Bioinspiring Chondrosia reniformis (Nardo, 1847) Collagen-Based Hydrogel: A New Extraction Method to Obtain a Sticky and Self-Healing Collagenous Material
Abstract
:1. Introduction
2. Results
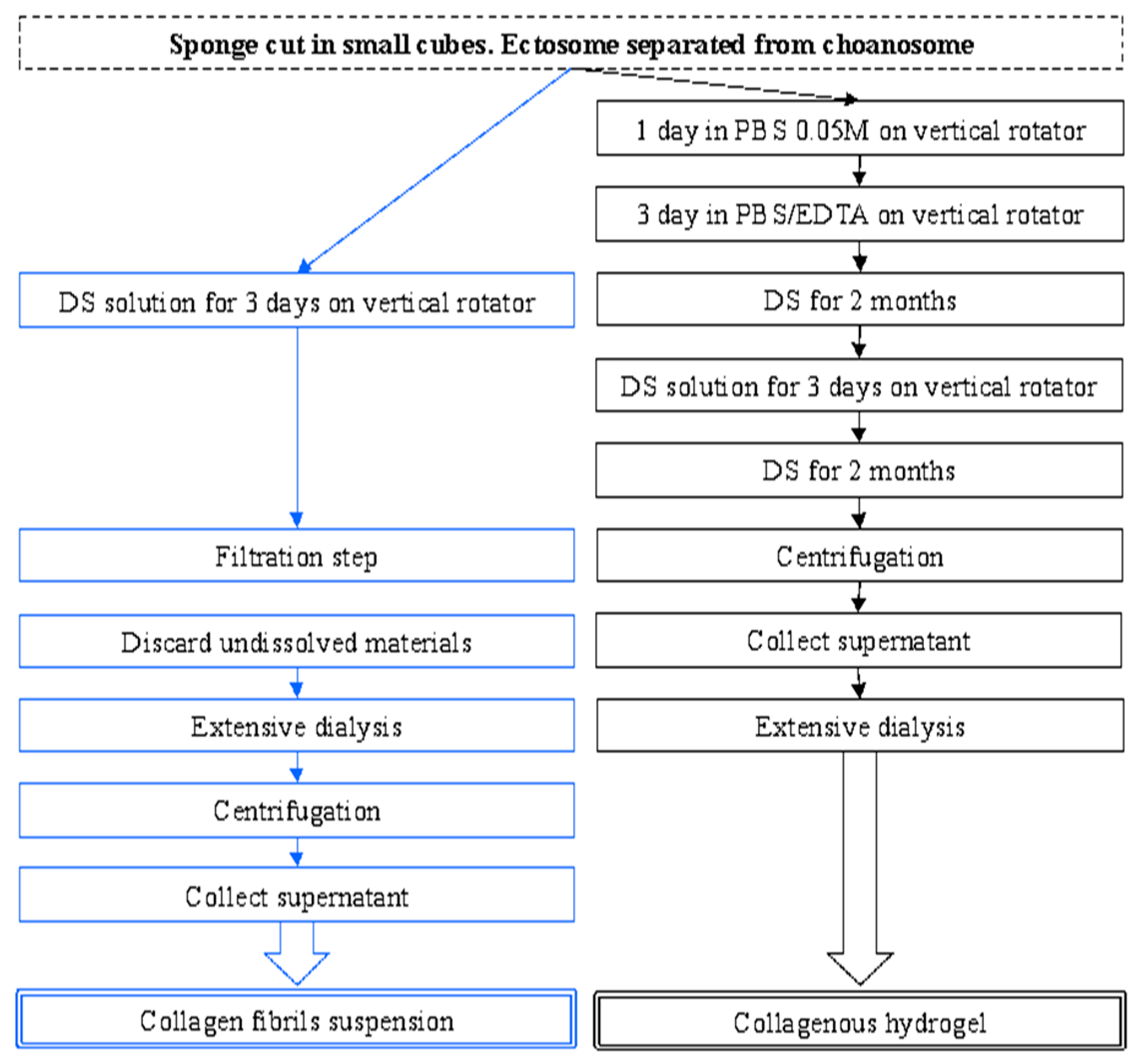
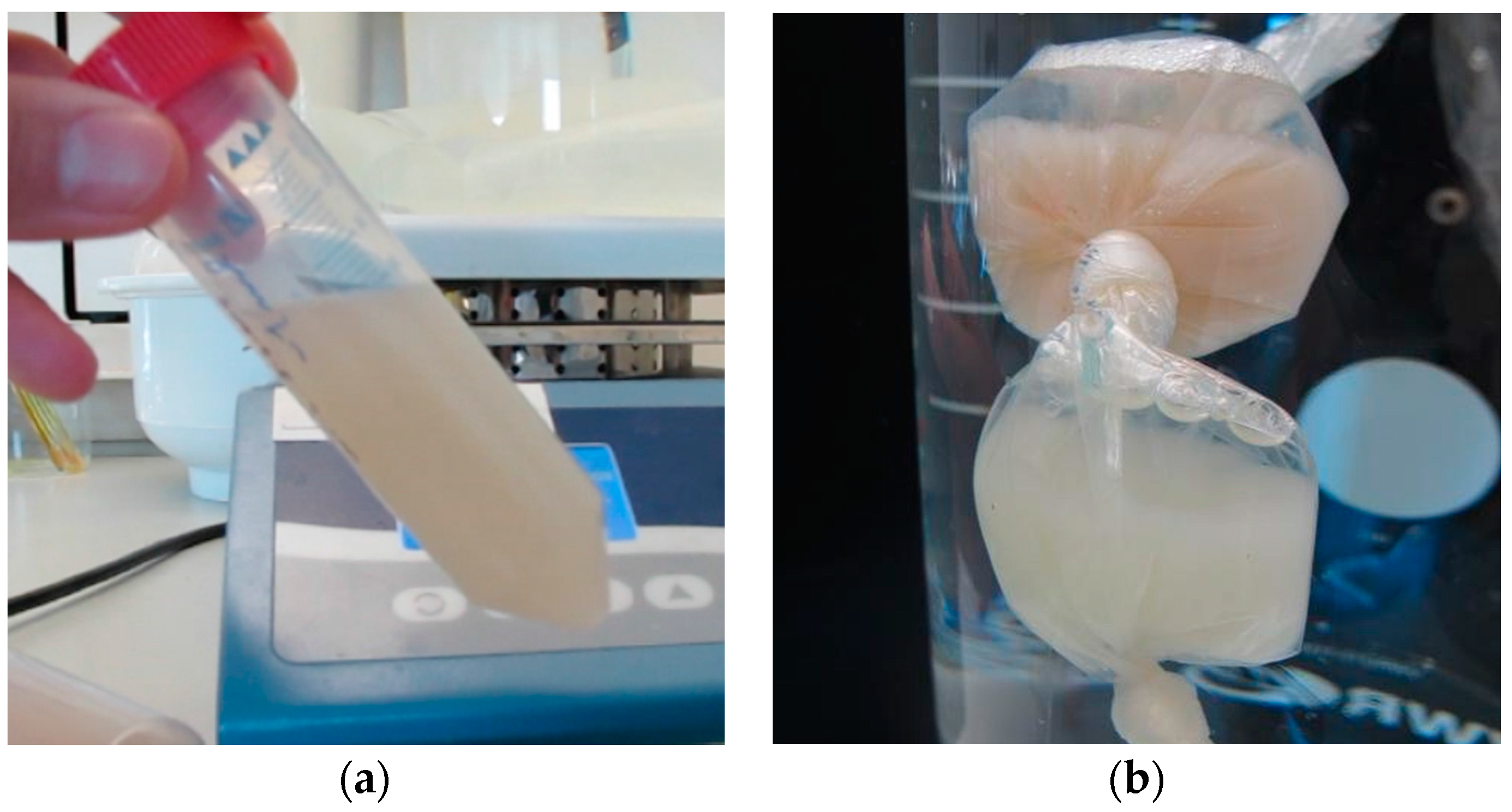
2.1. Collagen Extraction
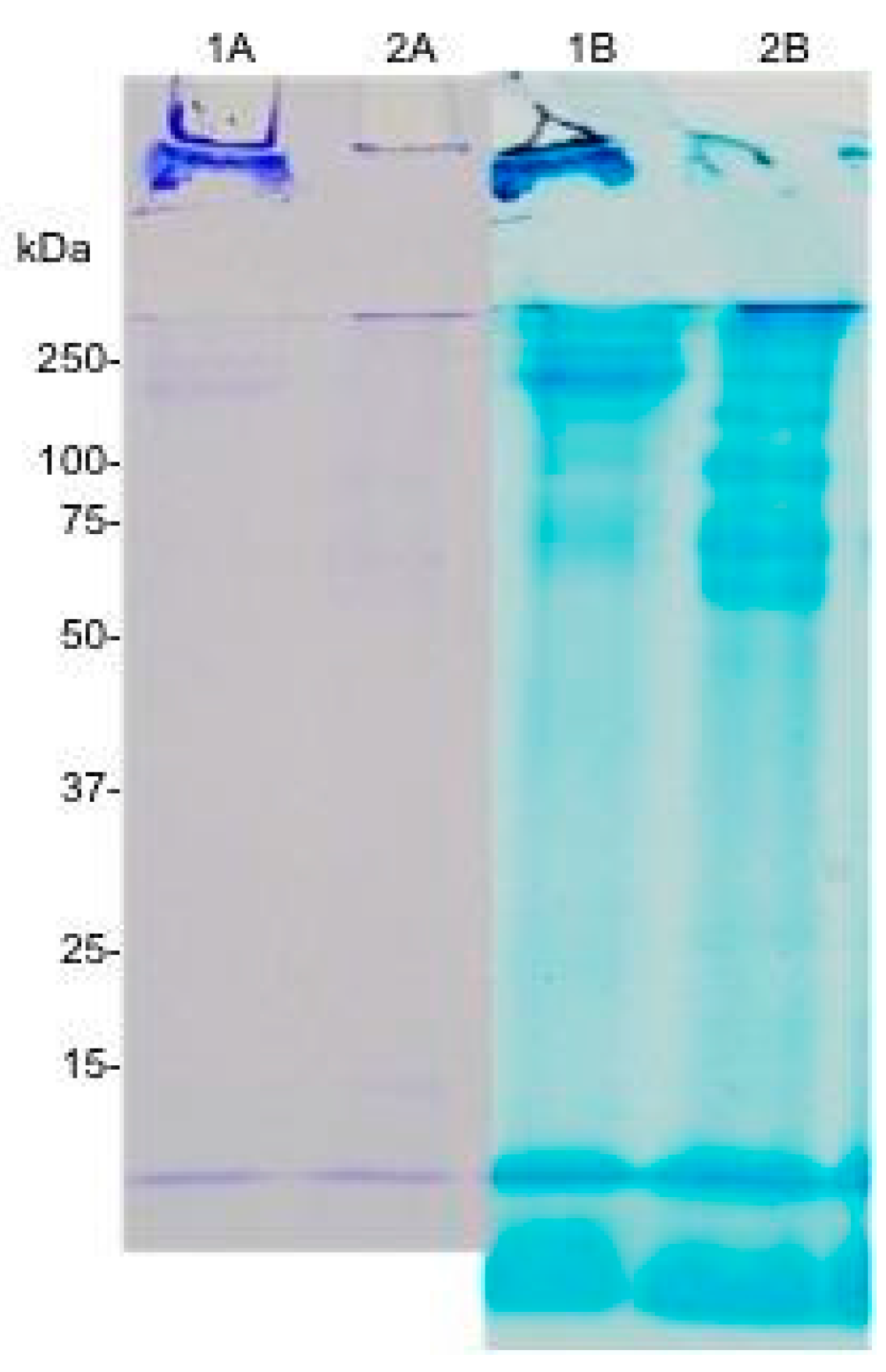
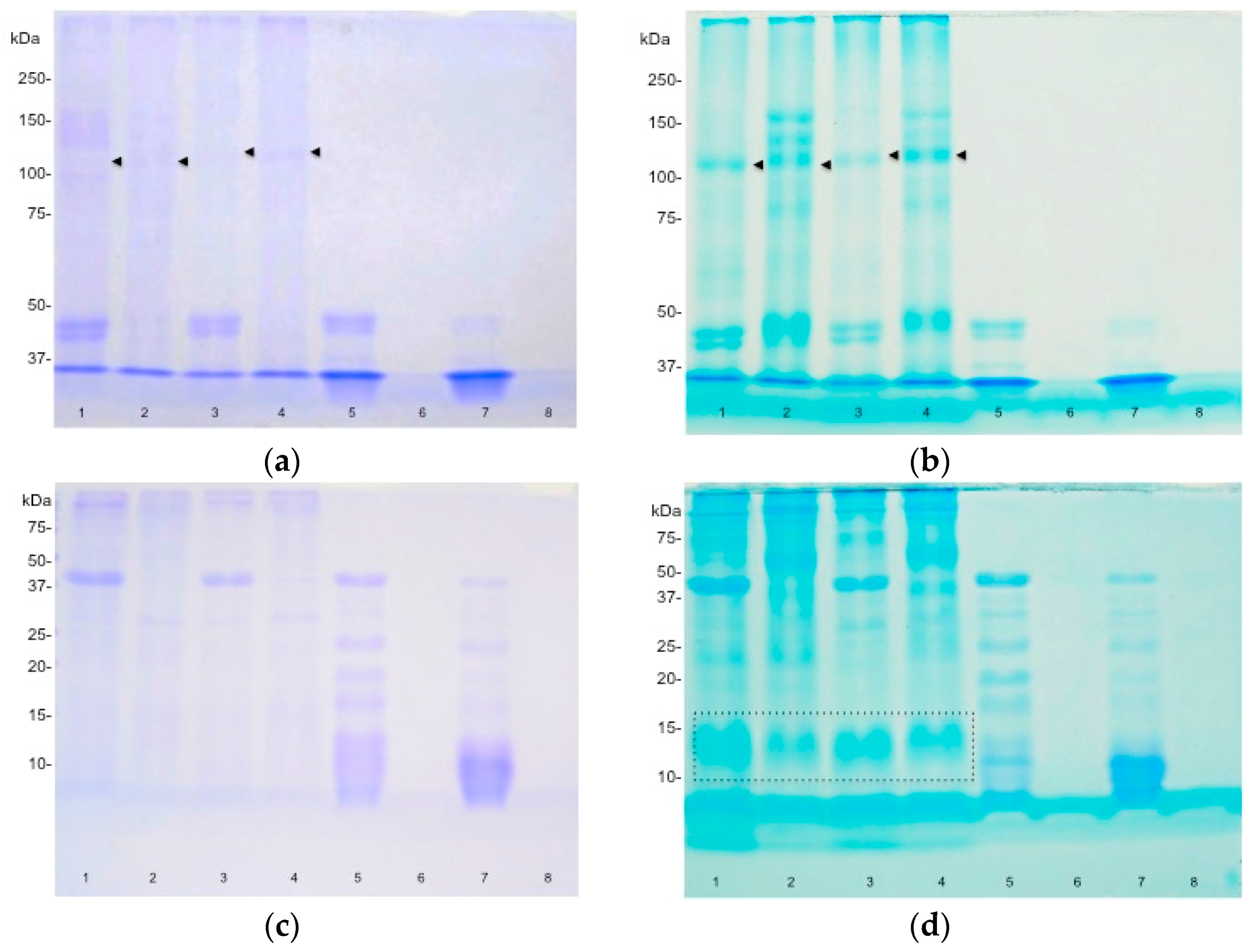
2.2. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.3. Effect of Pepsin Digestion
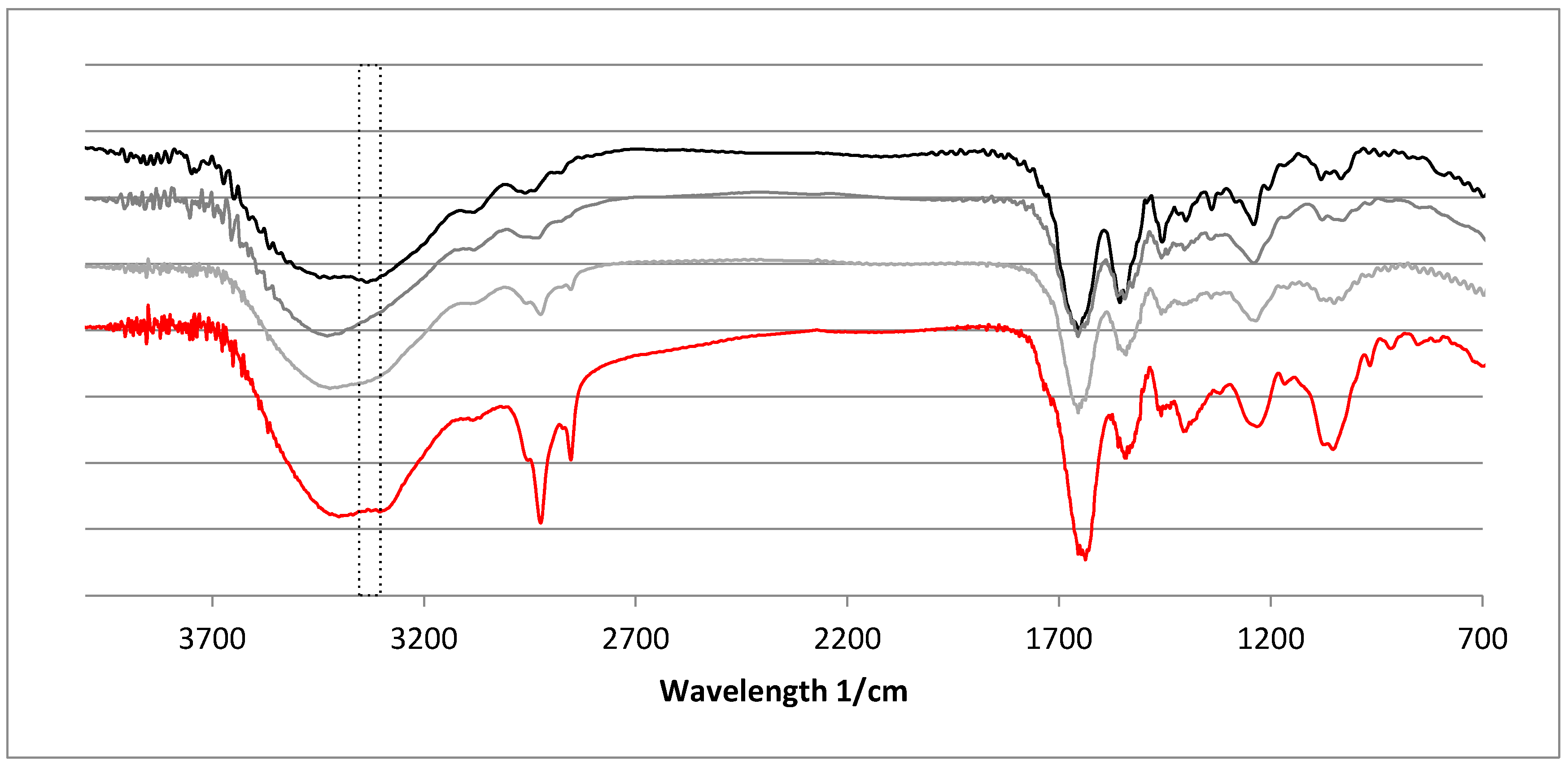
2.4. Fourier-Transformed Infrared Spectroscopy (FTIR)
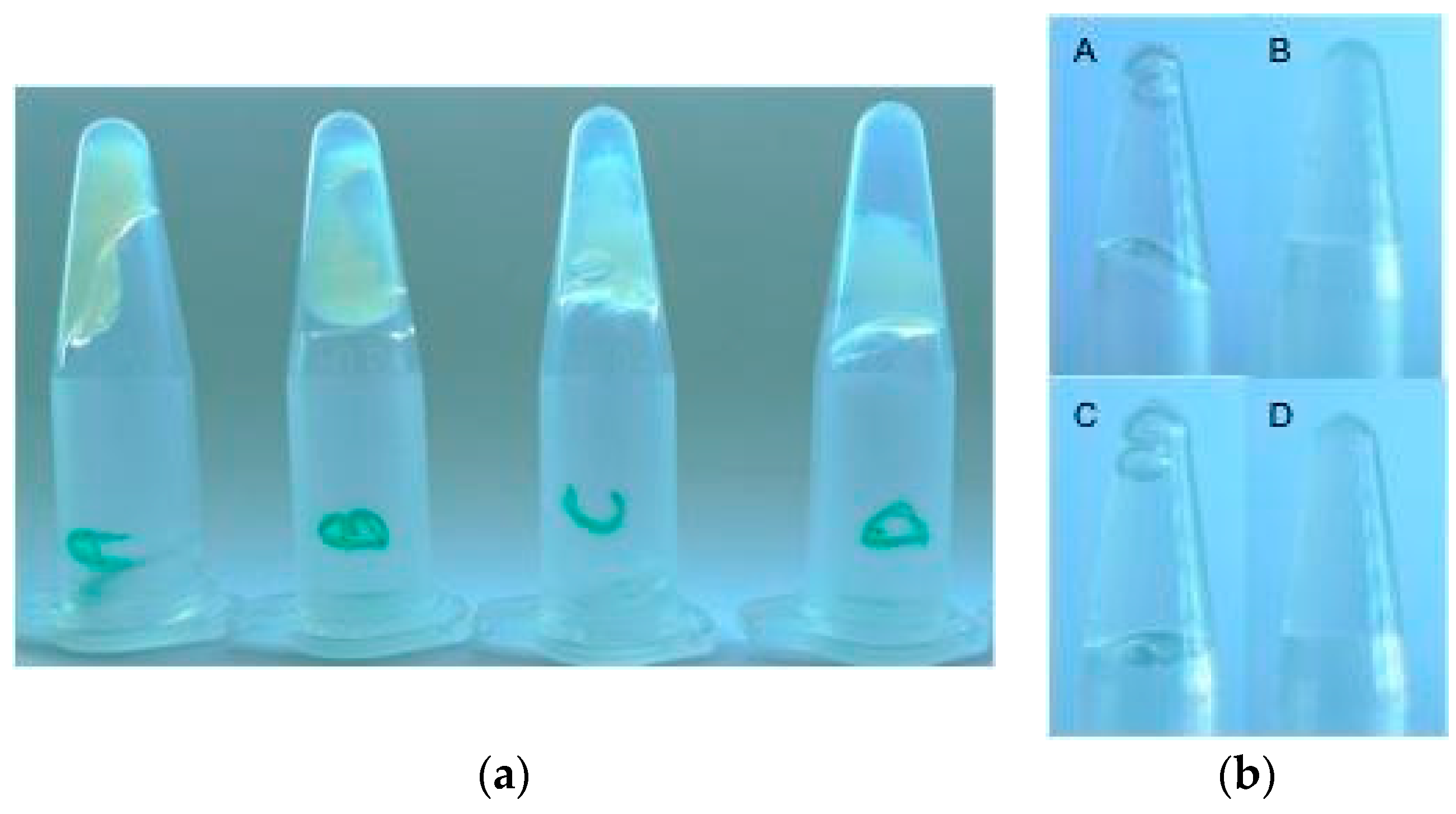
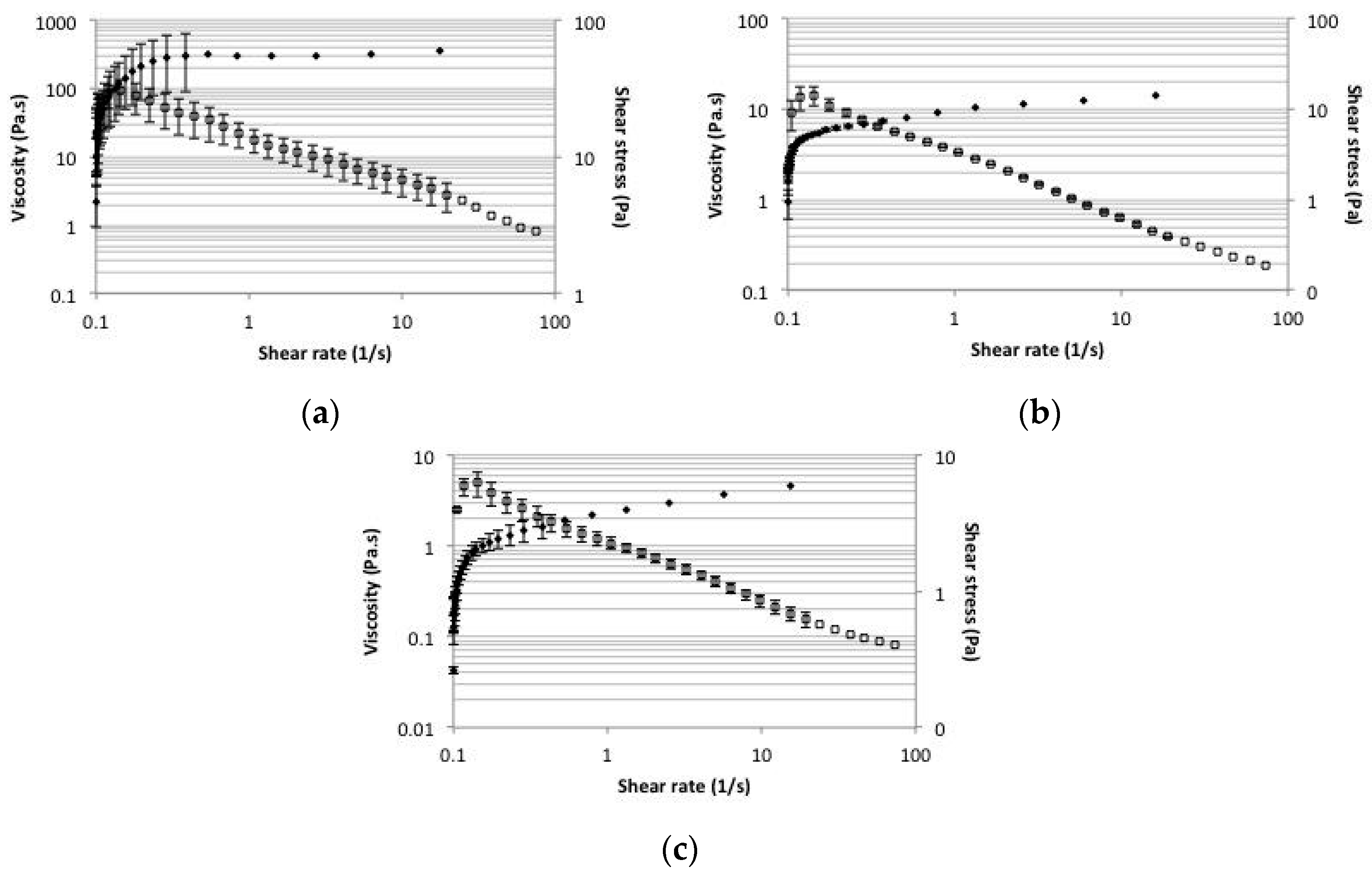
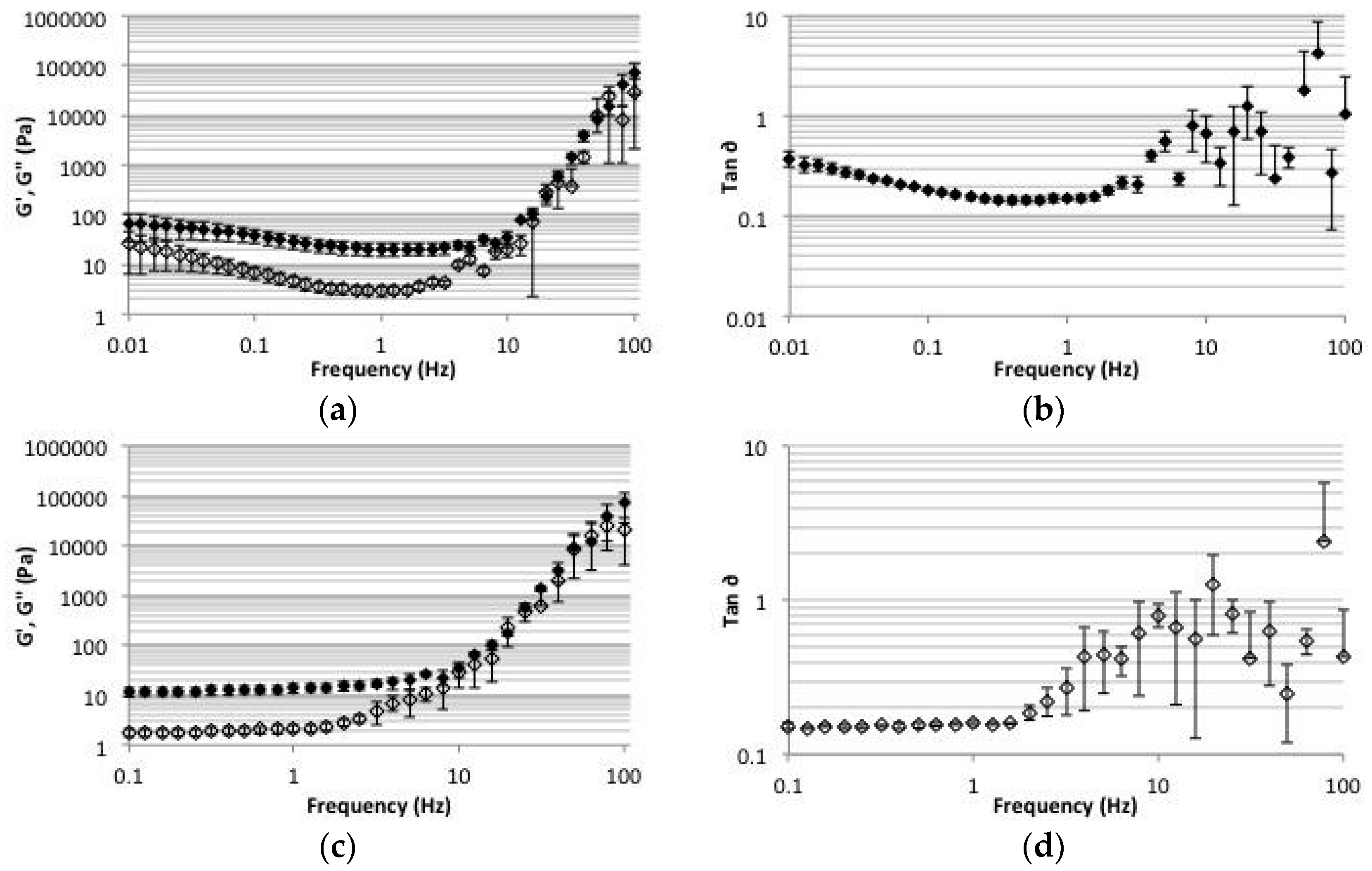
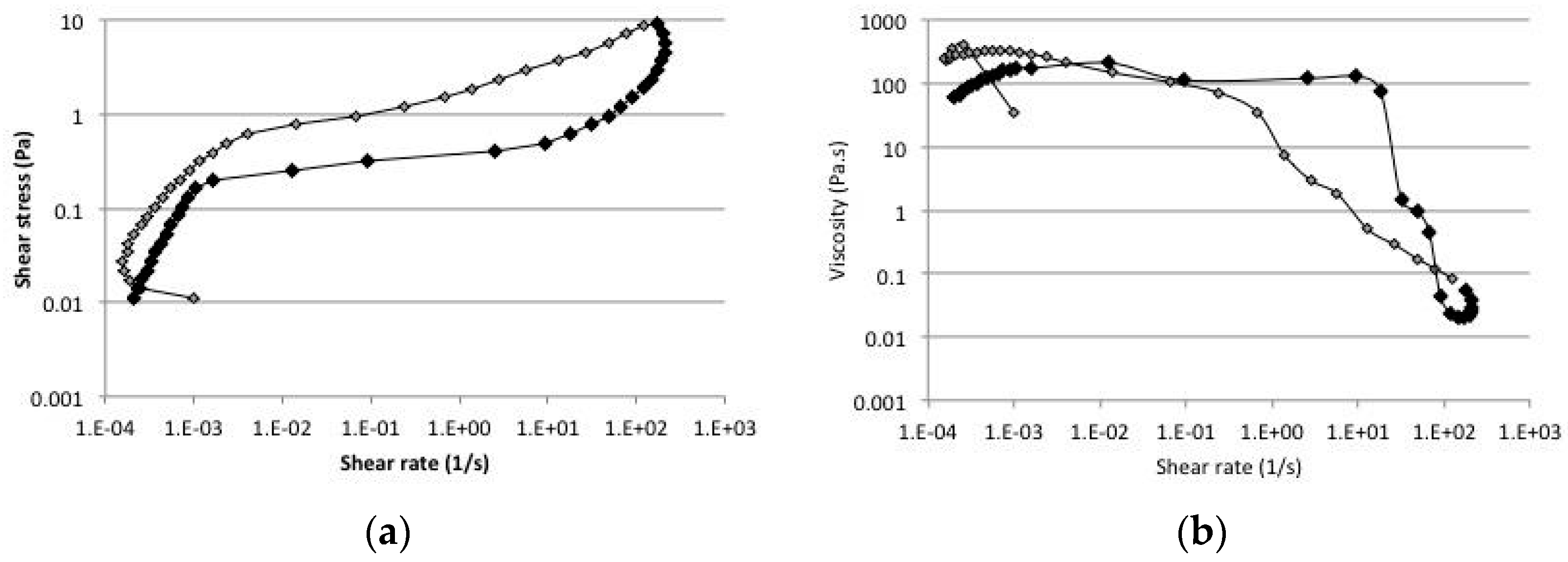
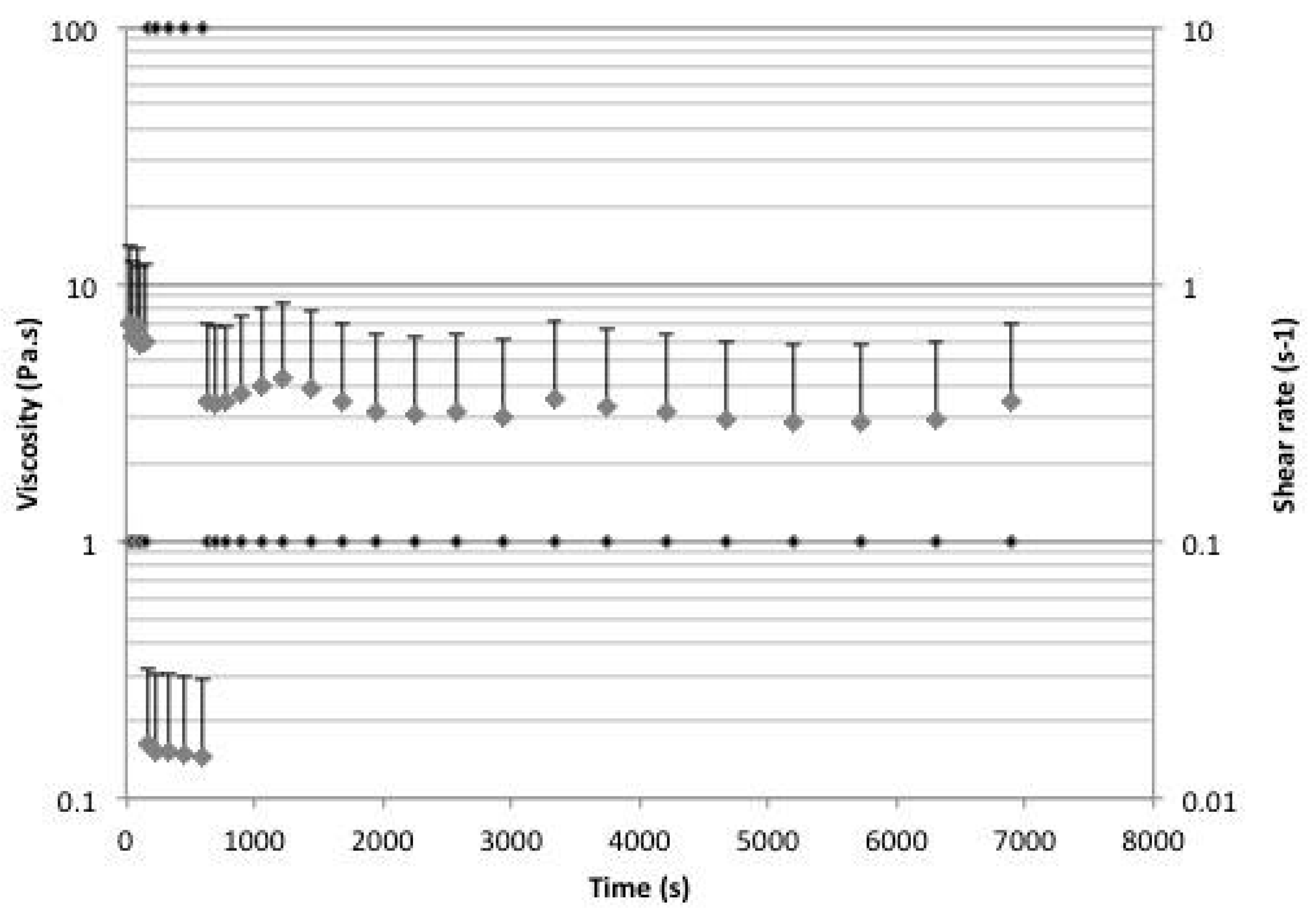
2.5. Rheology
3. Discussion
4. Materials and Methods
4.1. Sponge Sampling
4.2. Collagen Extraction
4.3. Collagen and Protein Quantification
4.4. Enzymatic Digestions of Collagen and GAGs Extraction
4.5. Electrophoresis
4.5.1. SDS-PAGE
4.5.2. Tris/Borate/EDTA Polyacrylamide Gel Electrophoresis (TBE-PAGE)
4.6. FTIR
4.7. Rheology
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Exposito, J.; Valcourt, U.; Cluzel, C.; Lethias, C. The Fibrillar Collagen Family. Int. J. Mol. Sci. 2010, 11, 407–426. [Google Scholar] [CrossRef] [PubMed]
- Syed, T.; Schierwater, B. The Evolution of the Placozoa: A New Morphological Model. Senckenberg. Lethaea 2002, 82, 315–324. [Google Scholar] [CrossRef]
- Clément, P. The Phylogeny of Rotifers: Molecular, Ultrastructural and Behavioural Data. Hydrobiologia 2003, 255, 527–544. [Google Scholar] [CrossRef]
- Junqueira, L.C.; Montes, G.S. Biology of collagen-proteoglycan interaction. Arch. Histol. Jpn. 1983, 46, 589–629. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.J.; Pels, E.; Schurmans, L.R.; Vrensen, G.F. A new three-dimensional model of the organization of proteoglycans and collagen fibrils in the human corneal stroma. Exp. Eye Res. 2004, 78, 493–501. [Google Scholar] [CrossRef]
