Immunoenhancement Effects of Glycosaminoglycan from Apostichopus japonicus: In Vitro and In Cyclophosphamide-Induced Immunosuppressed Mice Studies
Abstract
:1. Introduction
2. Results
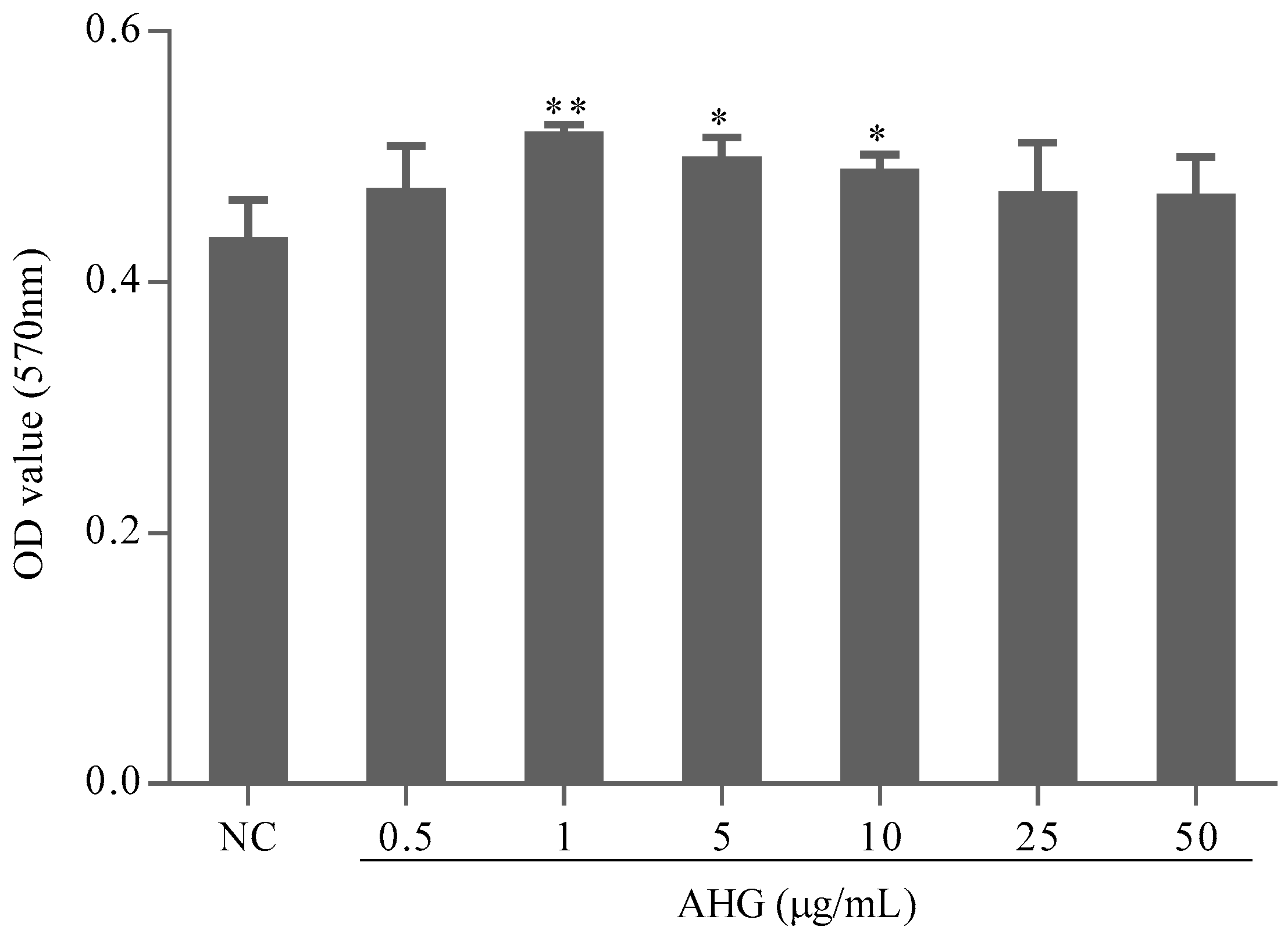
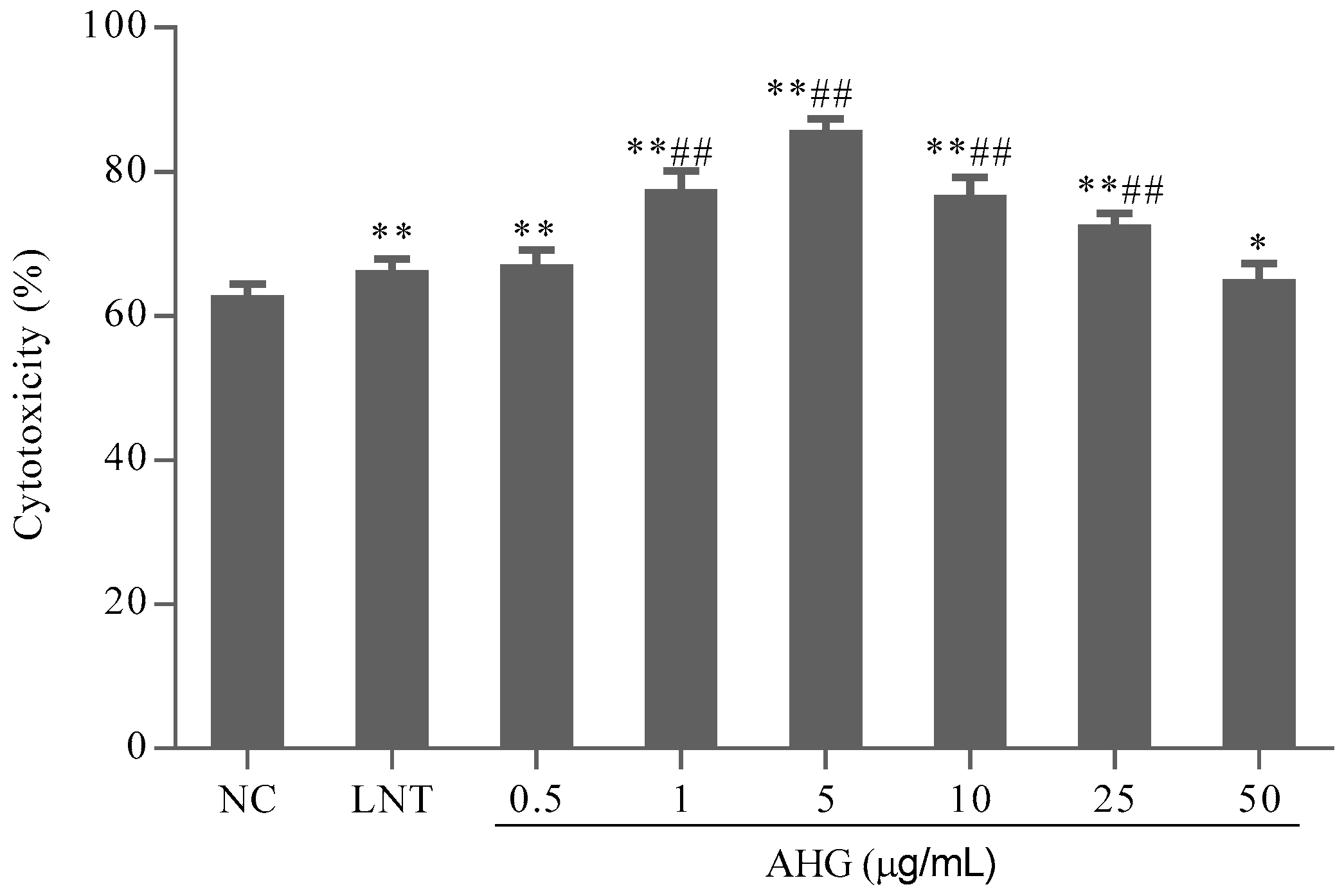
2.1. Cytotoxic Effect of AHG on Splenocytes
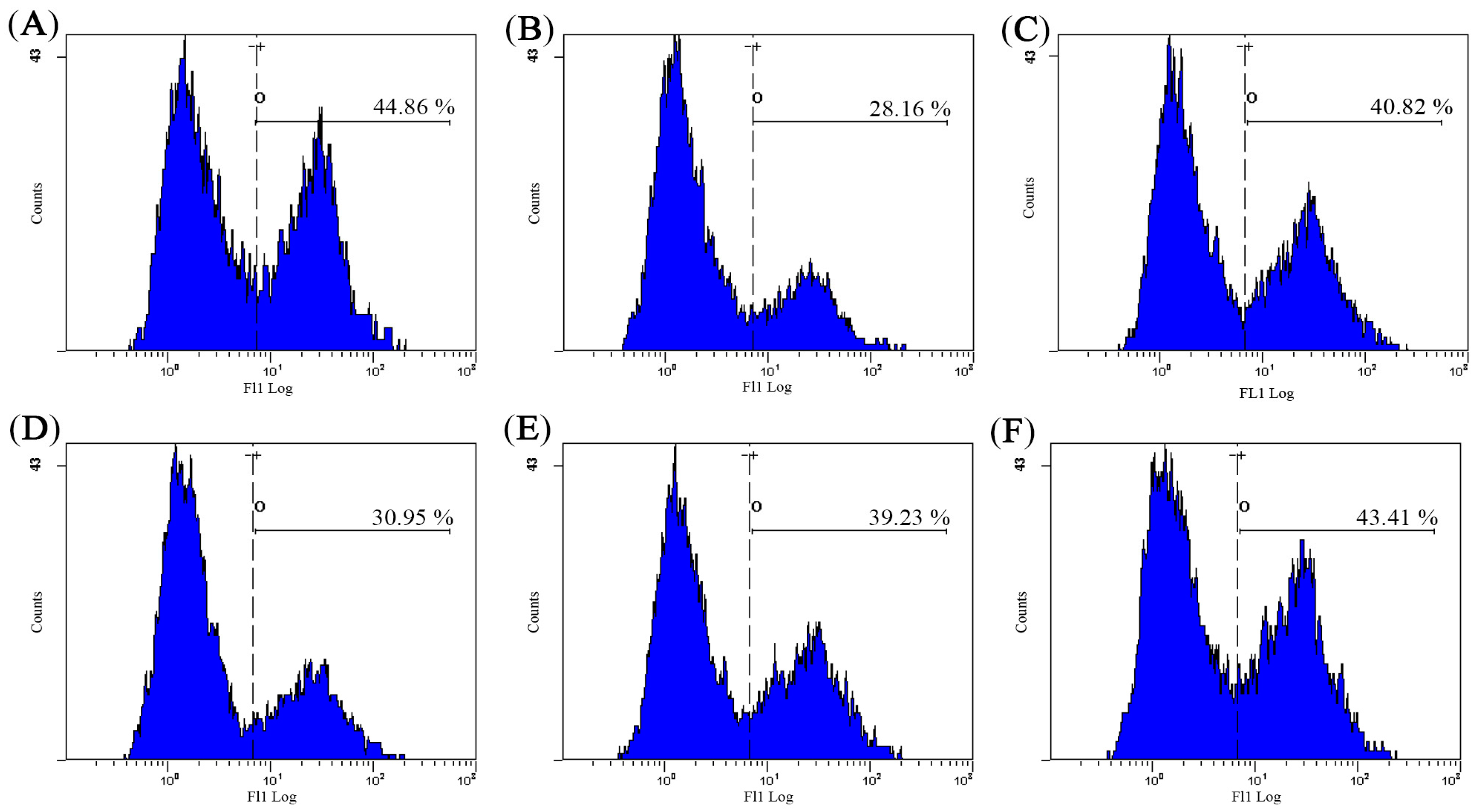
2.2. Effect of AHG on Splenic NK Cells Activity
2.3. Effect of AHG on Splenic CTLs Activity
2.4. Effect of AHG on Mitogen-Induced Splenic Lymphocyte Proliferation in CY-Treated Mice
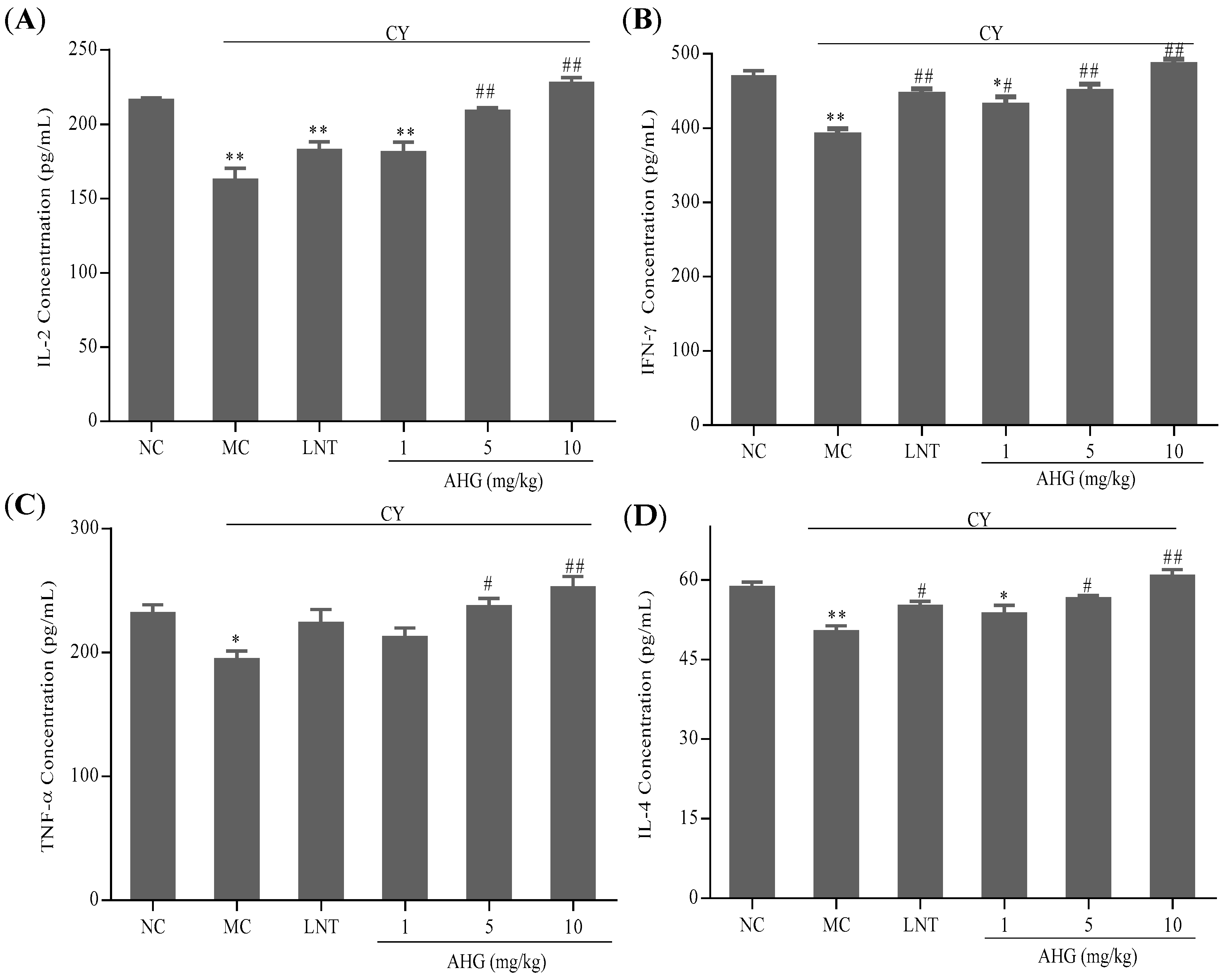
2.5. Effects of AHG on Cytokines Secretion of Splenocytes in CY-Treated Mice
2.6. Effect of AHG on Intracellular Free Ca2+ Concentration of Splenocytes in CY-Treated Mice
2.7. Effect of AHG on DTH Reaction in CY-Treated Mice
2.8. Antioxidant Activity of AHG in CY-Treated Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Animals
4.3. Immunomodulatory Activity of AHG In Vitro
4.3.1. Cell Preparation and Culture
4.3.2. Cytotoxic Effect of AHG on Splenocyte
4.3.3. Cytotoxicity Assays of NK Cell Activity of Splenocytes
4.3.4. Assay of CTL Activity of Splenocytes
4.3.5. MTT Assay
4.4. Immunomodulatory Activity of AHG In Vivo
4.4.1. Animal Experiments
4.4.2. Splenic Lymphocyte Proliferation Assay
4.4.3. Assay of Cytokine Levels
4.4.4. Measurement of Intracellular Ca2+
4.4.5. Assay of DTH Reaction (Footpad Reaction Test)
4.4.6. Biochemical Assays
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, H.; Wang, M.; Chen, J.; Tang, Y.; Dou, J.; Yu, J.; Xi, T.; Zhou, C. A polysaccharide from strongylocentrotus nudus eggs protects against myelosuppression and immunosuppression in cyclophosphamide-treated mice. Int. Immunopharmacol. 2011, 11, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Morenomendieta, S.; Guillén, D.; Hernándezpando, R.; Sánchez, S.; Rodríguezsanoja, R. Potential of glucans as vaccine adjuvants: A review of the α-glucans case. Carbohydr. Polym. 2017, 165, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, L.-M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr. Polym. 2009, 76, 349–361. [Google Scholar] [CrossRef]
