Antibacterial Effect of Eicosapentaenoic Acid against Bacillus cereus and Staphylococcus aureus: Killing Kinetics, Selection for Resistance, and Potential Cellular Target
Abstract
:1. Introduction
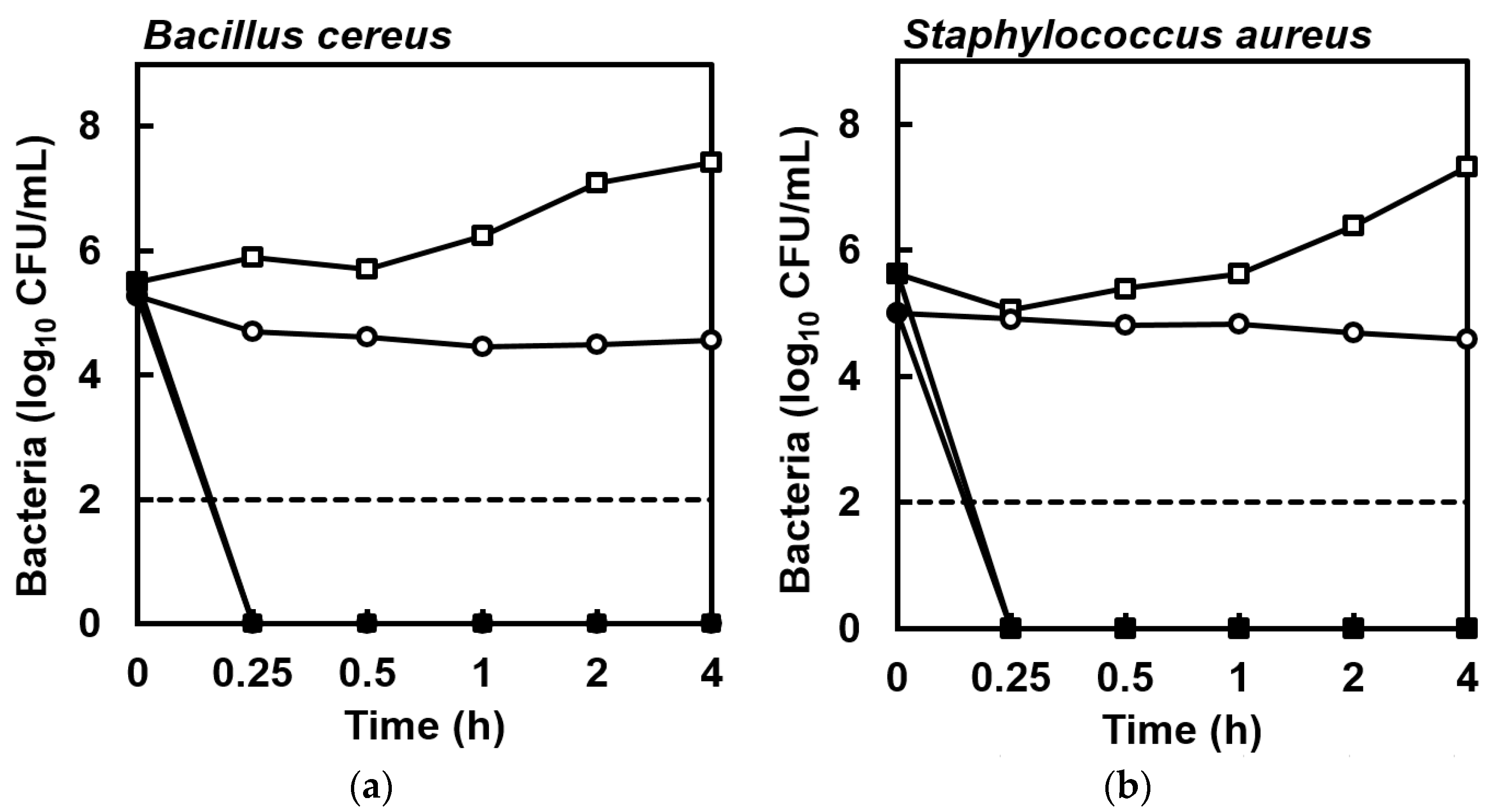
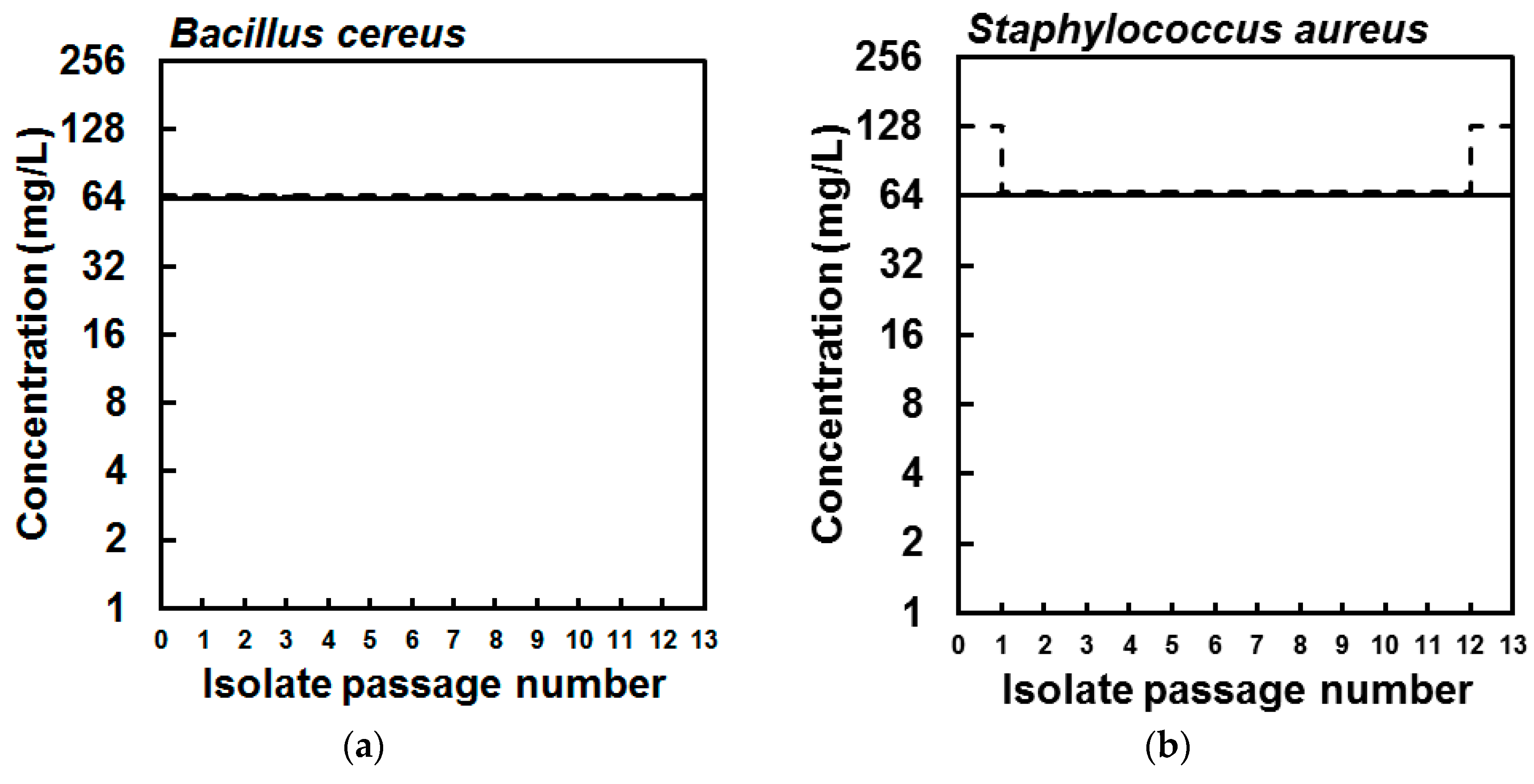
2. Results
3. Discussion
4. Materials and Methods
4.1. Reagents and Bacteria
4.2. Preparation of Bacterial Suspensions
4.3. Assessing Antibacterial Potency
4.4. Killing Kinetics
4.5. Selection of Bacterial Strains with Reduced Susceptibility to EPA
4.6. Leakage of A260-Absorbing Material from Bacterial Cells
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Desbois, A.P. Potential applications of antimicrobial fatty acids in medicine, agriculture and other industries. Recent Pat. Antiinfect. Drug Discov. 2012, 7, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Lawlor, K.C. Antibacterial activity of long-chain polyunsaturated fatty acids against Propionibacterium acnes and Staphylococcus aureus. Mar. Drugs 2013, 11, 4544–4557. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P. Antimicrobial properties of eicosapentaenoic acid (C20:5n-3). In Marine Microbiology: Bioactive Compounds and Biotechnological Applications; Kim, S.K., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2013; pp. 351–367. ISBN 978-3-527-33327-1. [Google Scholar]
- Sun, M.; Zhou, Z.; Dong, J.; Zhang, J.; Xia, Y.; Shu, R. Antibacterial and antibiofilm activities of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) against periodontopathic bacteria. Microb. Pathog. 2016, 99, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Crossley, K.B.; Jefferson, K.K.; Archer, G.L.; Fowler, V.G. (Eds.) Staphylococci in Human Disease, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2009; ISBN 978-1-4443-0847-1. [Google Scholar]
- Bottone, E.J. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef] [PubMed]
- Brogden, N.K.; Mehalick, L.; Fischer, C.L.; Wertz, P.W.; Brogden, K.A. The emerging role of peptides and lipids as antimicrobial epidermal barriers and modulators of local inflammation. Skin Pharmacol. Physiol. 2012, 25, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.B.; Yao, J.; Frank, M.W.; Jackson, P.; Rock, C.O. Membrane disruption by antimicrobial fatty acids releases low-molecular-weight proteins from Staphylococcus aureus. J. Bacteriol. 