Chitin Oligosaccharide (COS) Reduces Antibiotics Dose and Prevents Antibiotics-Caused Side Effects in Adolescent Idiopathic Scoliosis (AIS) Patients with Spinal Fusion Surgery
Abstract
:1. Introduction
2. Results
2.1. The Bacteria Isolated from AIS Patients
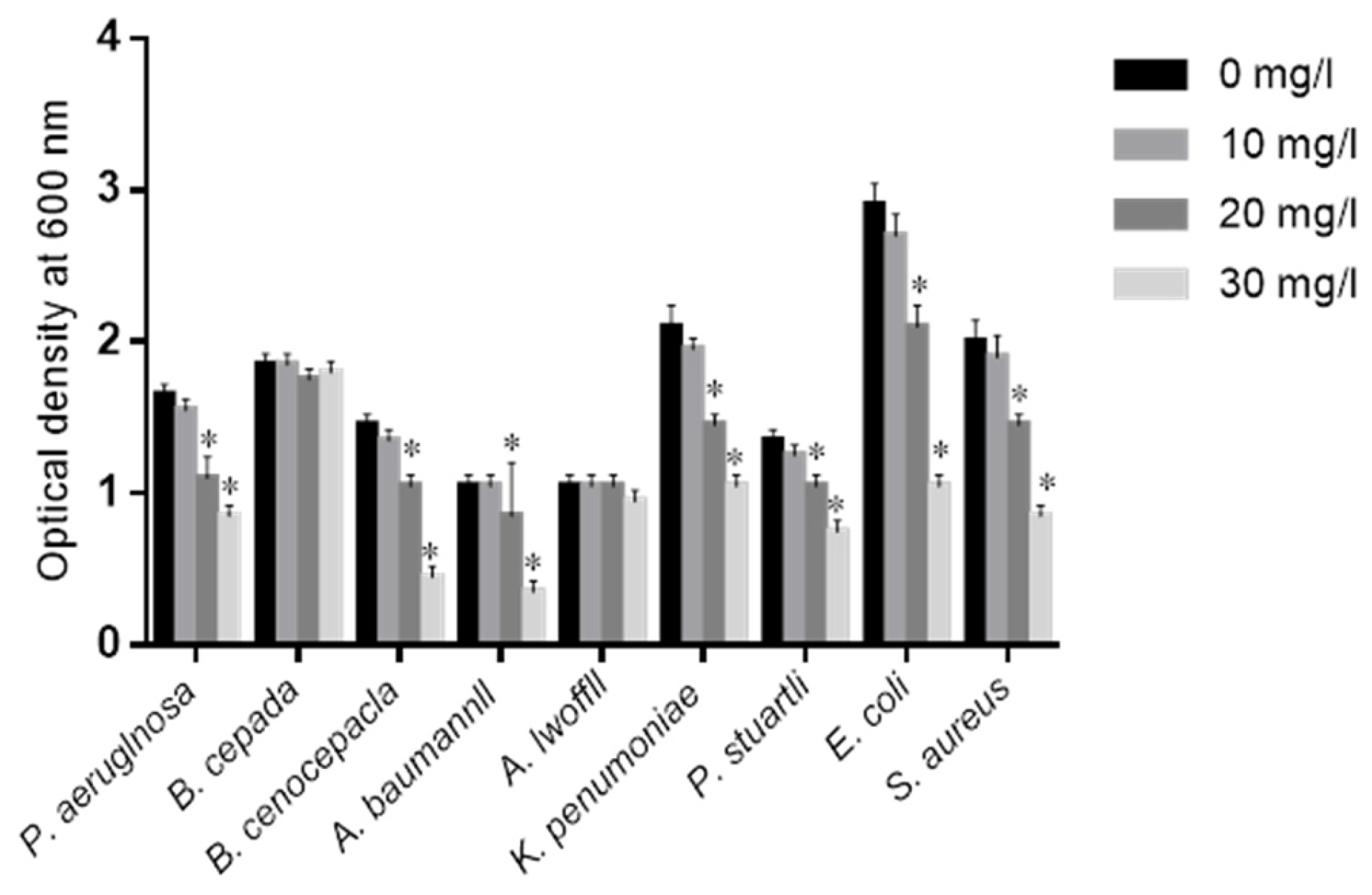
2.2. Effects of COS on Isolated Bacteria
2.3. COS Reduces the Antibiotics Resistance of MDR Bacteria
2.4. The Measurement of Resistance to COS
2.5. Baseline Characters
2.6. COS Were Potential Adjuvants of Antibiotics for Preventing Surgical Infection
2.7. COS Prevented the Side Effects Caused by Antibiotics
2.8. COS Improves the Biochemical Parameters of AIS Patients
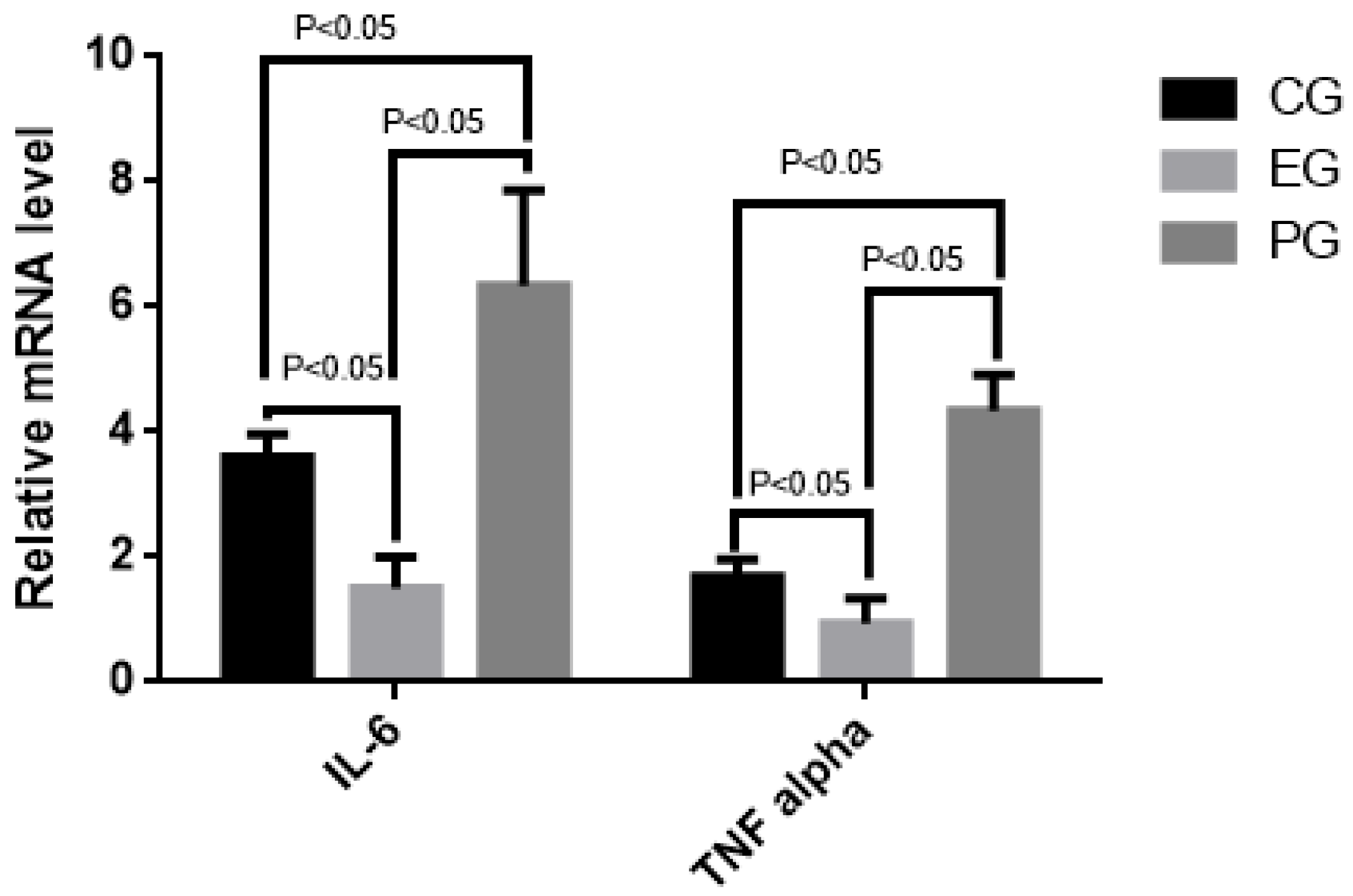
2.9. COS Reduced Relative mRNA Levels of Inflammatory Cytokines (IL-6 and TNF Alpha)
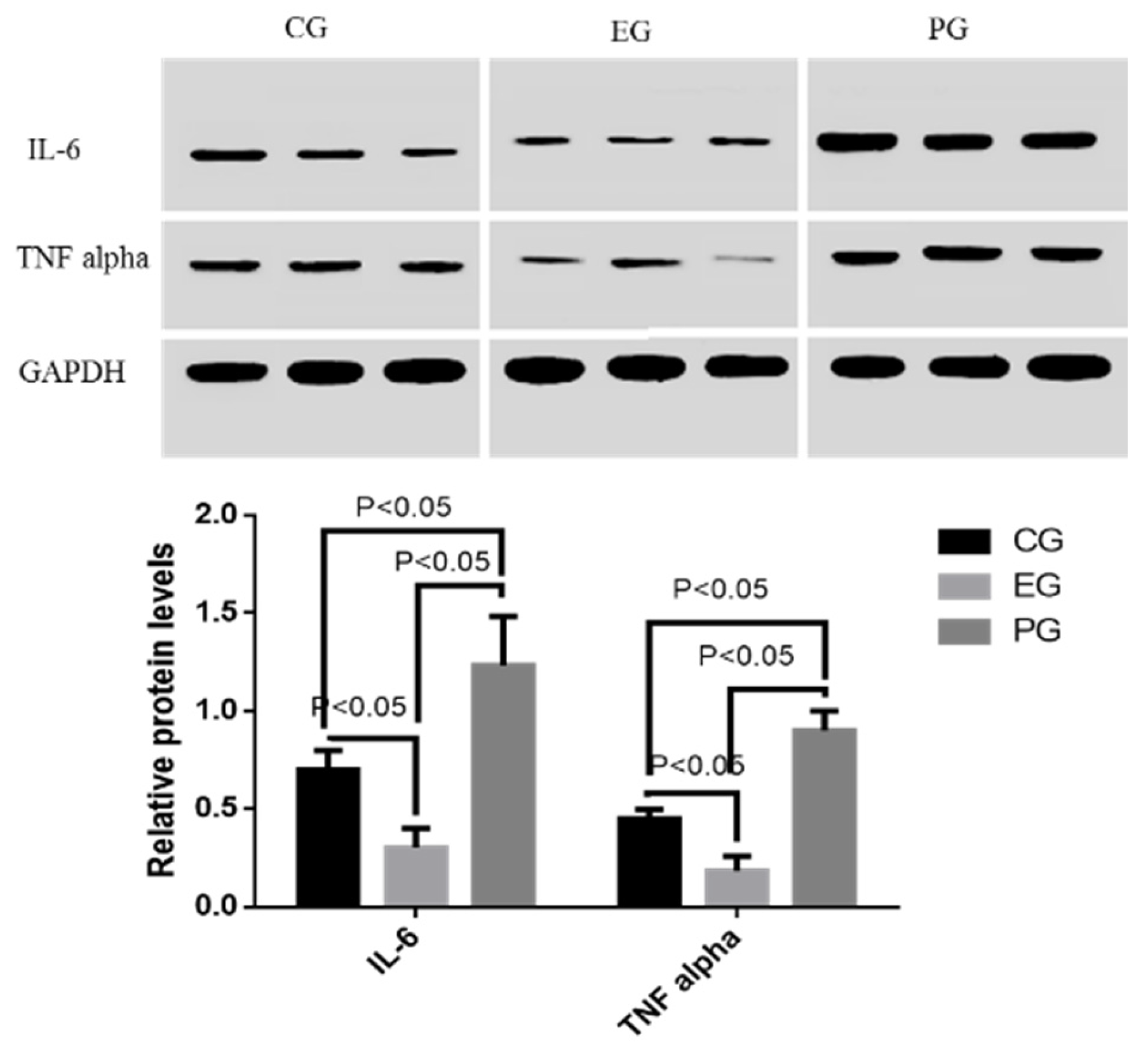
2.10. COS Reduced Relative Protein Levels of Inflammatory Cytokines (IL-6 and TNF Alpha)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Participants
4.3. Inclusion Criteria
4.4. Exclusion Criteria
4.5. Patients Grouping
4.6. Baseline Measurement
4.7. Measurement of Surgical Infection
4.8. Measurement of Anti-Bacteria Activities of COS
4.9. Measurement for the Resistance to COS after Long-Term Culture
4.10. Biochemical Analysis
4.11. qRT-PCR
4.12. Western Blot Analysis
4.13. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kasatpibal, N.; Whitney, J.D.; Dellinger, E.P.; Nair, B.G.; Pike, K.C. Failure to Redose Antibiotic Prophylaxis in Long Surgery Increases Risk of Surgical Site Infection. Surg. Infect. (Larchmt) 2016, 3, 1449. [Google Scholar]
- Salgado, M.; Fernandez, F.; Aviles, C.; Cordova, C. Erythromycin Seromadesis in Orthopedic Surgery. J. Orthop. Case Rep. 2016, 6, 92–94. [Google Scholar] [PubMed]
- Healey, K.R.; Zhao, Y.; Perez, W.B.; Lockhart, S.R.; Sobel, J.D.; Farmakiotis, D.; Kontoyiannis, D.P.; Sanglard, D.; Taj-Aldeen, S.J.; Alexander, B.D.; et al. Prevalent mutator genotype identified in fungal pathogen Candida glabrata promotes multi-drug resistance. Nat. Commun. 2016, 7, 11128. [Google Scholar] [CrossRef] [PubMed]
- Moyano Calvo, J.L.; Arellano Ganan, R.; Sempere Gutierrez, A.; Sanz Sacristan, J.; Teba del Pino, F.; Melon Rey, F.J.; Herrero Torres, L.; Pereira Sanz, I. Prophylaxis in prostatic surgery with Aztreonam. Our experience. Arch. Esp. Urol. 1992, 45, 519–521. [Google Scholar] [PubMed]
- Boffi, L.; Panebianco, R. A comparative study of 2 schedules of antibiotic prophylaxis using Ceftazidime in the prevention of infections in elective surgery of the biliary surgery. Preliminary results. Clin. Ter. 1992, 140, 265–271. [Google Scholar] [PubMed]
