An Extract from Shrimp Processing By-Products Protects SH-SY5Y Cells from Neurotoxicity Induced by Aβ25–35
Abstract
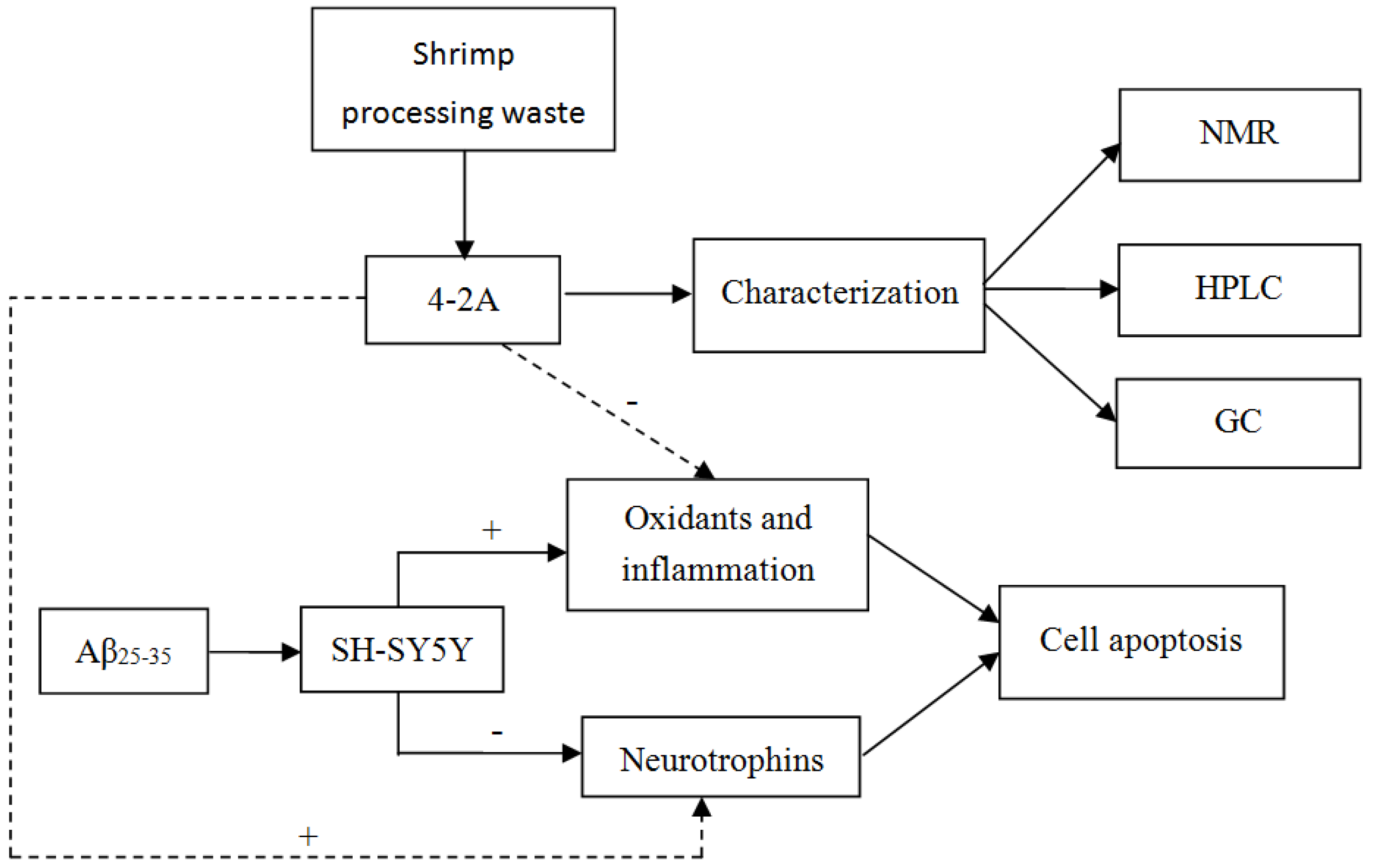
:1. Introduction
2. Results
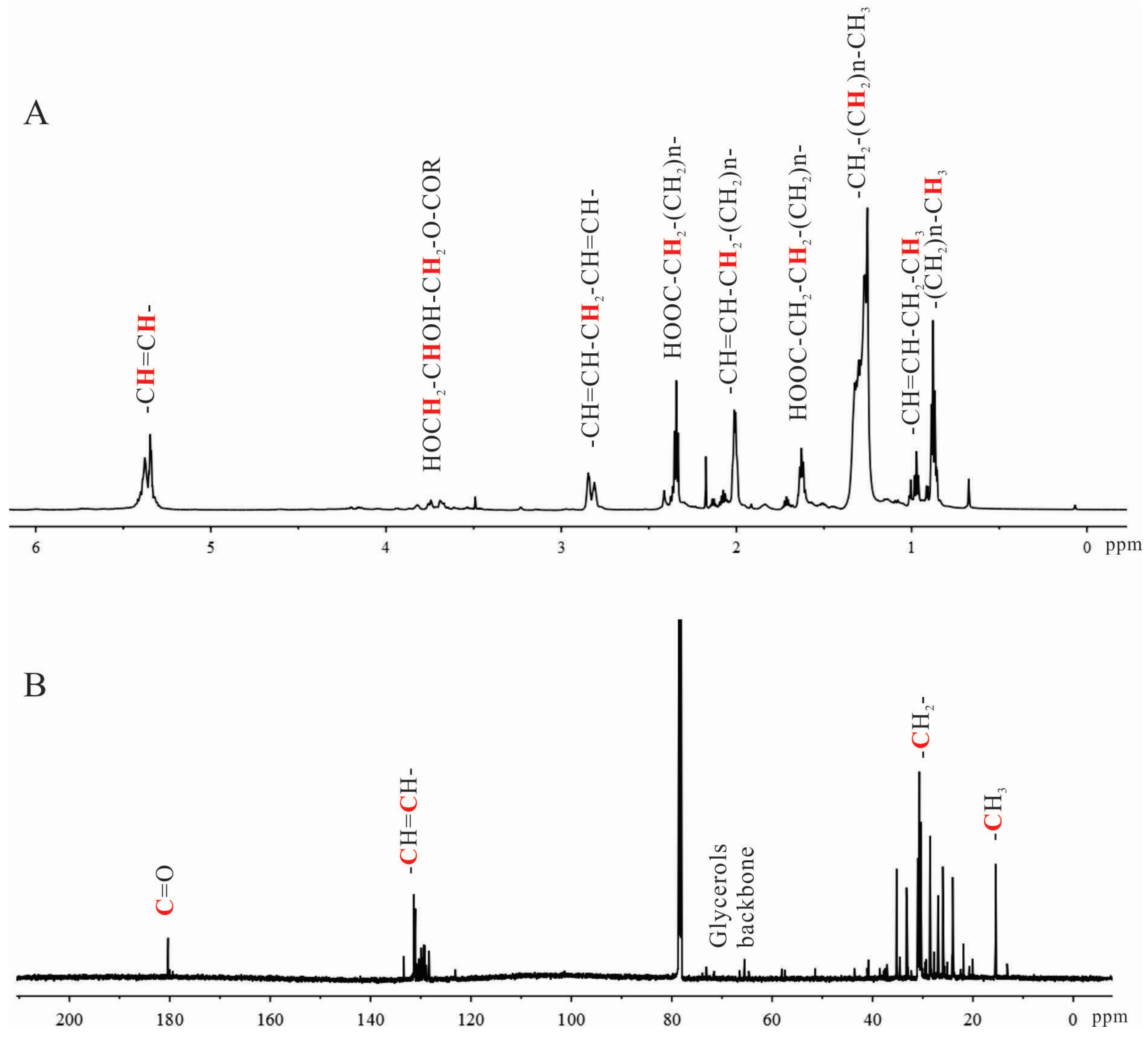
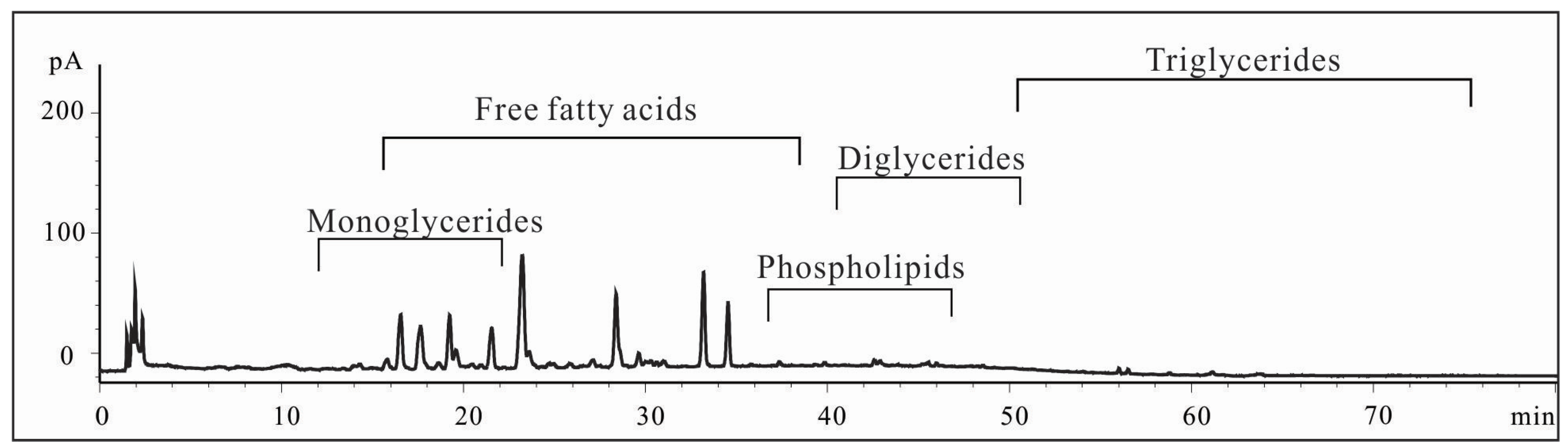
2.1. Characterization of Shrimp Extract 4-2A
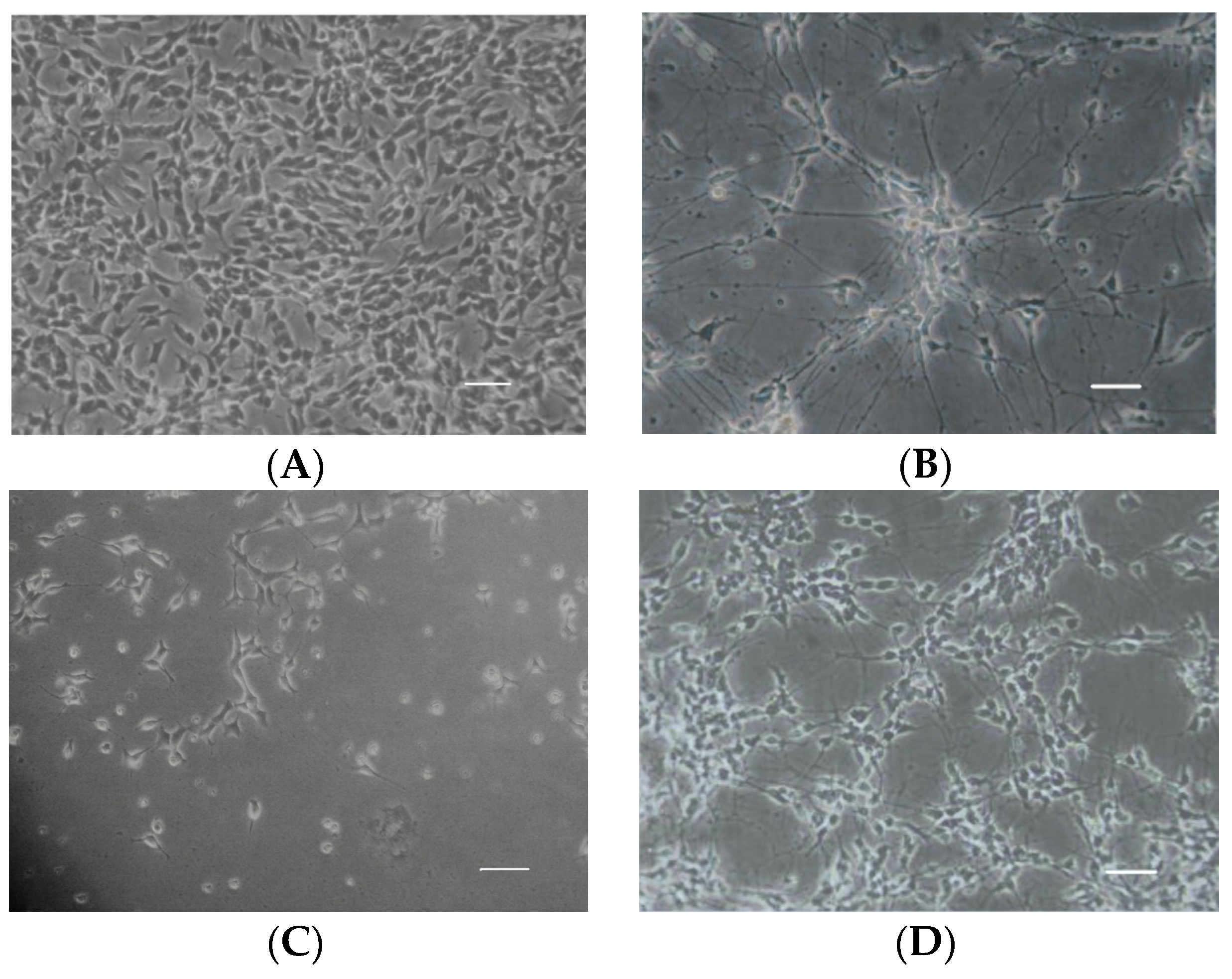
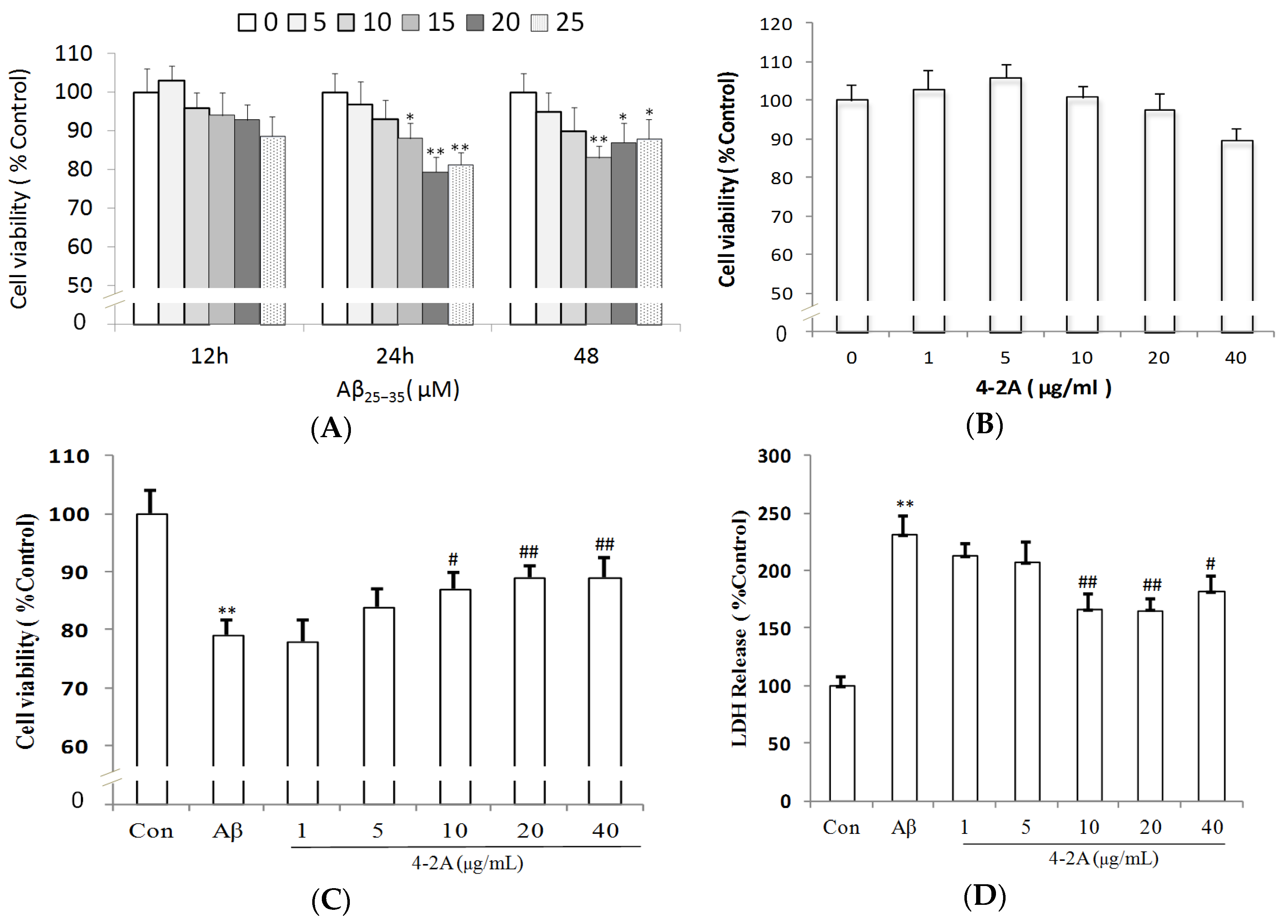
2.2. Aβ25–35-Induced Decrease in Neuronal Cell Viability Was Ameliorated by 4-2A
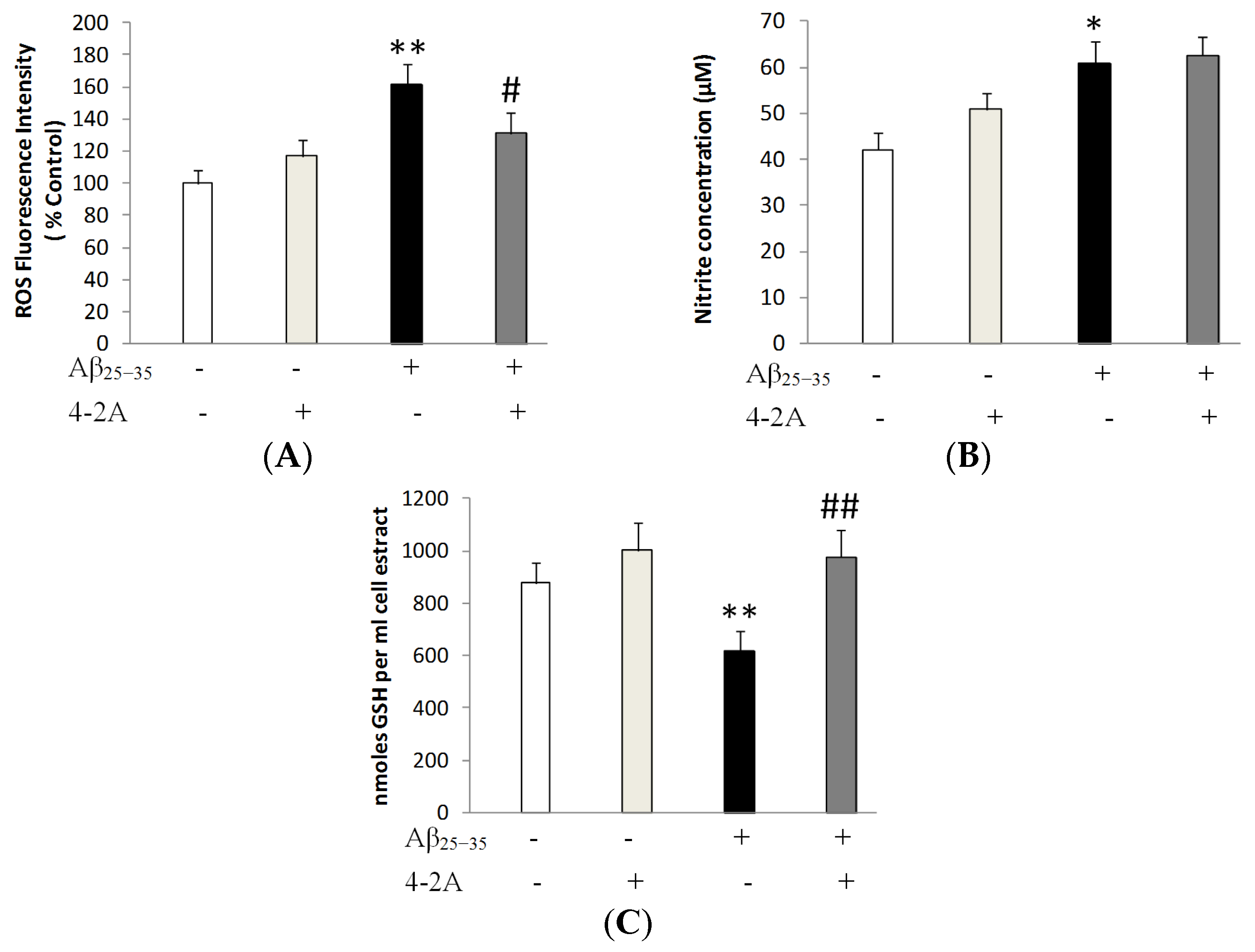
2.3. Treatment with 4-2A Attenuated the Changes in ROS, Nitric Oxide (NO) and Glutathione (GSH) Level Induced by Aβ25–35
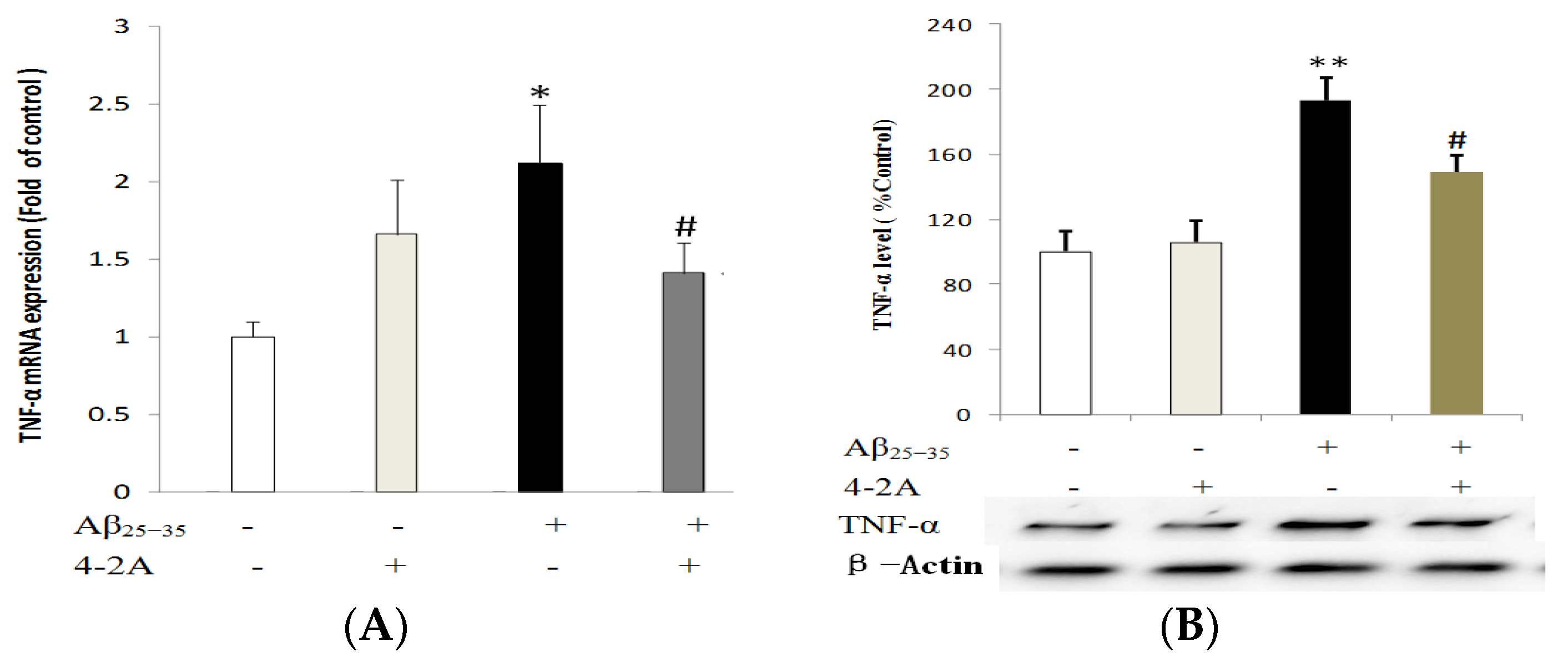
2.4. Treatment with 4-2A Attenuated the Aβ25–35-Inducued Increased Expression of Pro-Inflammatory Cytokine TNF-α
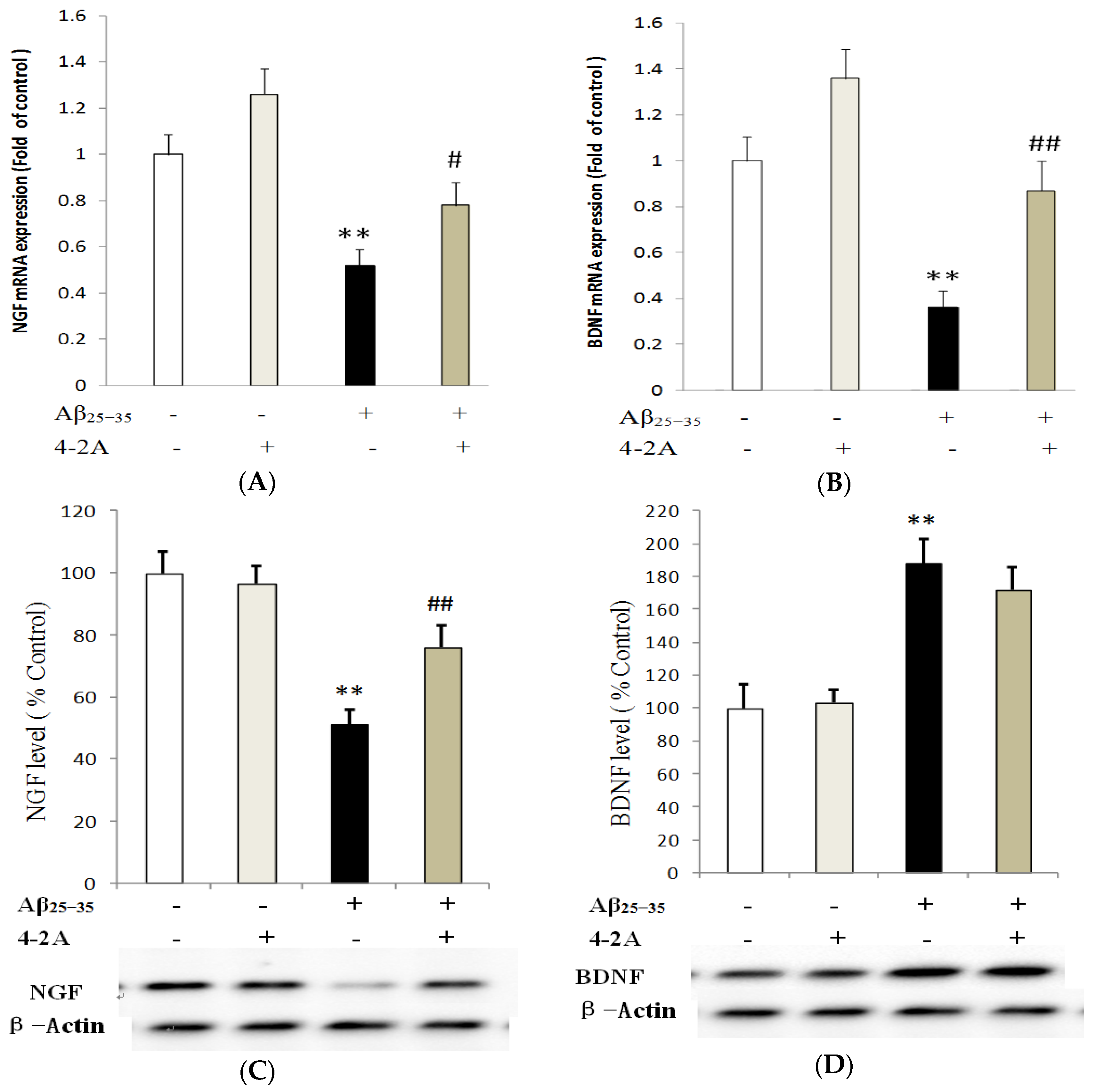
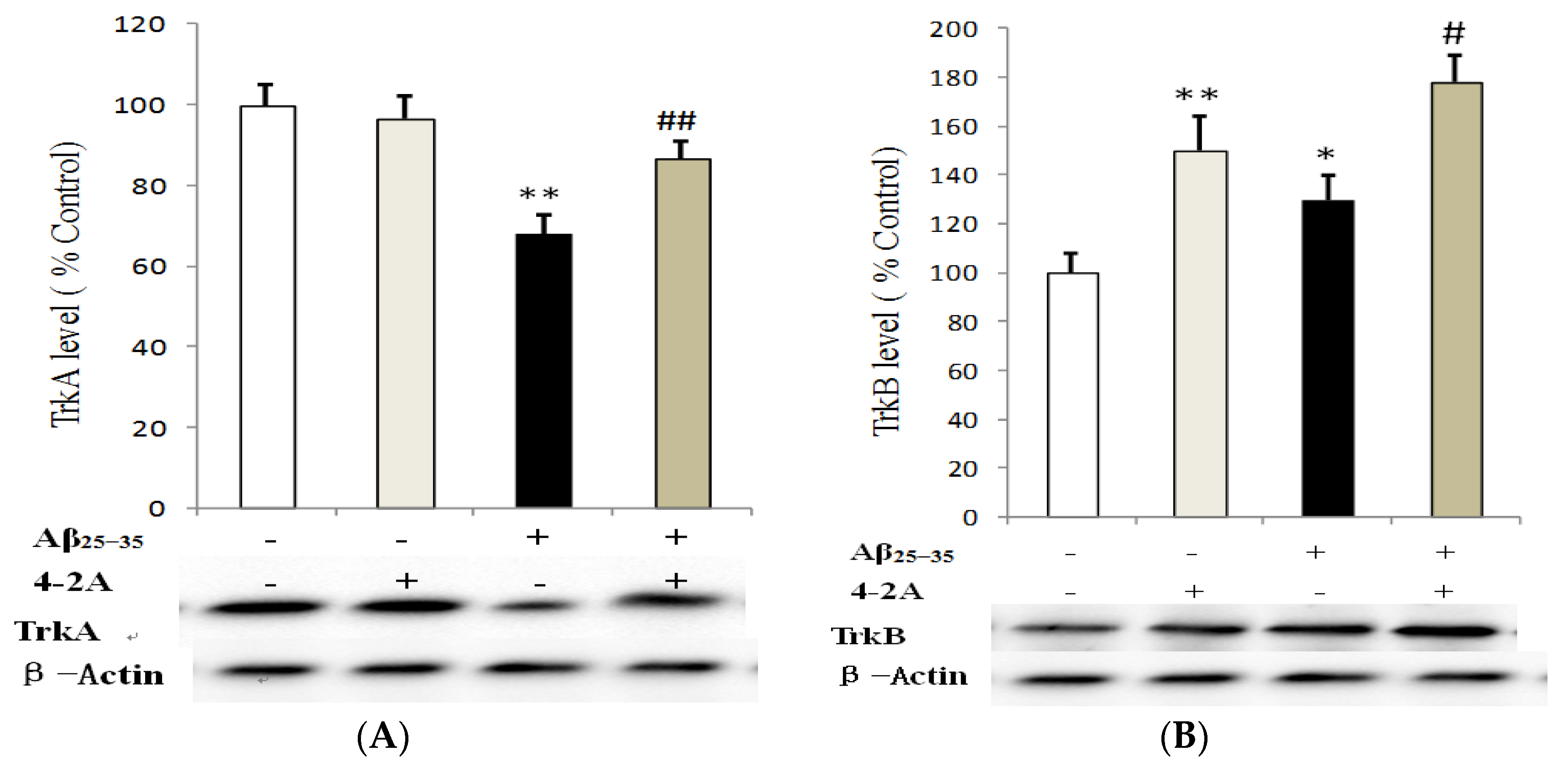
2.5. Effect of 4-2A on the Expression of Neurotrophins: NGF and BDNF and Their TrkA and TrkB Receptors
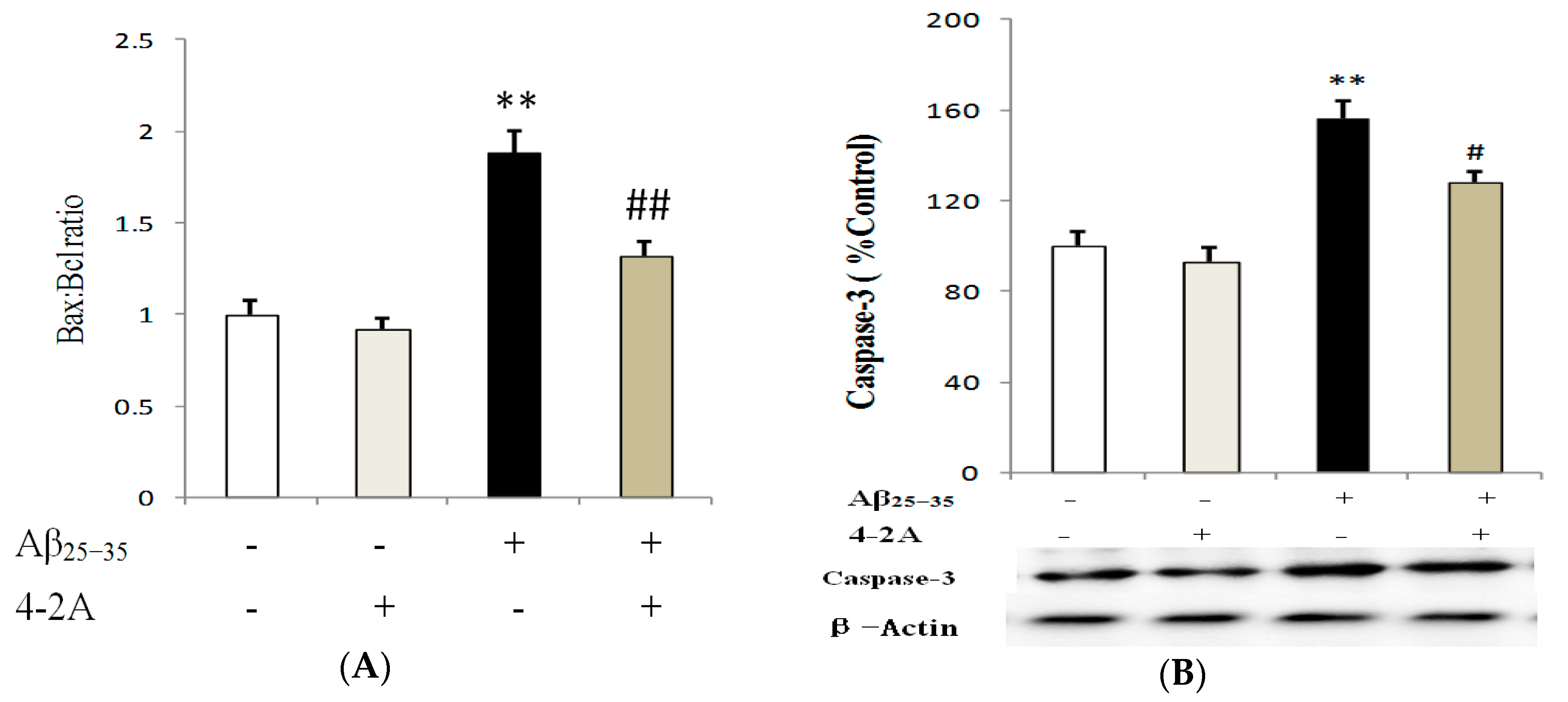
2.6. Treatment with 4-2A Regulated the Expression of Apoptosis Related Genes: Bax, Bcl-2 and Caspase-3