2012, 194, 5294–5304. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Peng, K.C.; Wu, J.L.; Chen, J.Y. Transgenic expression of salmon delta-5 and delta-6 desaturase in zebrafish muscle inhibits growth of Vibrio alginolyticus and affects fish immunomodulatory activity. Fish Shellfish Immunol. 2014, 39, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.; Ma, D.C.; Alvarez, S.; Faull, K.F. Antimicrobial lipids: Emerging effector molecules of innate host defense. World J. Immunol. 2015, 5, 51–61. [Google Scholar] [CrossRef]
- Mullen, A.; Loscher, C.E.; Roche, H.M. Anti-inflammatory effects of EPA and DHA are dependent upon time and dose-response elements associated with LPS stimulation in THP-1-derived macrophages. J. Nutr. Biochem. 2010, 21, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Shingel, K.I.; Faure, M.P.; Azoulay, L.; Roberge, C.; Deckelbaum, R.J. Solid emulsion gel as a vehicle for delivery of polyunsaturated fatty acids: Implications for tissue repair, dermal angiogenesis and wound healing. J. Tissue Eng. Regen. Med. 2008, 2, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Kao, M.C.; Fang, J.Y.; Zouboulis, C.C.; Zhang, L.; Gallo, R.L.; Huang, C.M. Antimicrobial property of lauric acid against Propionibacterium acnes: Its therapeutic potential for inflammatory acne vulgaris. J. Investig. Dermatol. 2009, 129, 2480–2488. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard M07-A8; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline M26-A Vol. 19 No. 18; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 1999. [Google Scholar]
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Bajpai, V.K.; Kim, H.R.; Kang, S.C. Antibacterial activity of eicosapentaenoic acid (EPA) against foodborne and food spoilage microorganisms. LWT Food Sci. Technol. 2007, 40, 1515–1519. [Google Scholar] [CrossRef]
- Desbois, A.P.; Mearns-Spragg, A.; Smith, V.J. A fatty acid from the diatom Phaeodactylum tricornutum is antibacterial against diverse bacteria including multi-resistant Staphylococcus aureus (MRSA). Mar. Biotechnol. 2009, 11, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Borst, P.; Loos, J.A.; Christ, E.J.; Slater, E.C. Uncoupling activity of long chain fatty acids. Biochim. Biophys. Acta 1962, 62, 509–518. [Google Scholar] [CrossRef]