- Wang, Y.; Li, H.; Chen, B. Pathogen distribution and drug resistance of nephrology patients with urinary tract infections. Saudi Pharm. J. 2016, 24, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Martinez-Puchol, S.; Palma, N.; Horna, G.; Ruiz-Roldan, L.; Pons, M.J.; Ruiz, J. Macrolide resistance mechanisms in Enterobacteriaceae: Focus on Azithromycin. Crit. Rev. Microbiol. 2017, 43, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Yin, O.Q.; Tomlinson, B.; Chow, M.S. Effect of multidrug resistance gene-1 (ABCB1) polymorphisms on the single-dose pharmacokinetics of cloxacillin in healthy adult Chinese men. Clin. Ther. 2009, 31, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Nwobu, R.A.; Dosunmu-Ogunbi, O.; Rotimi, V.O. Phage-types and resistance pattern of Staphylococcus aureus, isolated from clinical specimens, to penicillin and cloxacillin in a Lagos hospital. Cent. Afr. J. Med. 1986, 32, 155–158. [Google Scholar] [PubMed]
- Marshall, S.; Hujer, A.M.; Rojas, L.J.; Papp-Wallace, K.M.; Humphries, R.M.; Spellberg, B.; Hujer, K.M.; Marshall, E.K.; Rudin, S.D.; Perez, F.; et al. Can Ceftazidime/avibactam and Aztreonam overcome beta-lactam resistance conferred by metallo-beta-lactamases in Enterobacteriaceae? Antimicrob. Agents Chemother. 2017. [Google Scholar] [CrossRef] [PubMed]
- Braz, V.S.; Furlan, J.P.; Fernandes, A.F.; Stehling, E.G. Mutations in NalC induce MexAB-OprM overexpression resulting in high level of Aztreonam resistance in environmental isolates of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.E.; Slayden, R.A. Transient In Vivo Resistance Mechanisms of Burkholderia pseudomallei to Ceftazidime and Molecular Markers for Monitoring Treatment Response. PLoS Negl. Trop. Dis. 2017, 11, e0005209. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Chen, L.; Cheng, S.; Chavda, K.D.; Press, E.G.; Snyder, A.; Pandey, R.; Doi, Y.; Kreiswirth, B.N.; Nguyen, M.H.; et al. Emergence of Ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob. Agents Chemother. 2017, 61, e02097-16. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S. Clinical toleration and safety of Azithromycin. Am. J. Med. 1991, 91, 40S–45S. [Google Scholar] [CrossRef]
- Richa; Tandon, V.R.; Sharma, S.; Khajuria, V.; Mahajan, V.; Gillani, Z. Adverse drug reactions profile of antimicrobials: A 3-year experience, from a tertiary care teaching hospital of India. Indian J. Med. Microbiol. 2015, 33, 393–400. [Google Scholar]
- El Nekidy, W.; Dziamarski, N.; Soong, D.; Donaldson, C.; Ibrahim, M.; Kadri, A. Cloxacillin-induced seizure in a hemodialysis patient. Hemodial. Int. 2015, 19, E33–E36. [Google Scholar] [CrossRef] [PubMed]
- Wanjiru, M.M. Isolation and Characterization of Bacteria Pathogens in Blood and Stool Samples among Patients Presenting with Typhoid Fever Symptoms in Alupe, Busia County; Kenyatta University: Nairobi County, Kenya, 2013. [Google Scholar]
- Pazmino, P. Acute renal failure, skin rash, and eosinophilia associated with Aztreonam. Am. J. Nephrol. 1988, 8, 68–70. [Google Scholar] [PubMed]
