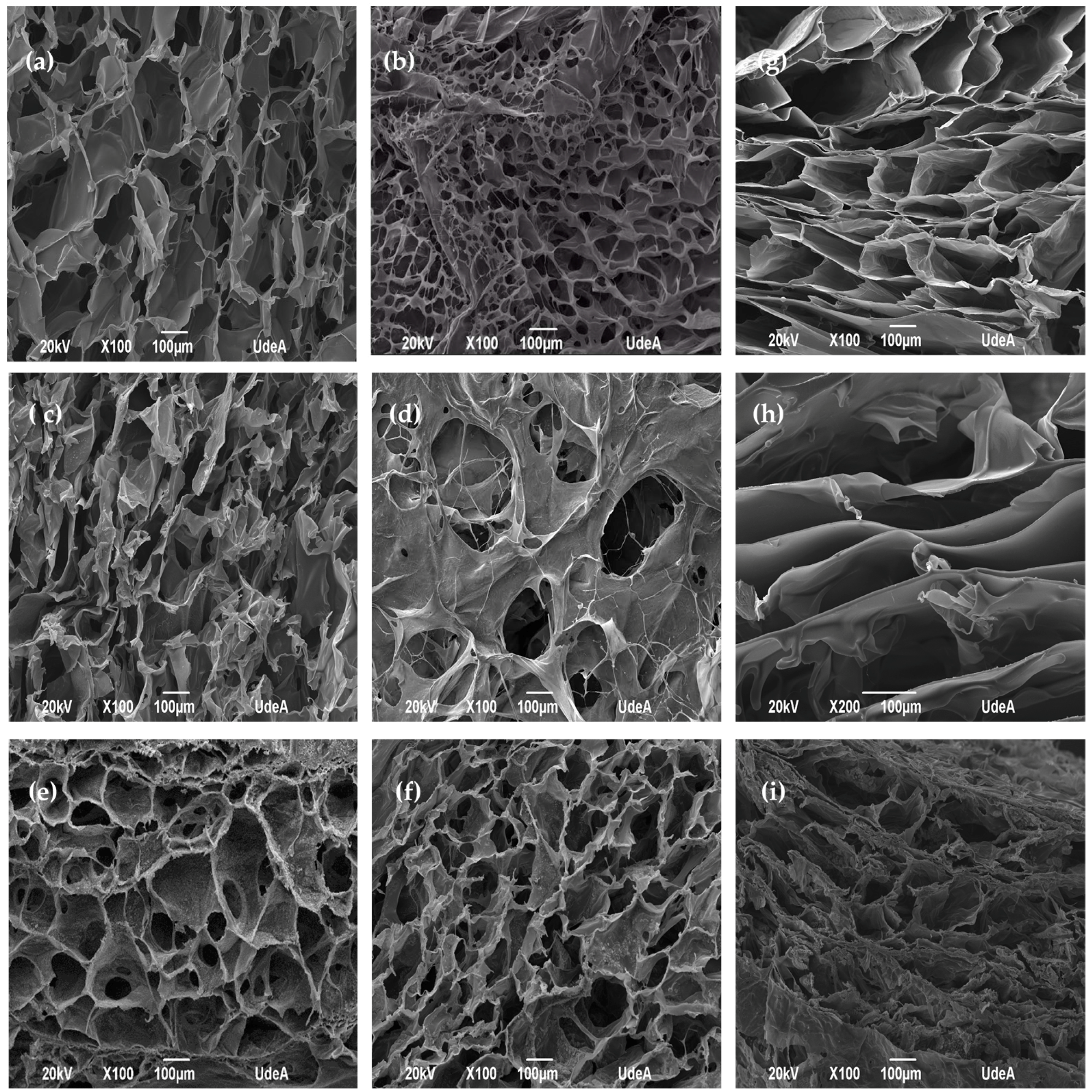
2.2. SEM Analysis
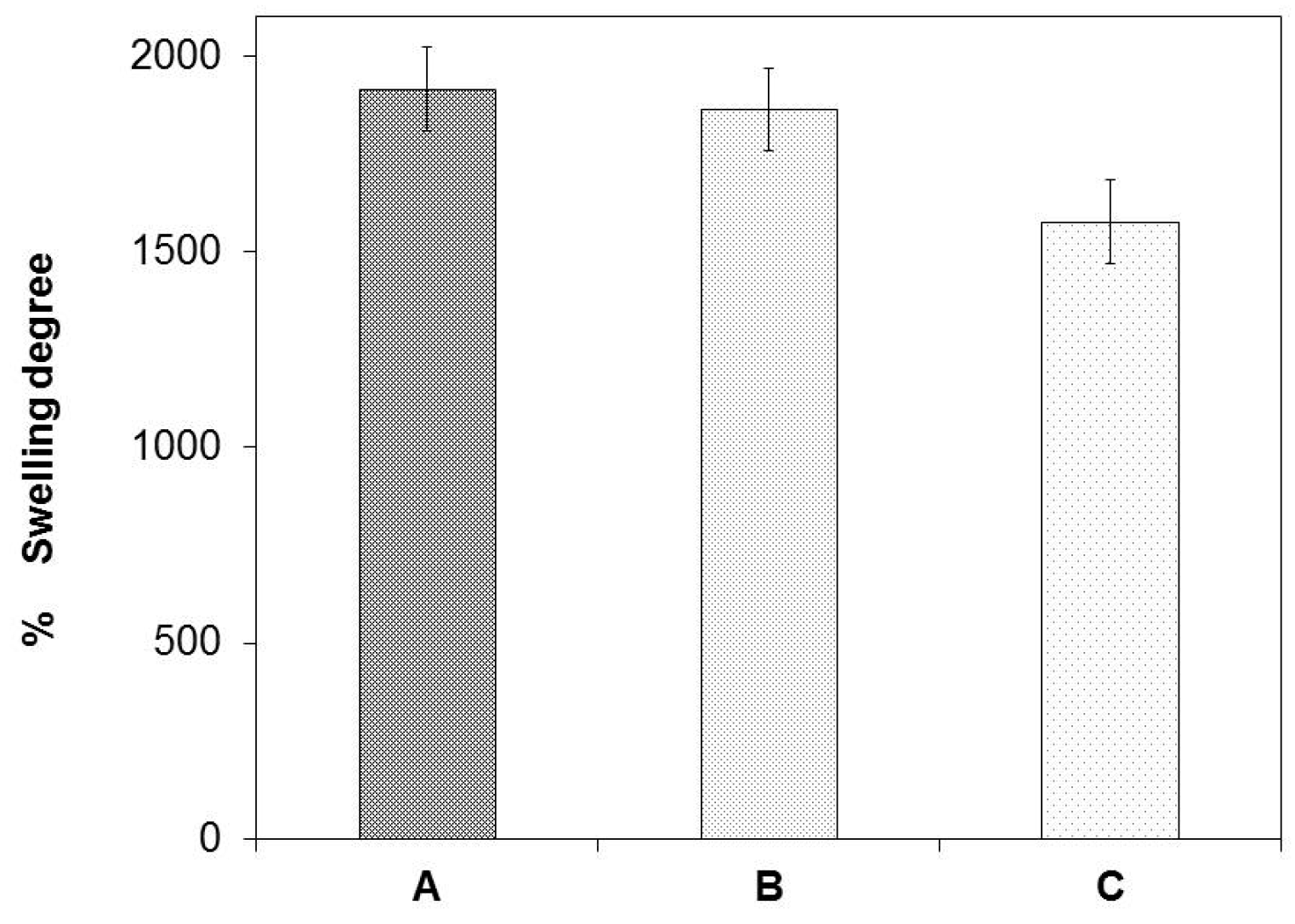
2.3. Swelling Degree
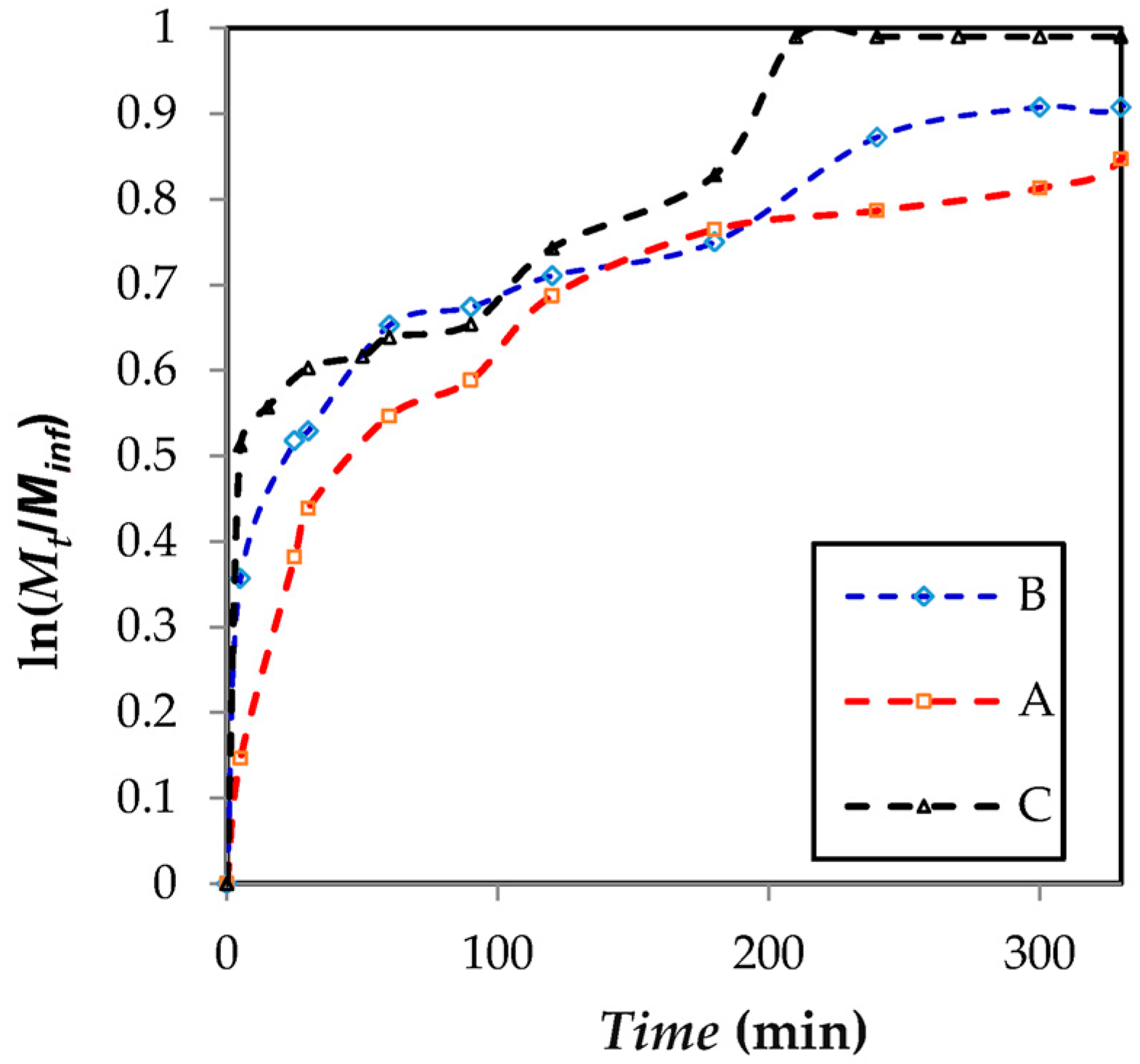
2.4. Release Assay
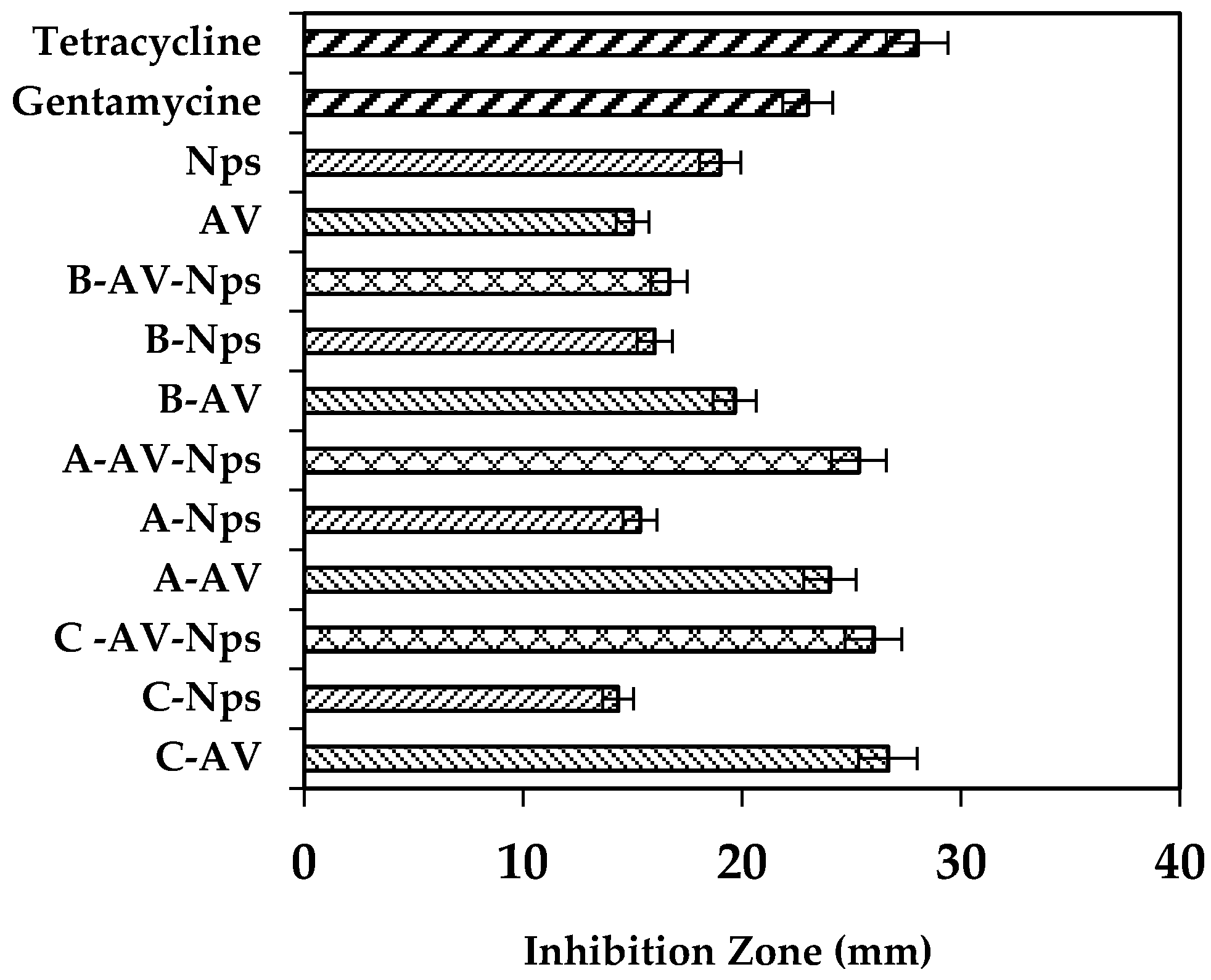
2.5. Antibacterial Activity
3. Materials and Methods
3.1. Materials
3.2. Extraction of Aloe vera Gel
3.3. Matrix Preparation
3.4. Loading of the Polymer Matrices with Aloe vera Gel and Incorporation of the Silver Nanoparticles
3.5. Matrix Characterization
3.5.1. ATR Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3.5.2. SEM Analysis
3.5.3. Release Kinetics Assay
3.5.4. Antibacterial Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Selig, H.F.; Lumenta, D.B.; Giretzlehner, M.; Jeschke, M.G.; Upton, D.; Kamolz, L.P. The properties of an “ideal” burn wound dressing—What do we need in daily clinical practice. Burns 2012, 38, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Nagoba, B.S.; Gandhi, R.C.; Hartalkar, A.R.; Wadher, B.J.; Selkar, S.P. Simple, effective and affordable approach for the treatment of burns infections. Burns 2010, 36, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Popa, E.G.; Gomes, M.E.; Cerqueira, M.; Marques, A.P.; Caridade, S.G.; Teixeira, P.; Sousa, C.; Mano, J.F.; Reis, R.L. An investigation of the potential application of chitosan/aloe-based membranes for regenerative medicine. Acta Biomater. 2013, 9, 6790–6797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, S.S.; Caridade, S.G.; Mano, J.F.; Reis, R.L. Effect of crosslinking in chitosan/Aloe vera-based membranes for biomedical applications. Carbohydr. Polym. 2013, 98, 581–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadtaghi, V.; Mohd, R.; Babak, S.; Ahmad, Z.A.; Mahamad, H.; Kok, B.T.; Zahra, G.; Parisa, A. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar]