- Boyaval, P.; Corre, C.; Dupuis, C.; Roussel, E. Effects of free fatty acids on propionic acid bacteria. Lait 1995, 75, 17–29. [Google Scholar] [CrossRef]
- Wojtczak, L.; Więckowski, M.R. The mechanisms of fatty acid induced proton permeability of the inner mitochondrial membrane. J. Bioenerg. Biomembr. 1999, 31, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Greenway, D.L.A.; Dyke, K.G.H. Mechanism of the inhibitory action of linoleic acid on the growth of Staphylococcus aureus. J. Gen. Microbiol. 1979, 115, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Sheu, C.W.; Freese, E. Effects of fatty acids on growth and envelope proteins of Bacillus subtilis. J. Bacteriol. 1972, 111, 516–524. [Google Scholar] [PubMed]
- Galbraith, H.; Miller, T.B. Physiological effects of long chain fatty acids on bacterial cells and their protoplasts. J. Appl. Bacteriol. 1973, 36, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Thormar, H.; Isaacs, C.E.; Brown, H.R.; Barshatzky, M.R.; Pessolano, T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1987, 31, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J. Membranes as targets of antimicrobial lipids. In Lipids and Essential Oils as Antimicrobial Agents; Halldor, T., Ed.; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 1–24. ISBN 978-0-470-74178-8. [Google Scholar]
- Arouri, A.; Mouritsen, O.G. Membrane-perturbing effect of fatty acids and lysolipids. Prog. Lipids Res. 2013, 52, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.R.; Mohamed, R.; Bian, L.; Routh, A.F.; Kokai-Kun, J.F.; Mond, J.J.; Tarkowski, A.; Foster, S.J. The Staphylococcus aureus surface protein IsdA mediates resistance to innate defenses of human skin. Cell Host Microbe 2007, 1, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Kenny, J.G.; Ward, D.; Josefsson, E.; Jonsson, I.M.; Hinds, J.; Rees, H.H.; Lindsay, J.A.; Tarkowski, A.; Horsburgh, M.J. The Staphylococcus aureus response to unsaturated long chain free fatty acids: Survival mechanisms and virulence implications. PLoS ONE 2009, 4, e4344. [Google Scholar] [CrossRef] [PubMed]
- Kohler, T.; Weidenmaier, C.; Peschel, A. Wall teichoic acid protects Staphylococcus aureus against antimicrobial fatty acids from human skin. J. Bacteriol. 2009, 191, 4482–4484. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, N.R.; Mehrtens, B.G.; Xiong, Z.; Kapral, F.A.; Boardman, J.L.; Rearick, J.I. Correlation of carotenoid production, decreased membrane fluidity, and resistance to oleic acid killing in Staphylococcus aureus 18Z. Infect. Immun. 1991, 59, 4332–4337. [Google Scholar] [PubMed]