- Wang, J.L.; Bi, Z.; Zou, J.W.; Gu, X.M. Combination therapy with lentinan improves outcomes in patients with esophageal carcinoma. Mol. Med. Rep. 2012, 5, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Mitsuhashi, N.; Saito, Y.; Takahashi, M.; Katano, S.; Shiojima, K.; Furuta, M.; Niibe, H. Effect of krestin (psk) as adjuvant treatment on the prognosis after radical radiotherapy in patients with non-small cell lung cancer. Anticancer Res. 1993, 13, 1815–1820. [Google Scholar] [PubMed]
- Cui, J.; Chisti, Y. Polysaccharopeptides of coriolus versicolor: Physiological activity, uses, and production. Biotechnol. Adv. 2003, 21, 109–122. [Google Scholar] [CrossRef]
- Fang, X.S.; Zhou, P. Clinical efficacy of injection astragalus polysaccharide on treatment primary live cancer patients. Chongqing Med. 2009, 38, 935–938. [Google Scholar]
- Ma, Y.L.; Li, H. Clinic effect of ginseng polysaccharide combined with radiation therapy in nasopharyngeal carcinoma patients. J. Mod. Oncol. 2015, 23, 1511–1514. [Google Scholar]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed]
- Minamiguchi, K.; Kitazato, K.T.; Nagase, H.; Sasaki, E.; Ohwada, K.; Kitazato, K. Depolymerized holothurian glycosaminoglycan (dhg), a novel alternative anticoagulant for hemodialysis, is safe and effective in a dog renal failure model. Kidney Int. 2003, 63, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Panagos, C.G.; Thomson, D.S.; Moss, C.; Hughes, A.D.; Kelly, M.S.; Liu, Y.; Chai, W.; Venkatasamy, R.; Spina, D.; Page, C.P. Fucosylated chondroitin sulfates from the body wall of the sea cucumber holothuria forskali: Conformation, selectin binding, and biological activity. J. Biol. Chem. 2014, 289, 28284–28298. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jin, S.J.; Cui, L.H.; Ji, X.J.; Yang, F.G. Immunomodulatory effect of stichopus japonicus acid mucopolysaccharide on experimental hepatocellular carcinoma in rats. Molecules 2013, 18, 7179–7193. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Wang, A.; Zhu, Z.; Tao, L.; Li, Y.; Zhou, L.; Chen, W.; Lu, Y. Holothurian glycosaminoglycan inhibits metastasis via inhibition of p-selectin in b16f10 melanoma cells. Mol. Cell. Biochem. 2015, 410, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Tao, L.; Wang, Y.; Zhang, F.; Li, M.; Huang, S.; Wang, A.; Chen, W.; Yue, Z.; Chen, L. Downregulation of integrins in cancer cells and anti-platelet properties are involved in holothurian glycosaminoglycan-mediated disruption of the interaction of cancer cells and platelets in hematogenous metastasis. J. Vasc. Res. 2015, 52, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xin, Y.N.; Luo, B.; Xuan, S.Y. Effects of glycosaminoglycan extracted from stichopus japonicus selenka on the expression of hbv-associated antigens in the liver of hbv transgenic mice. World Chin. J. Digestol. 2010, 18, 1201–1205. [Google Scholar]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Tsvetkova, E.A.; Shashkov, A.S.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. Structural characterization of fucosylated chondroitin sulfates from sea cucumbers apostichopus japonicus and actinopyga mauritiana. Carbohydr. Polym. 2016, 153, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Kariya, Y.; Watabe, S.; Kyogashima, M.; Ishihara, M.; Ishii, T. Structure of fucose branches in the glycosaminoglycan from the body wall of the sea cucumber stichopus japonicus. Carbohydr. Res. 1997, 297, 273–279. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Jiang, T.; Lv, Z. Novel branch patterns and anticoagulant activity of glycosaminoglycan from sea cucumber apostichopus japonicus. Int. J. Biol. Macromol. 2015, 72, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wang, Y.H.; Hu, J.F.; Lv, Z.H. The research of glycosaminoglycan fromapostichopus japonius on specific immune adjustment. Chin. J. Mar. Drugs 2015, 34, 53–58. [Google Scholar]