3. Discussion
4. Materials and Methods
4.1. Preparation of the Shrimp Extract 4-2A
4.2. Characterization of Shrimp Extract 4-2A
4.2.1. Characterization of 4-2A by NMR
4.2.2. Characterization of 4-2A by HPLC
4.2.3. GC Analysis of 4-2A
4.3. SH-SY5Y Culture and Differentiation
4.4. Experimental Design
4.5. Measurement of Cell Viability by MTT Assay
4.6. Cytotoxicity Assay by LDH Assay
4.7. Measurement of Oxidative Stress and Antioxidant Response
4.8. Determination of Gene Expression with Quantitative PCR
4.9. Analysis of Protein Expression by Western Blotting
4.10. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Selkoe, D.J. Alzheimer’s disease. Cold Spring Harbor Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Okello, E.J.; Harris, J.R. Experimental inhibition of fibrillogenesis and neurotoxicity by amyloid-beta (Abeta) and other disease-related peptides/proteins by plant extracts and herbal compounds. Sub-Cell. Biochem. 2012, 65, 295–326. [Google Scholar]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Moon, M.; Choi, J.G.; Park, G.; Kim, A.J.; Hur, J.; Lee, K.T.; Oh, M.S. Donepezil inhibits the amyloid-beta oligomer-induced microglial activation in vitro and in vivo. Neurotoxicology 2014, 40, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Tabner, B.J.; Turnbull, S.; King, J.E.; Benson, F.E.; El-Agnaf, O.M.; Allsop, D. A spectroscopic study of some of the peptidyl radicals formed following hydroxyl radical attack on beta-amyloid and alpha-synuclein. Free Radic. Res. 2006, 40, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.J.; Cho, S.; Seo, J.Y.; Lee, H.B.; Park, Y.I. Neuroprotective effects of the Phellinus linteus ethyl acetate extract against H2O2-induced apoptotic cell death of SK-N-MC cells. Nutr. Res. 2016, 36, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Sondag, C.M.; Dhawan, G.; Combs, C.K. Beta amyloid oligomers and fibrils stimulate differential activation of primary microglia. J. Neuroinflamm. 2009, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; McGeer, E.; McGeer, P.L. Activated human microglia stimulate neuroblastoma cells to upregulate production of beta amyloid protein and tau: Implications for Alzheimer’s disease pathogenesis. Neurobiol. Aging 2015, 36, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Watson, J.J.; Dawbarn, D. The neurotrophins and their role in Alzheimer’s disease. Curr. Neuropharmacol. 