- Xiong, Z.; Kapral, F.A. Carotenoid pigment levels in Staphylococcus aureus and sensitivity to oleic acid. J. Med. Microbiol. 1992, 37, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Marounek, M.; Skivanová, E.; Rada, V. Susceptibility of Escherichia coli to C2-C18 fatty acids. Folia Microbiol. 2003, 48, 731–735. [Google Scholar] [CrossRef]
- Petschow, B.W.; Batema, R.P.; Ford, L.L. Susceptibility of Helicobacter pylori to bactericidal properties of medium-chain monoglycerides and free fatty acids. Antimicrob. Agents Chemother. 1996, 40, 302–306. [Google Scholar] [PubMed]
- Obonyo, M.; Zhang, L.; Thamphiwatana, S.; Pornpattananangkul, D.; Fu, V.; Zhang, L. Antibacterial activities of liposomal linolenic acids against antibiotic-resistant Helicobacter pylori. Mol. Pharm. 2012, 9, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Q.; O’Connor, C.J.; Roberton, A.M. Antibacterial actions of fatty acids and monoglycerides against Helicobacter pylori. FEMS Immunol. Med. Microbiol. 2003, 36, 9–17. [Google Scholar] [CrossRef]
- Lacey, R.W.; Lord, V.L. Sensitivity of staphylococci to fatty acids: Novel inactivation of linolenic acid by serum. J. Med. Microbiol. 1981, 14, 41–49. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, P.N.T.; Desbois, A.P. Antibacterial Effect of Eicosapentaenoic Acid against Bacillus cereus and Staphylococcus aureus: Killing Kinetics, Selection for Resistance, and Potential Cellular Target. Mar. Drugs 2017, 15, 334. https://doi.org/10.3390/md15110334
Le PNT, Desbois AP. Antibacterial Effect of Eicosapentaenoic Acid against Bacillus cereus and Staphylococcus aureus: Killing Kinetics, Selection for Resistance, and Potential Cellular Target. Marine Drugs. 2017; 15(11):334. https://doi.org/10.3390/md15110334
Chicago/Turabian StyleLe, Phuc Nguyen Thien, and Andrew P. Desbois. 2017. "Antibacterial Effect of Eicosapentaenoic Acid against Bacillus cereus and Staphylococcus aureus: Killing Kinetics, Selection for Resistance, and Potential Cellular Target" Marine Drugs 15, no. 11: 334. https://doi.org/10.3390/md15110334