- Ma, Y.; Xin, L.; Tan, H.; Fan, M.; Li, J.; Jia, Y.; Ling, Z.; Chen, Y.; Hu, X. Chitosan membrane dressings toughened by glycerol to load antibacterial drugs for wound healing. Mater. Sci. Eng. C 2017. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Panawes, S.; Ekabutr, P.; Niamlang, P.; Pavasant, P.; Chuysinuan, P.; Supaphol, P. Antimicrobial mangosteen extract infused alginate-coated gauze wound dressing. J. Drug Deliv. Sci. Technol. 2017, 41, 182–190. [Google Scholar] [CrossRef]
- Pereira, R.; Mendes, A.; Bártolo, P. Alginate/Aloe vera hydrogel films for biomedical applications. Procedia CIRP 2013, 5, 210–215. [Google Scholar] [CrossRef]
- Momoh, F.U.; Babur, Z.; Chowdhry, J.C.; Mitchell, J.S. Boateng, Development and functional characterization of alginate dressing as potential protein delivery system for wound healing. Int. J. Biol. Macromol. 2015, 81, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhu, X.-K.; Xue, X.-T.; Wu, D.-Y. Hydrogel sheets of chitosan, honey and gelatin as burn wound dressings. Carbohydr. Polym. 2012, 88, 75–83. [Google Scholar] [CrossRef]
- Venkatesan, J.; Lee, J.Y.; Kang, D.S.; Anil, S.; Kim, S.K.; Shim, M.S.; Kim, D.G. Antimicrobial and anticancer activities of porous chitosan-alginate biosynthesized silver nanoparticles. Int. J. Biol. Macromol. 