- Anastasio, G.D.; Robinson, M.D.; Little, J.M., Jr.; Leitch, B.B.; Pettice, Y.L.; Norton, H.J. A comparison of the gastrointestinal side effects of two forms of erythromycin. J. Fam. Pract. 1992, 35, 517–523. [Google Scholar] [PubMed]
- Lee, S.; Jang, J.; Jeon, H.; Lee, J.; Yoo, S.M.; Park, J.; Lee, M.S. Latent Kaposi’s sarcoma-associated herpesvirus infection in bladder cancer cells promotes drug resistance by reducing reactive oxygen species. J. Microbiol. 2016, 54, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Aklog, Y.F.; Egusa, M.; Kaminaka, H.; Izawa, H.; Morimoto, M.; Saimoto, H.; Ifuku, S. Protein/CaCO(3)/Chitin Nanofiber Complex Prepared from Crab Shells by Simple Mechanical Treatment and Its Effect on Plant Growth. Int. J. Mol. Sci. 2016, 17, 1600. [Google Scholar] [CrossRef] [PubMed]
- Sayari, N.; Sila, A.; Abdelmalek, B.E.; Abdallah, R.B.; Ellouz-Chaabouni, S.; Bougatef, A.; Balti, R. Chitin and chitosan from the Norway lobster by-products: Antimicrobial and anti-proliferative activities. Int. J. Biol. Macromol. 2016, 87, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, S.; Ktari, N.; Hajji, S.; Nasri, M.; Sellami Kamoun, A. Alkaline proteases from a newly isolated Micromonospora chaiyaphumensis S103: Characterization and application as a detergent additive and for chitin extraction from shrimp shell waste. Int. J. Biol. Macromol. 2017, 94, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Martinez, A.; Lopez-Moya, F.; Lopez-Llorca, L.V. Cell wall composition plays a key role on sensitivity of filamentous fungi to chitosan. J. Basic Microbiol. 2016, 56, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, J.; Fan, X.; Chen, W.; Zhang, W. MicroRNA and dsRNA targeting chitin synthase A reveal a great potential for pest management of a hemipteran insect Nilaparvata lugens. Pest. Manag. Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Peters, W. Occurrence of chitin in Mollusca. Comp. Biochem. Physiol. B Comp. Biochem. 1972, 41, 541–544. [Google Scholar] [CrossRef]
- Geetha, P.; Sivaram, A.J.; Jayakumar, R.; Gopi Mohan, C. Integration of in silico modeling, prediction by binding energy and experimental approach to study the amorphous chitin nanocarriers for cancer drug delivery. Carbohydr. Polym. 2016, 142, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Ohnuma, T.; Numata, T.; Osawa, T.; Inanaga, H.; Okazaki, Y.; Shinya, S.; Kondo, K.; Fukuda, T.; Fukamizo, T. Crystal structure and chitin oligosaccharide-binding mode of a ‘loopful’ family GH19 chitinase from rye, Secale cereale, seeds. FEBS J. 2012, 279, 3639–3651. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and anti-inflammatory properties of chitin and chitosan oligosaccharides. J. Funct. Biomater. 