- Schuppan, D.; Schmid, M.; Somasundaram, R.; Ackermann, R.; Ruehl, M.; Nakamura, T.; Riecken, E.O. Collagens in the liver extracellular matrix bind hepatocyte growth factor. Gastroenterology 1988, 114, 139–152. [Google Scholar] [CrossRef]
- Czirok, A.; Rongish, B.; Little, C. Extracellular matrix dynamics during vertebrate axis formation. Dev. Biol. 2004, 268, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Di Lullo, G.; Sweeney, S.; Korkko, J.; Ala-Kokko, L.; San Antonio, J. Mapping the Ligand-binding Sites and Disease-associated Mutations on the Most Abundant Protein in the Human, Type I Collagen. J. Biol. Chem. 2001, 277, 4223–4231. [Google Scholar] [CrossRef] [PubMed]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- An, B.; Lin, Y.; Brodsky, B. Collagen interactions: Drug design and delivery. Adv. Drug Deliv. Rev. 2016, 97, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Swatschek, D.; Schatton, W.; Kellermann, J.; Muller, W.; Kreuter, J. Marine sponge collagen: Isolation, characterization and effects on the skin parameters surface-pH, moisture and sebum. Eur. J. Pharm. Biopharm. 2002, 53, 107–113. [Google Scholar] [CrossRef]
- Kim, S.; Mendis, E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Xinrong, P.; Ruiyue, Y.; Haifeng, Z.; Qiong, L.; Zhigang, L.; Junbo, W.; Yong, L. Preventive effect of marine collagen peptide on learning and memory impairment in SAMP8 Mice. Food Ferment. Ind. 2009, 35, 1–5. [Google Scholar]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 71, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Bentkover, S.H. The biology of facial fillers. Facial Plast. Surg. 2009, 25, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Han, X.L.; Li, Y. Effect of marine collagen peptides on long bone development in growing rats. J. Sci. Food Agric. 