- Hu, J.F.; Wang, Y.H.; Li, S.; Gao, Q.; Lv, Z.H. Effect of apostichopus japonicus glycosaminoglycan on the function of peritoneal macrophages in mice. Chin. J. Mar. Drugs 2014, 33, 21–26. [Google Scholar]
- Qiu, R.F.; Wang, Y.H.; Gao, Q.; Li, S.; Lv, Z.H. Effects of glycosaminoglycan fromapostichopus japonius on peripheral blood cells and bone marrow cell cycle of myelosuppressed anemia mice. Chin. J. Mar. Drugs 2015, 34, 47–52. [Google Scholar]
- Kos, F.J.; Engleman, E.G. Immune regulation: A critical link between nk cells and ctls. Immunol. Today 1996, 17, 174–176. [Google Scholar] [CrossRef]
- Whiteside, T.L.; Chikamatsu, K.; Nagashima, S.; Okada, K. Antitumor effects of cytolytic t lymphocytes (ctl) and natural killer (nk) cells in head and neck cancer. Anticancer Res. 1996, 16, 2357–2364. [Google Scholar] [PubMed]
- Henney, C.S.; Kuribayashi, K.; Kern, D.E.; Gillis, S. Interleukin-2 augments natural killer cell activity. Nature 1981, 291, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Good, M.F.; Powell, L.W.; Halliday, J.W. Il-2 and il-4 can co-modulate the generation of cytotoxic t cells through cd8- cd4- splenic lymphocytes. Immunology 1989, 67, 225–230. [Google Scholar] [PubMed]
- Moretta, L.; Bottino, C.; Cantoni, C.; Mingari, M.C.; Moretta, A. Human natural killer cell function and receptors. Curr. Opin. Pharmacol. 2001, 1, 387–391. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, S.H.; Shin, J.G.; Kim, G.S.; Son, Y.O.; Park, S.W.; Kwon, B.H.; Kim, D.W.; Lee, C.H.; Sol, M.Y.; et al. Direct injection of immature dendritic cells into irradiated tumor induces efficient antitumor immunity. Int. J. Cancer 2004, 109, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Noh, Y.W.; Kim, K.D.; Yong, S.J.; Choe, Y.K.; Lim, J.S. Activated natural killer cell-mediated immunity is required for the inhibition of tumor metastasis by dendritic cell vaccination. Exp. Mol. Med. 2004, 36, 428–443. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Nie, W.; Fan, S.; Zhang, J.; Wang, Y.; Lu, J.; Jin, L. A polysaccharide from sargassum fusiforme protects against immunosuppression in cyclophosphamide-treated mice. Carbohydr. Polym. 2012, 90, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.W.; Han, C.J.; Rhee, Y.K.; Lee, Y.C.; Shin, K.S.; Shin, J.S.; Lee, K.T.; Hong, H.D. Cheonggukjang polysaccharides enhance immune activities and prevent cyclophosphamide-induced immunosuppression. Int. J. Biol. Macromol. 2015, 72, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tong, X.; Li, P.; Cao, H.; Su, W. Immuno-enhancement effects of shenqi fuzheng injection on cyclophosphamide-induced immunosuppression in balb/c mice. J. Ethnopharmacol. 2012, 139, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-E. Lactobacillus plantarum hy7712 ameliorates cyclophosphamide-induced immunosuppression in mice. J. Microbiol. Biotechnol. 2013, 23, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, K.M.; Guruvayoorappan, C. Acacia ferruginea inhibits cyclophosphamide-induced immunosuppression and urotoxicity by modulating cytokines in mice. J. Immunotoxicol. 2015, 12, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Sweet, M.J.; Hume, D.A. Signal integration between ifngamma and tlr signalling pathways in macrophages. Immunobiology 2006, 211, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Gessani, S.; Belardelli, F. Ifn-gamma expression in macrophages and its possible biological significance. Cytokine Growth Factor Rev. 1998, 9, 117–123. [Google Scholar] [CrossRef]
- Cohen, M.C.; Cohen, S. Cytokine function: A study in biologic diversity. Am. J. Clin. Pathol. 1996, 105, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Natural inhibitors of tumour necrosis factor-α production, secretion and function. Planta Med. 2000, 66, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Feske, S. Calcium signals in lymphocyte activation and disease. Nat. Rev. Immunol. 2009, 7, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 1995, 80, 259–268. [Google Scholar] [CrossRef]