2015, 6, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Moon, M.E.; Park, H.S.; Im, S.Y.; Kim, Y.H. Chitosan oligosaccharide (COS) inhibits LPS-induced inflammatory effects in RAW 264.7 macrophage cells. Biochem. Biophys. Res. Commun. 2007, 358, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.D.; Sacha, P.T.; Ojdana, D.; Wieczorek, A.; Tryniszewska, E. Prevalence of resistance to aminoglycosides and fluoroquinolones among Pseudomonas aeruginosa strains in a University Hospital in Northeastern Poland. Braz. J. Microbiol. 2014, 45, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Zhang, L.; Weng, Y.; Chen, R.; Zhu, F.; Jin, Y.; Cheng, Z.; Jin, S.; Wu, W. PA3297 Counteracts Antimicrobial Effects of Azithromycin in Pseudomonas aeruginosa. Front. Microbiol. 2016, 7, 317. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Quintanilla, M.; Carretero-Ledesma, M.; Moreno-Martinez, P.; Martin-Pena, R.; Pachon, J.; McConnell, M.J. Lipopolysaccharide loss produces partial colistin dependence and collateral sensitivity to Azithromycin, rifampicin and vancomycin in Acinetobacter baumannii. Int. J. Antimicrob. Agents 2015, 46, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Chantratita, N.; Rholl, D.A.; Sim, B.; Wuthiekanun, V.; Limmathurotsakul, D.; Amornchai, P.; Thanwisai, A.; Chua, H.H.; Ooi, W.F.; Holden, M.T.; et al. Antimicrobial resistance to Ceftazidime involving loss of penicillin-binding protein 3 in Burkholderia pseudomallei. Proc. Natl. Acad. Sci. USA 2011, 108, 17165–17170. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Hu, Y.; Pan, Y.; Liang, H.; Wang, H.; Wang, X.; Hao, Q.; Yang, X.; Yang, X.; Xiao, X. Novel plasmid and its variant harboring both a blaNDM-1 gene and type IV secretion system in clinical isolates of Acinetobacter lwoffii. Antimicrob. Agents Chemother. 2012, 56, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.W.; Xu, X.Y.; Xu, J.; Yuan, J.Y.; Wu, W.K.; Zhang, N.; Chen, Z.L. Multi-drug resistant uropathogenic Escherichia coli and its treatment by Chinese medicine. Chin. J. Integr. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.; Siadat, S.D.; Shahcheraghi, F.; Vaziri, F.; Japoni-Nejad, A.; Vand Yousefi, J.; Rajaei, B.; Harifi Mood, E.; Ebrahim zadeh, N.; Moshiri, A.; et al. Variability in gene cassette patterns of class 1 and 2 integrons associated with multi drug resistance patterns in Staphylococcus aureus clinical isolates in Tehran-Iran. BMC Microbiol. 2015, 15, 152. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.P.; Wilson, K.T. Polyamine- and NADPH-dependent generation of ROS during Helicobacter pylori infection: A blessing in disguise. Free Radic. Biol. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Broxton, C.N.; Culotta, V.C. SOD Enzymes and Microbial Pathogens: Surviving the Oxidative Storm of Infection. PLoS Pathog. 