2017, 98, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, H.C.; Oh, J.S.; Shin, B.A.; Oh, C.S.; Park, R.D.; Yang, K.S.; Cho, C.S. Polyelectroylte complex composed of chitosan and sodium alginate for wound dressing application. J. Biomater. Sci. Polym. 1999, 10, 543–556. [Google Scholar] [CrossRef]
- Wang, L.; Khor, E.; Wee, A.; Lim, L.Y. Chitosan-alginate PEC membrane as a wound dressing: Assessment of incisional wound healing. J. Biomed. Mater. Res. 2002, 63, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, M. Chitosan-alginate as scaffolding material for cartilage tissue engineering. J. Biomed. Mater. Res. A 2005, 75, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Conzatti, G.; Faucon, D.; Castel, M.; Ayadi, F.; Cavalie, S.; Tourrette, A. Alginate/chitosan polyelectrolyte complexes: A comparative study of the influence of the drying step on physicochemical properties. Carbohydr. Polym. 2017, 172, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Anusuya, T. Investigation on Curcumin nanocomposite for wound dressing. Int. J. Biol. Macromol. 2017, 98, 366–378. [Google Scholar] [CrossRef]
- Montaser, A.S.; Abdel-Mohsen, A.M.; Ramadan, M.A.; Sleem, A.A.; Sahffie, N.M.; Jancar, J.; Hebeish, A. Preparation and characterization of alginate/silver/nicotinamide nanocomposites for treating diabetic wounds. Int. J. Biol. Macromol. 2016, 92, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Mayur, G.S.; Rajashree, C.M.; Jolly, M.S.; Vijay, B.S. Reversed chitosan-alginate polyelectrolyte complex for stability improvement of alpha-amylase: Optimization and physicochemical characterization. Eur. J. Pharm. Biopharm. 