- Hogan, P.G.; Chen, L.; Nardone, J.; Rao, A. Transcriptional regulation by calcium, calcineurin, and nfat. Genes Dev. 2003, 17, 2205. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Nie, S.P.; Wang, J.Q.; Huang, D.F.; Li, W.J.; Xie, M.Y. Signaling pathway involved in the immunomodulatory effect of ganoderma atrum polysaccharide in spleen lymphocytes. J. Agric. Food Chem. 2015, 63, 2734–2740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.D.; Yin, Y.X.; Wei, Q. Immunopotentiation on murine spleen lymphocytes induced by polysaccharide fraction of panax ginseng via upregulating calcineurin activity. APMIS 2010, 118, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Jacysyn, J.F.; Abrahamsohn, I.A.; Macedo, M.S. Modulation of delayed-type hypersensitivity during the time course of immune response to a protein antigen. Immunology 2001, 102, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Kong, M.; Zhang, P.; Sun, Q.; Chen, K. Immune-enhancing activity of extracellular polysaccharides isolated from rhizopus nigricans. Carbohydr. Polym. 2016, 148, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, R.; Liu, Y.; Xiao, J.; Liu, L.; Wei, Z.; Yi, Y.; Zhang, M.; Liu, D. Dietary litchi pulp polysaccharides could enhance immunomodulatory and antioxidant effects in mice. Int. J. Biol. Macromol. 2016, 92, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Meng, X.Y.; Yang, R.L.; Qin, T.; Wang, X.Y.; Zhang, K.Y.; Fei, C.Z.; Li, Y.; Hu, Y.; Xue, F.Q. Cordyceps militaris polysaccharides can enhance the immunity and antioxidation activity in immunosuppressed mice. Carbohydr. Polym. 2012, 89, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, S.Z.; Ying, H.Z.; Dai, X.Y.; Li, X.X.; Yu, C.H.; Ye, H.C. Chemical characterization and immunostimulatory effects of a polysaccharide from polygoni multiflori radix praeparata in cyclophosphamide-induced anemic mice. Carbohydr. Polym. 2012, 88, 1476–1482. [Google Scholar] [CrossRef]