2010, 90, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Moreira-Silva, J.; Marques, A.; Domingues, A.; Bayon, Y.; Reis, R. Marine Origin Collagens and Its Potential Applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weadock, K.; Miller, E.; Bellincampi, L.; Zawadsky, J.; Dunn, M. Physical crosslinking of collagen fibers: Comparison of ultraviolet irradiation and dehydrothermal treatment. J. Biomed. Mater. Res. 1995, 29, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Dewavrin, J.; Hamzavi, N.; Shim, V.; Raghunath, M. Tuning the architecture of three-dimensional collagen hydrogels by physiological macromolecular crowding. Acta Biomater. 2014, 10, 4351–4359. [Google Scholar] [CrossRef] [PubMed]
- Berthod, F.; Hayek, D.; Damour, O.; Collombel, C. Collagen synthesis by fibroblasts cultured within a collagen sponge. Biomaterials 1993, 14, 749–754. [Google Scholar] [CrossRef]
- Berthod, F.; Saintigny, G.; Chretien, F.; Hayek, D.; Collombel, C.; Damour, O. Optimization of thickness, pore size and mechanical properties of a biomaterial designed for deep burn coverage. Clin. Mater. 1994, 15, 259–265. [Google Scholar] [CrossRef]
- Osborne, C.; Barbenel, J.; Smith, D.; Savakis, M.; Grant, M. Investigation into the tensile properties of collagen/chondroitin-6-sulphate gels: The effect of crosslinking agents and diamines. Med. Biol. Eng. Comput. 1998, 36, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Gough, J.; Scotchford, C.; Downes, S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly(vinyl alcohol) films is by the mechanism of apoptosis. J. Biomed. Mater. Res. 2002, 61, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Zeugolis, D.; Paul, G.; Attenburrow, G. Cross-linking of extruded collagen fibers—A biomimetic three-dimensional scaffold for tissue engineering applications. J. Biomed. Mater. Res. Part A 2009, 89, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Sundararaghavan, H.; Monteiro, G.; Lapin, N.; Chabal, Y.; Miksan, J.; Shreiber, D. Genipin-induced changes in collagen gels: Correlation of mechanical properties to fluorescence. J. Biomed. Mater. Res. Part A 2008, 87, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Chandran, P.; Paik, D.; Holmes, J. Structural Mechanism for Alteration of Collagen Gel Mechanics by Glutaraldehyde Crosslinking. Connect. Tissue Res. 2012, 53, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Walters, B.; Stegemann, J. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta Biomater. 2014, 10, 1488–1501. [Google Scholar] [CrossRef] [PubMed]