2007, 65, 215–232. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grondalh, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Bueno, C.Z.; Dias, A.M.; Sousa, H.J.; Braga, M.E.; Moraes, A.M. Control of the properties of porous chitosan–alginate membranes through the addition of different proportions of Pluronic F68. Mater. Sci. Eng. C 2014, 44, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Xiaoxia, L.; Hongguo, X.; Junzhang, L.; Weiyang, X.; Xiaojun, M. Characterization and biodegradation of chitosan-alginate polyelectrolyte complexes. Polym. Degrad. Stab. 2009, 94, 1–6. [Google Scholar] [CrossRef]
- Kemal, B.; Ayse, Z.A.; Zelal, A.; Bahattin, M.B. Chitosan/alginate crosslinked hydrogels: Preparation, characterization and application for cell growth purposes. Int. J. Biol. Macromol. 2013, 59, 342–348. [Google Scholar] [CrossRef]
- Skoog, D.; Holler, F.; Crouch, S. Principios de Análisis Instrumental; Cengage Learning: Boston, MA, USA, 2008. [Google Scholar]
- Li, C.C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modelling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Han, J.; Zhou, Z.; Yin, R.; Yang, D.; Nie, J. Alginate-chitosan/hydroxyapatite polyelectrolyte complex porous scaffolds: Preparation and characterization. Int. J. Biol. Macromol. 2010, 46, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Luo, H.; Zhang, Y.; Zhou, Y.; Ye, Z.; Tan, W.; Lang, M. Fabrication of three-dimensional poly(ε-caprolactone) scaffolds with hierarchical pore structures for tissue engineering. Mater. Sci. Eng. C 