- Vida, C.; González, E.M.; De, L.F.M. Increase of oxidation and inflammation in nervous and immune systems with aging and anxiety. Curr. Pharm. Des. 2014, 20, 4656–4678. [Google Scholar] [CrossRef] [PubMed]
- Simic, M.G.; Bergtold, D.S.; Karam, L.R. Generation of oxy radicals in biosystems. Mutat. Res. 1989, 214, 3–12. [Google Scholar] [CrossRef]
- Puertollano, M.A.; Puertollano, E.; de Cienfuegos, G.Á.; de Pablo, M.A. Dietary antioxidants: Immunity and host defense. Curr. Top. Med. Chem. 2011, 11, 1752–1766. [Google Scholar] [CrossRef] [PubMed]



| Group | Dose (mg/kg) | Malondialdehyde (MDA, nmol/mg pro) | Superoxidase Dismutase (SOD, U/mg pro) | Total Antioxidant Capacity (T-AOC, U/mg pro) | Glutathione Peroxidase (GSH-PX, U/mg pro) | Catalase (CAT, U/mg pro) |
|---|---|---|---|---|---|---|
| NC | 1.40 ± 0.18 | 383.26 ± 15.34 | 4.30 ± 0.23 | 213.22 ± 15.56 | 51.07 ± 3.78 | |
| MC | 3.09 ± 0.18 ** | 280.17 ± 33.59 ** | 2.46 ± 0.22 ** | 147.68 ± 19.88 ** | 23.79 ± 3.91 ** | |
| LNT | 1 | 2.06 ± 0.14 **## | 368.63 ± 27.40 ## | 4.11 ± 0.14 ## | 211.75 ± 22.31 ## | 34.72 ± 3.13 **## |
| AHG | 1 | 2.81 ± 0.17 **# | 283.71 ± 13.60 ** | 2.89 ± 0.26 ** | 156.36 ± 25.89 ** | 24.60 ± 1.81 ** |
| 5 | 2.17 ± 0.13 **## | 322.16 ± 27.94 ** | 3.58 ± 0.25 ## | 191.47 ± 18.69 *# | 43.43 ± 2.39 *## | |
| 10 | 1.60 ± 0.10 ## | 385.12 ± 25.21 ## | 4.31 ± 0.15 ## | 220.12 ± 30.57 ## | 53.19 ± 2.73 ## |
| Group | Dose (mg/kg) | MDA (nmol/mg pro) | SOD (U/mg pro) | T-AOC (U/mg pro) | GSH-PX (U/mg pro) | CAT (U/mg pro) |
|---|---|---|---|---|---|---|
| NC | 3.39 ± 0.23 | 256.93 ± 22.22 | 2.83 ± 0.09 | 83.01 ± 7.46 | 39.90 ± 3.01 | |
| MC | 6.34 ± 0.13 ** | 187.77 ± 18.34 ** | 1.28 ± 0.1 ** | 57.11 ± 3.53 ** | 21.83 ± 2.56 ** | |
| LNT | 1 | 3.64 ± 0.27 ## | 251.19 ± 32.64 # | 2.83 ± 0.01 ## | 80.06 ± 2.33 ## | 40.28 ± 3.74 ## |