- Orban, J.; Wilson, L.; Kofroth, J.; El-Kurdi, M.; Maul, T.; Vorp, D. Crosslinking of collagen gels by transglutaminase. J. Biomed. Mater. Res. Part A 2004, 68, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Wollensak, G.; Spoerl, E.; Seiler, T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophthalmol. 2003, 135, 620–627. [Google Scholar] [CrossRef]
- Fernandes-Silva, S.; Moreira-Silva, J.; Silva, T.; Perez-Martin, R.; Sotelo, C.; Mano, J.; Duarte, A.; Reis, R. Porous Hydrogels From Shark Skin Collagen Crosslinked under Dense Carbon Dioxide Atmosphere. Macromol. Biosci. 2013, 13, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Lazoski, C.; Solé-Cava, A.M.; Boury-Esnault, N.; Klautau, M.; Russo, C.A.M. Cryptic speciation in a high flow scenario in the oviparous marine sponge Chondrosia reniformis. Mar. Biol. 2001, 139, 421–429. [Google Scholar]
- Uriz, M.; Turon, X.; Becerro, M.; Agell, G. Siliceous spicules and skeleton frameworks in sponges: Origin, diversity, ultrastructural patterns, and biological functions. Microsc. Res. Tech. 2003, 62, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Maldonado, M.; Spindler, K.; Eckert, C.; Hanke, T.; Born, R.; Goebel, C.; Simon, P.; Heinemann, S.; Worch, H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (demospongia: Porifera). J. Exp. Zool. B Mol. Dev. Evol. 2007, 308, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Aouacheria, A.; Geourjon, C.; Aghajari, N.; Navratil, V.; Deleage, G.; Lethias, C.; Exposito, J. Insights into Early Extracellular Matrix Evolution: Spongin Short Chain Collagen-Related Proteins Are Homologous to Basement Membrane Type IV Collagens and Form a Novel Family Widely Distributed in Invertebrates. Mol. Biol. Evol. 2006, 23, 2288–2302. [Google Scholar] [CrossRef] [PubMed]