2013, 33, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Koosehgol, S.; Ebrahimian-Hosseinabadi, M.; Alizadeh, M.; Zamanian, A. Preparation and characterization of in situ chitosan/polyethylene glycol fumarate/thymol hydrogel as an effective wound dressing. Mater. Sci. Eng. C 2017, 79, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-W.; Tabata, Y.; Ikada, Y. Fabrication of porous gelatin scaffolds for tissue engineering. Biomateriales 1999, 20, 1339–1344. [Google Scholar] [CrossRef]
- Silva, S.S.; Oliveira, M.B.; Mano, J.F.; Reis, R.L. Bio-inspired Aloe vera sponges for biomedical applications. Carbohydr. Polym. 2014, 112, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Tang, Y. Honey/PVA hybrid wound dressings with controlled release of antibiotics: Structural, physico-mechanical and in vitro biomedical studies. Mater. Sci. Eng. C 2017, 77, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Straccia, M.C.S.; Ayala, G.G.; Romano, I.; Oliva, A.; Laurienzo, P. Alginate Hydrogels Coated with Chitosan for Wound Dressing. Mar. Drugs 2015, 13, 2890–2908. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Tian, F.; Yang, J.; He, C.H.; Xing, N.; Li, F. Chitosan and alginate polyelectrolyte complex membranes and their properties for wound dressing application. J. Mater. Sci. Mater. Med. 2010, 21, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.H.; Kim, D.H.; Kim, T.W.; Shin, K.K.; Jin, S.J.; Park, H.C.; Yoon, S.Y. In vivo evaluation of porous hydroxyapatite/chitosan-alginate composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2012, 51, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tang, X.; Wang, Y.; Guo, S. Smart gelation of chitosan solution in the presence of NaHCO3 for injectable drug delivery system. Int. J. Pharm. 