2011, 9, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Ciaramella, A.; Salani, F.; Bizzoni, F.; Orfei, M.D.; Langella, R.; Angelucci, F.; Spalletta, G.; Taddei, A.R.; Caltagirone, C.; Bossu, P. The stimulation of dendritic cells by amyloid beta 1-42 reduces BDNF production in Alzheimer’s disease patients. Brain Behav. Immun. 2013, 32, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Poon, W.W.; Carlos, A.J.; Aguilar, B.L.; Berchtold, N.C.; Kawano, C.K.; Zograbyan, V.; Yaopruke, T.; Shelanski, M.; Cotman, C.W. beta-Amyloid (Abeta) oligomers impair brain-derived neurotrophic factor retrograde trafficking by down-regulating ubiquitin C-terminal hydrolase, UCH-L1. J. Biol. Chem. 2013, 288, 16937–16948. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, A.; Ekavali. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Luchtman, D.W.; Meng, Q.; Song, C. Ethyl-eicosapentaenoate (E-EPA) attenuates motor impairments and inflammation in the MPTP-probenecid mouse model of Parkinson’s disease. Behav. Brain Res. 2012, 226, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Manku, M.S.; Horrobin, D.F. Long-chain polyunsaturated fatty acids modulate interleukin-1beta-induced changes in behavior, monoaminergic neurotransmitters, and brain inflammation in rats. J. Nutr. 2008, 138, 954–963. [Google Scholar] [PubMed]
- Song, C. Essential fatty acids as potential anti-inflammatory agents in the treatment of affective disorders. Mod. Trends Pharmacopsychiatry 2013, 28, 75–89. [Google Scholar]
- Song, C.; Li, X.; Leonard, B.E.; Horrobin, D.F. Effects of dietary n-3 or n-6 fatty acids on interleukin-1beta-induced anxiety, stress, and inflammatory responses in rats. J. Lipid Res. 2003, 44, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Li, L.; Liu, Y.; Pu, J.; Zhang, S.; Yu, J.; Zhao, J.; Liu, P. Oleate blocks palmitate-induced abnormal lipid distribution, endoplasmic reticulum expansion and stress, and insulin resistance in skeletal muscle. Endocrinology 2011, 152, 2206–2218. [Google Scholar] [CrossRef] [PubMed]