- Bonasoro, F.; Wilkie, I.C.; Bavestrello, G.; Cerrano, C.; Candia Carnevali, M.D. Dynamic structure of the mesohyl in the sponge Chondrosia reniformis (Porifera, Demospongiae). Zoomorphology 2001, 121, 109–121. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Parma, L.; Bonasoro, F.; Bavestrello, G.; Cerrano, C.; Candia Carnevali, M.D. Mechanical adaptability of a sponge extracellular matrix: Evidence for cellular control of mesohyl stiffness in Chondrosia reniformis Nardo. J. Exp. Biol. 2006, 209, 4436–4443. [Google Scholar] [CrossRef] [PubMed]
- Fassini, D.; Parma, L.; Lembo, F.; Candia Carnevali, M.; Wilkie, I.; Bonasoro, F. The reaction of the sponge Chondrosia reniformis to mechanical stimulation is mediated by the outer epithelium and the release of stiffening factor(s). Zoology 2014, 117, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Ehrlich, H.; Douglas, T.; Heinemann, C.; Worch, H.; Schatton, W.; Hanke, T. Ultrastructural Studies on the Collagen of the Marine Sponge Chondrosia reniformis Nardo. Biomacromolecules 2007, 8, 3452–3457. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Ehrlich, H.; Knieb, C.; Hanke, T. Biomimetically inspired hybrid materials based on silicified collagen. Int. J. Mater. Res. 2007, 98, 603–608. [Google Scholar] [CrossRef]
- Barros, A.; Aroso, I.; Silva, T.; Mano, J.; Duarte, A.; Reis, R. Water and Carbon Dioxide: Green Solvents for the Extraction of Collagen/Gelatin from Marine Sponges. ACS Sustain. Chem. Eng. 2015, 3, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.; Barros, A.; Aroso, I.; Fassini, D.; Silva, T.; Reis, R.; Duarte, A. Extraction of Collagen/Gelatin from the Marine Demosponge Chondrosia reniformis (Nardo, 1847) Using Water Acidified with Carbon Dioxide—Process Optimization. Ind. Eng. Chem. Res. 2016, 55, 6922–6930. [Google Scholar] [CrossRef]
- Garrone, R.; Huc, A.; Junqua, S. Fine structure and physicochemical studies on the collagen of the marine sponge Chondrosia reniformis Nardo. J. Ultrastruct. Res. 1975, 52, 261–275. [Google Scholar] [CrossRef]