2016, 12, e1005295. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Fu, X.; Huang, L.; Ma, Y.; Ding, X.; Zhu, L.; Zhu, G. The synergistic antiviral effects of GSH in combination with acyclovir against BoHV-1 infection in vitro. Acta Virol. 2016, 60, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Ikuabe, P.O.; Ebuenyi, I.D.; Harry, T.C. Limited Elevations in Antituberculosis Drug-Induced Serum Alanine Aminotransferase (Alt) Levels in a Cohort of Nigerians on Treatment for Pulmonary Tuberculosis and Hiv Infection in Yenagoa. Niger. J. Med. 2015, 24, 103–107. [Google Scholar] [PubMed]
- Costa, M.M.; Franca, R.T.; Da Silva, A.S.; Paim, C.B.; Paim, F.; do Amaral, C.H.; Dornelles, G.L.; da Cunha, J.P.; Soares, J.F.; Labruna, M.B.; et al. Rangelia vitalii: Changes in the enzymes ALT, CK and AST during the acute phase of experimental infection in dogs. Rev. Bras. Parasitol. Vet. 2012, 21, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Catar, R.; Witowski, J.; Zhu, N.; Lucht, C.; Derrac Soria, A.; Uceda Fernandez, J.; Chen, L.; Jones, S.A.; Fielding, C.A.; Rudolf, A.; et al. IL-6 Trans-Signaling Links Inflammation with Angiogenesis in the Peritoneal Membrane. J. Am. Soc. Nephrol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Lee, R.W.; Khaw, P.T.; Brocchini, S.; Dick, A.D.; Copland, D.A. An anti-TNF-alpha antibody mimetic to treat ocular inflammation. Sci. Rep. 2016, 6, 36905. [Google Scholar] [CrossRef] [PubMed]
- Izumi, R.; Azuma, K.; Izawa, H.; Morimoto, M.; Nagashima, M.; Osaki, T.; Tsuka, T.; Imagawa, T.; Ito, N.; Okamoto, Y.; et al. Chitin nanofibrils suppress skin inflammation in atopic dermatitis-like skin lesions in NC/Nga mice. Carbohydr. Polym. 2016, 146, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.H.; Mok, J.Y.; Park, K.H.; Hwang, H.M.; Song, M.S.; Lee, D.; Lee, M.H.; Lee, W.Y.; Chai, K.Y.; Jang, S.I. Inhibitory effect of dibutyryl chitin ester on nitric oxide and prostaglandin E(2) production in LPS-stimulated RAW 264.7 cells. Arch. Pharm. Res. 2012, 35, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Wagener, J.; Malireddi, R.S.; Lenardon, M.D.; Köberle, M.; Vautier, S.; MacCallum, D.M.; Biedermann, T.; Schaller, M.; Netea, M.G.; Kanneganti, T.-D. Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS Pathog. 2014, 10, e1004050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youxi, Z.; Huihui, J.; Zhiming, R.; Yizhi, J.; Yanling, C.; Yanhe, M. High level expression of Saccharomyces cerevisiae chitinase (ScCTS1) in Pichia pastoris for degrading chitin. Int. J. Agric. Biol. Eng. 2015, 8, 142–150. [Google Scholar]
- Pasquini, G.; Cecchi, F.; Bini, C.; Molino-Lova, R.; Vannetti, F.; Castagnoli, C.; Paperini, A.; Boni, R.; Macchi, C.; Crusco, B.; et al. The outcome of a modified version of the Cheneau brace in adolescent idiopathic scoliosis (AIS) based on SRS and SOSORT criteria: A retrospective study. Eur. J. Phys. Rehabil. Med. 2016, 52, 618–629. [Google Scholar] [PubMed]