- Coll, T.; Eyre, E.; Rodriguez-Calvo, R.; Palomer, X.; Sanchez, R.M.; Merlos, M.; Laguna, J.C.; Vazquez-Carrera, M. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J. Biol. Chem. 2008, 283, 11107–11116. [Google Scholar] [CrossRef] [PubMed]
- Yuzefovych, L.; Wilson, G.; Rachek, L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: Role of oxidative stress. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E1096–E1105. [Google Scholar] [CrossRef] [PubMed]
- Bento-Abreu, A.; Tabernero, A.; Medina, J.M. Peroxisome proliferator-activated receptor-alpha is required for the neurotrophic effect of oleic acid in neurons. J. Neurochem. 2007, 103, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Frigolet, M.E.; Gutierrez-Aguilar, R. The Role of the Novel Lipokine Palmitoleic Acid in Health and Disease. Adv. Nutr. 2017, 8, 173S–181S. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Campos, H.; McGarvey, S.; Wu, Z.; Goldberg, R.; Baylin, A. Adipose tissue palmitoleic acid and obesity in humans: Does it behave as a lipokine? Am. J. Clin. Nutr. 2011, 93, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Tchoukanova, N.; Benoit, G. Method for extracting organic solids and oil from marine organisms enriched with astaxanthin. U.S. Patent App. 14/776,481, 2014. [Google Scholar]
- Jiao, G.; Hui, J.; Burton, I.; Thibault, M.-H.; Pelletier, C.; Boudreau, J.; Tchoukanova, N.; Subramanian, B.; Djaoued, Y.; Ewart, S.; et al. Characterization of Shrimp Oil from Pandalus borealis by High Performance Liquid Chromatography and High Resolution Mass Spectrometry. Mar. Drugs 2015, 13, 3849–3876. [Google Scholar] [CrossRef] [PubMed]
- Morioka, N.; Zhang, F.F.; Nakamura, Y.; Kitamura, T.; Hisaoka-Nakashima, K.; Nakata, Y. Tumor necrosis factor-mediated downregulation of spinal astrocytic connexin43 leads to increased glutamatergic neurotransmission and neuropathic pain in mice. Brain Behav. Immunity 2015, 49, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G. TNF-alpha signaling in glaucomatous neurodegeneration. Prog. Brain Res. 2008, 173, 409–421. [Google Scholar] [PubMed]
- Gunstone, F.; Knothe, G. NMR Spectroscopy of Fatty Acids and Their Derivatives. AOCS Lipid Library. Available online: http://lipidlibrary.aocs.org/Analysis/content.cfm?ItemNumber=40256 (accessed on 21 March 2017).