- Matsumura, T.; Shinmei, M.; Nagai, Y. Disaggregation of connective tissue: Preparation of fibrous components from sea cucumber body wall and calf skin. J. Biochem. 1973, 73, 155–162. [Google Scholar] [PubMed]
- Pozzolini, M.; Scarfì, S.; Mussino, F.; Ferrando, S.; Gallus, L.; Giovine, M. Molecular Cloning, Characterization, and Expression Analysis of a Prolyl 4-Hydroxylase from the Marine Sponge Chondrosia reniformis. Mar. Biotechnol. 2015, 17, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Bavestrello, G.; Cerrano, C.; Cattaneo-Vietti, R.; Sarà, M.; Calabria, F.; Cortesogno, L. Selective incorporation of foreign material in Chondrosia reniformis (Porifera, Demospongiae). Ital. J. Zool. 1996, 63, 215–220. [Google Scholar] [CrossRef]
- Bavestrello, G.; Benatti, U.; Calcinai, B.; Cattaneo-Vietti, R.; Cerrano, C.; Favre, A.; Giovine, M.; Lanza, S.; Pronzato, R.; Sarà, M. Body polarity and mineral selectivity in the demosponge Chondrosia reniformis. Biol. Bull. 1998, 195, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Muyonga, J.; Cole, C.; Duodu, K. Characterisation of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 85, 81–89. [Google Scholar] [CrossRef]
- Tronci, G.; Doyle, A.; Russell, S.; Wood, D. Triple-helical collagen hydrogels via covalent aromatic functionalisation with 1,3-phenylenediacetic acid. J. Mater. Chem. B 2013, 1, 5478–5488. [Google Scholar] [CrossRef] [PubMed]
- Silva-Júnior, Z.; Botta, S.; Ana, P.; França, C.; Fernandes, K.; Mesquita-Ferrari, R.; Deana, A.; Bussadori, S. Effect of papain-based gel on type I collagen—Spectroscopy applied for microstructural analysis. Sci. Rep. 2015, 5, 11448. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Li, Y.; Li, G. Effect of concentration and temperature on the rheological behavior of collagen solution. Int. J. Biol. Macromol. 2008, 42, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhang, M.; Li, G. Rheological properties of collagen/hydroxypropyl methylcellulose (COL/HPMC) blended solutions. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Gelse, K. Collagens-structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen Tissue Engineering: Development of Novel Biomaterials and Applications. Pediatr. Res. 2008, 63, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Fassini, D.; Parma, L.; Wilkie, I.C.; Bavestrello, G.; Bonasoro, F.; Carnevali, M.D.C. Ecophysiology of mesohyl creep in the demosponge Chondrosia reniformis (Porifera: Chondrosida). J. Exp. Mar. Biol. Ecol. 2012, 428, 24–31. [Google Scholar] [CrossRef]
- Imhoff, J.M.; Garrone, R. Solubilization and Characterization of Chondrosia reniformis Sponge Collagen. Connect. Tissue Res. 1983, 11, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Pallela, R.; Boja, S.; Janapala, V. Biochemical and biophysical characterization of collagens of marine sponge, Ircinia fusca (Porifera: Demospongiae: Irciniidae). Int. J. Biol. Macromol. 2011, 49, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Riesgo, A.; Farrar, N.; Windsor, P.; Giribet, G.; Leys, S. The Analysis of Eight Transcriptomes from All Poriferan Classes Reveals Surprising Genetic Complexity in Sponges. Mol. Biol. Evol. 2014, 31, 1102–1120. [Google Scholar] [CrossRef] [PubMed]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of Collagen I Hydrogels for Bioengineered Tissue Microenvironments: Characterization of Mechanics, Structure, and Transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Ghobril, C.; Grinstaff, M.W. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: A tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.; Hilt, J.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, E.R. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Nandini, C.; Itoh, N.; Sugahara, K. Novel 70-kDa Chondroitin Sulfate/Dermatan Sulfate Hybrid Chains with a Unique Heterogenous Sulfation Pattern from Shark Skin, Which Exhibit Neuritogenic Activity and Binding Activities for Growth Factors and Neurotrophic Factors. J. Biol. Chem. 2004, 280, 4058–4069. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Novoa-Carballal, R.; Reis, R.; Pashkuleva, I. Following the enzymatic digestion of chondroitin sulfate by a simple GPC analysis. Anal. Chim. Acta 2015, 885, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]