- Khan, S.; Tøndervik, A.; Sletta, H.; Klinkenberg, G.; Emanuel, C.; Onsøyen, E.; Myrvold, R.; Howe, R.A.; Walsh, T.R.; Hill, K.E. Overcoming drug resistance with alginate oligosaccharides able to potentiate the action of selected antibiotics. Antimicrob. Agents Chemother. 2012, 56, 5134–5141. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.S.; Mikulca, J.A.; Cloutier, D.J.; Bliss, C.A.; Steenbergen, J.N. Outcomes of high-dose levofloxacin therapy remain bound to the levofloxacin minimum inhibitory concentration in complicated urinary tract infections. BMC Infect. Dis. 2016, 16, 710. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.S.; Berridge, M.V. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: A simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J. Immunol. Methods 2000, 238, 59–68. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5’-dithiobis(2-nitrobenzoic acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef]
| Antibiotics | COS | PA | BC | BC1 | AB | AL | KP | EC | SA | PS |
|---|---|---|---|---|---|---|---|---|---|---|
| Azithromycin | 0 | 7 | 30 | 3 | 17 | 0.2 | 500 | 8 | 0.1 | 8 |
| 10 | 3 | 16 | 2 | 4 | 0.1 | 250 | 4 | 0.1 | 2 | |
| 20 | 2 | 5 | 1 | 0.5 | 0.1 | 60 | 1 | 0.1 | 1 | |
| 30 | 0.5 | 0.1 | 0.5 | 0.02 | 0.1 | 32 | 0.5 | 0.1 | 0.1 | |
| Erythromycin | 0 | 250 | 60 | 68 | 9 | 0.2 | 250 | 30 | 0.1 | 20 |
| 10 | 126 | 48 | 38 | 4 | 0.1 | 120 | 18 | 0.1 | 16 | |
| 20 | 30 | 17 | 16 | 1 | 0.1 | 65 | 8 | 0.1 | 0.1 | |
| 30 | 6 | 9 | 8 | 0.3 | 0.1 | 20 | 4 | 0.1 | 0.1 | |
| Cloxacillin | 0 | 1000 | 120 | 39 | 2 | 0.1 | 2 | 4 | 0.1 | 0.1 |
| 10 | 1000 | 60 | 16 | 2 | 0.1 | 2 | 4 | 0.1 | 0.1 | |
| 20 | 1000 | 17 | 8 | 1 | 0.1 | 1 | 4 | 0.1 | 0.1 | |
| 30 | 1000 | 2 | 2 | 1 | 0.1 | 1 | 2 | 0.1 | 0.1 | |
| Aztreonam | 0 | 34 | 250 | 129 | 678 | 30 | 4000 | 1000 | 512 | 0.1 |
| 10 | 14 | 100 | 32 | 312 | 15 | 2000 | 510 | 234 | 0.1 | |
| 20 | 9 | 8 | 3 | 250 | 4 | 500 | 250 | 249 | 0.1 | |
| 30 | 3 | 2 | 1 | 126 | 1 | 250 | 64 | 244 | 0.1 | |
| Ceftazidime | 0 | 19 | 60 | 8 | 778 | 2 | 4230 | 4123 | 12 | 8 |
| 10 | 8 | 8 | 4 | 546 | 2 | 2078 | 3124 | 8 | 4 | |
| 20 | 1 | 2 | 1 | 312 | 0.5 | 1536 | 2341 | 2 | 2 | |
| 30 | 0.5 | 0.25 | 0.25 | 156 | 0.1 | 1324 | 1097 | 1 | 1 |
| COS | PA | BC | BC1 | AB | AL | KP | EC | SA | PS |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 10 ± 1.2 | 5 ± 0.6 | 8 ± 1 | 20 ± 2.6 | 4 ± 0.6 | 106 ± 11 | 9 ± 1.1 | 2 ± 0.1 | 11 ± 1.3 |
| 10 | 12 ± 1.5 | 5 ± 0.9 | 9 ± 1.1 | 18 ± 2.2 | 4 ± 0.8 | 100 ± 10 | 11 ± 0.8 | 2 ± 0.2 | 9 ± 1.6 |
| 20 | 11 ± 1.4 | 6 ± 0.7 | 10 ± 1.2 | 17 ± 2.4 | 3 ± 0.4 | 95 ± 9 | 12 ± 0.9 | 3 ± 0.2 | 12 ± 1.5 |