2011, 414, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Brazel, C.S.; Peppas, N.A. Mechanisms of solute and drug transport in relaxing, swellable, hydrophilic glassy polymers. Polymer 1999, 40, 3383–3398. [Google Scholar] [CrossRef]
- Lacerda, L.; Parize, A.L.; Fávere, V.; Laranjeira, M.C.; Stulzer, H.K. Development and evaluation of pH-sensitive sodium alginate/chitosan microparticles containing the antituberculosis drug rifampicin. Mater. Sci. Eng. C 2014, 39, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhan, C.; Fan, L.; Wang, L.; Zheng, H. Preparation of dual crosslinked alginate-chitosan blend gel beads and in vitro controlled release in oral site-specific drug delivery system. Int. J. Pharm. 2007, 336, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Mishra, A. Antibacterial activities of crude extract of Aloe barbadensis to clinically isolated bacterial pathogens. Appl. Biomochem. Biotechnol. 2010, 160, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, J.; Zhao, Y. High intensity ultrasound assisted heating to improve solubility, antioxidant and antibacterial properties of chitosan-fructose Maillard reaction products. LWT Food Sci. Technol. 2014, 60, 253–262. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; Hudson, S.H.; Rojas, O.J. Antibacterial wounds dressing nanofiber mats from multicomponent (chitosan/Silver-Nps/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef] [PubMed]