| AHG | 1 | 5.74 ± 0.12 **## | 208.02 ± 12.10 ** | 1.61 ± 0.08 **## | 61.62 ± 7.57 *# | 22.36 ± 1.56 ** |
| 5 | 4.49 ± 0.30 **## | 246.20 ± 22.57 # | 2.70 ± 0.08 ## | 71.39 ± 4.57 *# | 34.43 ± 1.34 **## | |
| 10 | 3.44 ± 0.19 ## | 256.86 ± 28.75 ## | 3.28 ± 0.04 ## | 83.39 ± 2.59 ## | 40.66 ± 4.63 ## |
| Group | Dose (mg/kg) | MDA (nmol/mg pro) | SOD (U/mg pro) | T-AOC (U/mg pro) | GSH-PX (U/mg pro) | CAT (U/mg pro) |
|---|---|---|---|---|---|---|
| NC | 2.42 ± 0.25 | 86.14 ± 0.57 | 3.32 ± 0.20 | 164.77 ± 12.47 | 51.75 ± 0.81 | |
| MC | 4.38 ± 0.34 ** | 66.03 ± 1.04 ** | 1.72 ± 0.17 ** | 130.53 ± 3.38 ** | 36.50 ± 1.34 ** | |
| LNT | 1 | 2.85 ± 0.26 *## | 84.91 ± 1.44 *## | 3.51 ± 0.22 ## | 163.27 ± 8.48 ## | 52.34 ± 2.25 ## |
| AHG | 1 | 3.93 ± 0.25 ** | 77.59 ± 0.66 **## | 2.40 ± 0.32 *## | 136.90 ± 4.91 **## | 41.95 ± 0.94 **## |
| 5 | 2.94 ± 0.25 *## | 82.68 ± 1.94 **## | 2.83 ± 0.21 ## | 142.05 ± 7.90 **## | 45.81 ± 2.57 **## | |
| 10 | 2.31 ± 0.17 ## | 89.75 ± 0.99 **## | 3.47 ± 0.18 ## | 158.40 ± 8.64 ## | 53.00 ± 1.38 *## |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yang, S.; Wang, Y.; Jiang, T.; Li, S.; Lv, Z. Immunoenhancement Effects of Glycosaminoglycan from Apostichopus japonicus: In Vitro and In Cyclophosphamide-Induced Immunosuppressed Mice Studies. Mar. Drugs 2017, 15, 347. https://doi.org/10.3390/md15110347
Wang H, Yang S, Wang Y, Jiang T, Li S, Lv Z. Immunoenhancement Effects of Glycosaminoglycan from Apostichopus japonicus: In Vitro and In Cyclophosphamide-Induced Immunosuppressed Mice Studies. Marine Drugs. 2017; 15(11):347. https://doi.org/10.3390/md15110347
Chicago/Turabian StyleWang, Han, Shuang Yang, Yuanhong Wang, Tingfu Jiang, Shuai Li, and Zhihua Lv. 2017. "Immunoenhancement Effects of Glycosaminoglycan from Apostichopus japonicus: In Vitro and In Cyclophosphamide-Induced Immunosuppressed Mice Studies" Marine Drugs 15, no. 11: 347. https://doi.org/10.3390/md15110347