| 30 | 12 ± 1.3 | 5.5 ± 0.6 | 10 ± 1.3 | 21 ± 1.6 | 5 ± 0.6 | 112 ± 12 | 14 ± 1.5 | 4 ± 0.4 | 13 ± 1.1 |
| p values | 0.56 | 0.73 | 0.4 | 0.23 | 0.18 | 0.09 | 0.15 | 0.10 | 0.27 |
| Parameters | CG, Mean (SD; Range) | EG, Mean (SD; Range) | PG, Mean (SD; Range) | p |
|---|---|---|---|---|
| Age, years | 12.03 (SD 2.64; 10–16) | 11.80 (SD 2.28; 10–16) | 12.37 (SD 2.70; 11–26) | 0.86 |
| Gender, male/female | 74/30 | 76/28 | 70/34 | 0.65 |
| BMI | 18.7 (SD 2.8; 13.42–28.27) | 18.93 (SD 3.62; 12.29–30.49) | 0.28 | |
| Smoking | 23 | 20 | 18 | 0.65 |
| Scoliosis curve type (MT/DM/DT/TM/TL) | 58/22/19/0/5 | 55/25/17/2/5 | 59/21/16/4/4 | 0.26 |
| Mean number of levels fused per patient | 9.26 (SD 2.06; 7–13) | 9.38 (SD 2.21; 7–13) | 9.57 (SD 2.23; 7–13) | 0.78 |
| Surgical duration (minutes) | 253.88 (SD 62.15; 198–487) | 287.95 (SD 61.85; 178–483) | 279.94 (SD 76.7; 196–437) | 0.53 |
| Mean number of anchor points per patient | 10.1 (SD 2.49; 67–20) | 11.1 (SD 2.69; 7–21) | 9.2(SD 2.06; 7–18) | 0.39 |
| Post-operative transfusion, mL | Blood: 198.26 (SD 149.80; 0–745) FFP: 21.32 (SD 77.23; 0–545) | Blood: 218.26 (SD 169.80; 0–721) FFP: 20.42 (SD 76.63; 0–521) | Blood: 207.68 (SD 282.43; 0–986) FFP: 22.48 (SD 75.37; 0–530) | 0.21 |
| Post-operative spine drain, mL | Total drain: 218.01 (SD 221.76; 5–1500) | Total drain: 473.02 (SD 355.40; 5–1320) | Total drain: 463.01 (SD 345.90; 5–1640) | 0.41 |
| Post-operative duration of drain in situ, days | 3.2 (SD 0.68; 2–7) | 3.0 (SD 0.61; 2–7) | 3.3 (SD 0.75; 2–7) | 0.36 |
| Instrument | CG | EG | PG | p-Value |
|---|---|---|---|---|
| All pedicle screw constructs | 35 | 30 | 34 | 0.73 |
| Pedicle screws and hook constructs | 42 | 48 | 46 | 0.69 |
| All hook constructs | 10 | 10 | 10 | 1.00 |
| Pedicle screws, hooks and sublaminar wire construct | 11 | 10 | 8 | 0.77 |
| Pedicle screw and sublaminar wire construct | 6 | 6 | 6 | 1.00 |
| Levels Fusion | T6-L2 | T2-L1 | T2-L2 | T5-L4 | T7-L2 | T6-12 | T4-11 | |
|---|---|---|---|---|---|---|---|---|
| Numbers | CG | 35 | 24 | 12 | 9 | 10 | 8 | 6 |
| EG | 33 | 26 | 13 | 8 | 11 | 7 | 6 | |
| PG | 34 | 25 | 14 | 7 | 9 | 8 | 7 | |
| p-value | 1 | |||||||
| Surgical duration (minutes) | CG | 250 ± 45 | 263 ± 51 | 298 ± 46 | 305 ± 62 | 527 ± 71 | 296 ± 43 | 248 ± 32 |
| EG | 242 ± 49 | 251 ± 59 | 277 ± 53 | 311 ± 53 | 512 ± 63 | 280 ± 37 | 239 ± 28 | |
| PG | 256 ± 40 | 266 ± 52 | 302 ± 43 | 299 ± 47 | 531 ± 58 | 301 ± 26 | 254 ± 21 | |
| p-value | 0.91 | 0.85 | 0.69 | 0.27 | 0.53 | 0.44 | 0.75 | |
| Blood loss (mL) | CG | 537 ± 214 | 587 ± 196 | 716 ± 243 | 436 ± 178 | NA | NA | NA |
| EG | 498 ± 253 | 502 ± 245 | 642 ± 215 | 451 ± 210 | NA | NA | NA | |
| PG | 399 ± 128 | 546 ± 237 | 597 ± 204 | 407 ± 185 | NA | NA | NA | |
| p-value | 0.12 | 0.26 | 0.37 | 0.55 | ||||