| Matrix | Alginate Solution 1% (v/v) (Alg1) | Chitosan Solution 1% (v/v) (Chit 1) | Chitosan Solution 1.5% (v/v) (Chit 1.5) | NaHCO3 | Aloe vera Gel AV | Nanoparticles AgNps |
|---|---|---|---|---|---|---|
| A | 75% | 25% | * | * | * | * |
| B | 75% | * | 25% | * | * | * |
| C | 25% | 75% | * | 0.12 M | * | * |
| A-AV | 75% | 25% | * | * | Yes | * |
| B-AV | 75% | * | 25% | * | Yes | * |
| C-AV | 25% | 75% | * | 0.12 M | Yes | * |
| A-Nps | 75% | 25% | * | * | * | Yes |
| B-Nps | 75% | * | 25% | * | * | Yes |
| C-Nps | 25% | 75% | * | 0.12 M | * | Yes |
| A-AV-Nps | 75% | 25% | * | * | Yes | Yes |
| B-AV-Nps | 75% | * | 25% | * | Yes | Yes |
| C-AV-Nps | 25% | 75% | * | 0.12 M | Yes | Yes |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez Chabala, L.F.; Cuartas, C.E.E.; López, M.E.L. Release Behavior and Antibacterial Activity of Chitosan/Alginate Blends with Aloe vera and Silver Nanoparticles. Mar. Drugs 2017, 15, 328. https://doi.org/10.3390/md15100328
Gómez Chabala LF, Cuartas CEE, López MEL. Release Behavior and Antibacterial Activity of Chitosan/Alginate Blends with Aloe vera and Silver Nanoparticles. Marine Drugs. 2017; 15(10):328. https://doi.org/10.3390/md15100328
Chicago/Turabian StyleGómez Chabala, Luisa Fernanda, Claudia Elena Echeverri Cuartas, and Martha Elena Londoño López. 2017. "Release Behavior and Antibacterial Activity of Chitosan/Alginate Blends with Aloe vera and Silver Nanoparticles" Marine Drugs 15, no. 10: 328. https://doi.org/10.3390/md15100328