| Batch | Dry Weight (mg/mL) | Sircol (µg/mL) | BCA (µg/mL) |
|---|---|---|---|
| 1 | 6.92 | 17.02 | 133.04 |
| 2 | 10.6 | 7.94 | 87.91 |
| 3 | 9.6 | 6.59 | 66.12 |
| Peak Wave Number/cm−1 | Assignment | Reference | ||
|---|---|---|---|---|
| Sigma Type I | Ch | Ec | ||
| 3414 | 3414 | 3421 | NH S, coupled with H-bond | [47] |
| 3325 | 3331 | as NH | [38] | |
| 3080 | 3082 | 3082 | NH S | [48] |
| 2951 | 2956 | 2962 | CHx | [38] |
| 2926 | 2924 | 2935 | as CH2 | [47] |
| 2852 | 2848 | s CH2 | [47] | |
| 1666 | 1654 | 1660 | C=O | [38] |
| 1651 | 1647 | 1654 | C=O S/HB coupled with COO- | [47] |
| 1556 | 1560 | 1556 | NH B coupled with CN S | [38] |
| 1545 | 1540 | 1544 | ||
| 1450 | 1448 | 1450 | Pyrrolidine ring | [49] |
| 1458 | 1458 | 1458 | CH2 B, CH2 S | [38] |
| 1400 | 1396 | 1404 | ||
| 1339 | 1340 | 1340 | CN S | [38] |
| 1235 | 1240 | 1240 | CH2 | [49] |
| 1081 | 1082 | 1078 | CO S | [38] |
| 1033 | 1031 | 1033 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fassini, D.; Duarte, A.R.C.; Reis, R.L.; Silva, T.H. Bioinspiring Chondrosia reniformis (Nardo, 1847) Collagen-Based Hydrogel: A New Extraction Method to Obtain a Sticky and Self-Healing Collagenous Material. Mar. Drugs 2017, 15, 380. https://doi.org/10.3390/md15120380
Fassini D, Duarte ARC, Reis RL, Silva TH. Bioinspiring Chondrosia reniformis (Nardo, 1847) Collagen-Based Hydrogel: A New Extraction Method to Obtain a Sticky and Self-Healing Collagenous Material. Marine Drugs. 2017; 15(12):380. https://doi.org/10.3390/md15120380
Chicago/Turabian StyleFassini, Dario, Ana Rita C. Duarte, Rui L. Reis, and Tiago H. Silva. 2017. "Bioinspiring Chondrosia reniformis (Nardo, 1847) Collagen-Based Hydrogel: A New Extraction Method to Obtain a Sticky and Self-Healing Collagenous Material" Marine Drugs 15, no. 12: 380. https://doi.org/10.3390/md15120380