| Spine drain (mL) | CG | 270 ± 123 | 346 ± 188 | 164 ± 49 | 105 ± 31 | 541 ± 103 | 90 ± 21 | 100 ± 17 |
| EG | 244 ± 105 | 320 ± 171 | 154 ± 33 | 96 ± 18 | 501 ± 83 | 84 ± 13 | 93 ± 11 | |
| PG | 252 ± 98 | 305 ± 124 | 137 ± 41 | 99 ± 27 | 527 ± 66 | 89 ± 17 | 102 ± 22 | |
| p-value | 0.18 | 0.29 | 0.08 | 0.57 | 0.62 | 0.71 | 0.41 | |
| Wound Category | CG | EG | PG | P1 | P2 | P3 |
|---|---|---|---|---|---|---|
| Spine wound | 1 | 2 | 10 | 1 | 0.004 | 0.005 |
| Iliac wound | 0 | 1 | 3 | 1 | 0.245 | 0.607 |
| Side Effects | CG | EG | PG | P1 | P2 | P3 |
|---|---|---|---|---|---|---|
| gastric upset | 15 | 3 | 1 | 0.003 | 0.000 | 0.614 |
| nausea | 13 | 1 | 0 | 0.001 | 0.000 | 1.000 |
| headache | 15 | 3 | 1 | 0.003 | 0.000 | 0.614 |
| vomiting | 20 | 4 | 2 | 0.000 | 0.000 | 0.679 |
| diarrhea | 7 | 0 | 0 | 0.210 | 0.210 | 1.000 |
| abdominal pain | 10 | 0 | 1 | 0.004 | 0.005 | 1.000 |
| seizure | 3 | 0 | 1 | 0.245 | 0.614 | 1.000 |
| chills | 6 | 1 | 1 | 0.124 | 0.124 | 1.000 |
| malaise | 5 | 1 | 1 | 0.214 | 0.214 | 1.000 |
| anxiety | 6 | 0 | 1 | 0.038 | 0.124 | 1.000 |
| fever | 8 | 0 | 1 | 0.012 | 0.041 | 1.000 |
| Stages | Group (n = 10) | SOD (U/mL) | GSH (pg/mL) | ALT (pg/mL) | AST (pg/mL) |
|---|---|---|---|---|---|
| Before experiment | CG | 26.24 ± 3.16 | 22.15 ± 2.04 | 44.12 ± 10.13 | 100.32 ± 25.67 |
| EG | 24.25 ± 4.16 | 23.23 ± 1.93 | 47.79 ± 8.40 | 108.26 ± 19.64 | |
| PG | 25.34 ± 2.32 | 21.28 ± 1.74 | 43.79 ± 12.36 | 103.42 ± 24.48 | |
| p-value | 0.56 | 0.74 | 0.12 | 0.68 | |
| After experiment | CG | 22.73 ± 3.28 Δ,● | 21.09 ± 3.06 Δ | 48.31 ± 8.25 Δ,● | 118.36 ± 26.82 Δ,● |
| EG | 28.29 ± 5.13 *,● | 25.44 ± 2.01 *,● | 38.1 9± 6.25 *,● | 76.34 ± 14.38 *,● | |
| PIG | 15.32 ± 5.71 *,Δ | 14.26 ± 4.39 *,Δ | 53.69 ± 18.31 *,Δ | 89.46 ± 27.52 *,Δ | |
| p-value | 0.01 | 0.01 | 0.01 | 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, Y.; Xu, J.; Zhou, H.; Dong, R.; Kang, M.; Zhao, J. Chitin Oligosaccharide (COS) Reduces Antibiotics Dose and Prevents Antibiotics-Caused Side Effects in Adolescent Idiopathic Scoliosis (AIS) Patients with Spinal Fusion Surgery. Mar. Drugs 2017, 15, 70. https://doi.org/10.3390/md15030070
Qu Y, Xu J, Zhou H, Dong R, Kang M, Zhao J. Chitin Oligosaccharide (COS) Reduces Antibiotics Dose and Prevents Antibiotics-Caused Side Effects in Adolescent Idiopathic Scoliosis (AIS) Patients with Spinal Fusion Surgery. Marine Drugs. 2017; 15(3):70. https://doi.org/10.3390/md15030070
Chicago/Turabian StyleQu, Yang, Jinyu Xu, Haohan Zhou, Rongpeng Dong, Mingyang Kang, and Jianwu Zhao. 2017. "Chitin Oligosaccharide (COS) Reduces Antibiotics Dose and Prevents Antibiotics-Caused Side Effects in Adolescent Idiopathic Scoliosis (AIS) Patients with Spinal Fusion Surgery" Marine Drugs 15, no. 3: 70. https://doi.org/10.3390/md15030070