- Millucci, L.; Ghezzi, L.; Bernardini, G.; Santucci, A. Conformations and biological activities of amyloid beta peptide 25-35. Curr. Protein Pept. Sci. 2010, 11, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Sohanaki, H.; Baluchnejadmojarad, T.; Nikbakht, F.; Roghani, M. Pelargonidin improves memory deficit in amyloid beta25-35 rat model of Alzheimer’s disease by inhibition of glial activation, cholinesterase, and oxidative stress. Biomed. Pharmacother. 2016, 83, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Vedagiri, A.; Thangarajan, S. Mitigating effect of chrysin loaded solid lipid nanoparticles against Amyloid beta25-35 induced oxidative stress in rat hippocampal region: An efficient formulation approach for Alzheimer’s disease. Neuropeptides 2016, 58, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, Z.; Baluchnejadmojarad, T.; Nikbakht, F.; Mansouri, M.; Roghani, M. Riluzole ameliorates learning and memory deficits in Abeta25-35-induced rat model of Alzheimer’s disease and is independent of cholinoceptor activation. Biomed. Pharmacother. 2017, 87, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Fedotova, J.; Soultanov, V.; Nikitina, T.; Roschin, V.; Ordyan, N.; Hritcu, L. Cognitive-enhancing activities of the polyprenol preparation Ropren(R) in gonadectomized beta-amyloid (25-35) rat model of Alzheimer’s disease. Physiol. Behav. 2016, 157, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Song, C.; Zuo, P. The mechanism of memory impairment induced by Abeta chronic administration involves imbalance between cytokines and neurotrophins in the rat hippocampus. Curr. Alzheimer Res. 2011, 8, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Rojas, K.; Espinosa, B.; Chavez, R.; Zenteno, E.; Limon, D.; Guevara, J. Aminoguanidine treatment ameliorates inflammatory responses and memory impairment induced by amyloid-beta 25-35 injection in rats. Neuropeptides 2014, 48, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yao, L.; Zhou, H.; Qu, S.; Zeng, X.; Zhou, D.; Zhou, Y.; Li, X.; Liu, Z. Neuroprotection against Abeta25-35-induced apoptosis by Salvia miltiorrhiza extract in SH-SY5Y cells. Neurochem. Int. 2014, 75, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Briyal, S.; Shepard, C.; Gulati, A. Endothelin receptor type B agonist, IRL-1620, prevents beta amyloid (Abeta) induced oxidative stress and cognitive impairment in normal and diabetic rats. Pharmacol. Biochem. Behav. 2014, 120, 65–72. [Google Scholar] [PubMed]
- Cioanca, O.; Hritcu, L.; Mihasan, M.; Trifan, A.; Hancianu, M. Inhalation of coriander volatile oil increased anxiolytic-antidepressant-like behaviors and decreased oxidative status in beta-amyloid (1-42) rat model of Alzheimer’s disease. Physiol. Behav. 2014, 131, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Suganthy, N.; Devi, K.P. Protective effect of catechin rich extract of Rhizophora mucronata against β-amyloid-induced toxicity in PC12 cells. J. Appl. Biomed. 2016, 14, 137–146. [Google Scholar] [CrossRef]
- Arimon, M.; Takeda, S.; Post, K.L.; Svirsky, S.; Hyman, B.T.; Berezovska, O. Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol. Dis. 2015, 84, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Shanbin, G.; Ling, M.; Yuan, W.; Ying, X.; Ping, Z. Sevoflurane aggregates cognitive dysfunction and hippocampal oxidative stress induced by beta-amyloid in rats. Life Sci. 2015, 143, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Padayachee, E.; Ngqwala, N.; Whiteley, C.G. Association of beta-amyloid peptide fragments with neuronal nitric oxide synthase: Implications in the etiology of Alzheimers disease. J. Enzyme Inhib. Med. Chem. 2012, 27, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Gamper, N.; Ooi, L. Redox and nitric oxide-mediated regulation of sensory neuron ion channel function. Antioxid. Redox Signal. 2015, 22, 486–504. [Google Scholar] [CrossRef] [PubMed]
- Ooi, L.; Gigout, S.; Pettinger, L.; Gamper, N. Triple cysteine module within M-type K+ channels mediates reciprocal channel modulation by nitric oxide and reactive oxygen species. J. Neurosci. 2013, 33, 6041–6046. [Google Scholar] [CrossRef] [PubMed]
- Shadfar, S.; Hwang, C.J.; Lim, M.S.; Choi, D.Y.; Hong, J.T. Involvement of inflammation in Alzheimer’s disease pathogenesis and therapeutic potential of anti-inflammatory agents. Arch. Pharm. Res. 2015, 38, 2106–2119. [Google Scholar] [CrossRef] [PubMed]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; He, H.; Liu, Z.J.; Cao, Z.G.; Wang, X.Y.; Yang, K.; Fang, Y.; Han, M.; Zhang, C.; Huo, F.Y. Effects of TNF-alpha on Cementoblast Differentiation, Mineralization, and Apoptosis. J. Dent. Res. 2015, 94, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Taepavarapruk, P.; Song, C. Reductions of acetylcholine release and nerve growth factor expression are correlated with memory impairment induced by interleukin-1beta administrations: Effects of omega-3 fatty acid EPA treatment. J. Neurochem. 2010, 112, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhang, Y.; Dong, Y. Acute and subacute IL-1beta administrations differentially modulate neuroimmune and neurotrophic systems: Possible implications for neuroprotection and neurodegeneration. J. Neuroinflamm. 2013, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, F.; Carboni, L.; Carretta, D.; Candeletti, S.; Romualdi, P. Treatment with the neurotoxic Abeta (25-35) peptide modulates the expression of neuroprotective factors Pin1, Sirtuin 1, and brain-derived neurotrophic factor in SH-SY5Y human neuroblastoma cells. Exp. Toxicol. Pathol. 2016, 68, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Loo, D.T.; Copani, A.; Pike, C.J.; Whittemore, E.R.; Walencewicz, A.J.; Cotman, C.W. Apoptosis is induced by beta-amyloid in cultured central nervous system neurons. Proc. Natl. Acad. Sci. USA 1993, 90, 7951–7955. [Google Scholar] [CrossRef] [PubMed]
- McGeer, E.G.; McGeer, P.L. Neuroinflammation in Alzheimer’s disease and mild cognitive impairment: A field in its infancy. J. Alzheimer’s Dis. JAD 2010, 19, 355–361. [Google Scholar] [PubMed]
- Asadi, F.; Jamshidi, A.H.; Khodagholi, F.; Yans, A.; Azimi, L.; Faizi, M.; Vali, L.; Abdollahi, M.; Ghahremani, M.H.; Sharifzadeh, M. Reversal effects of crocin on amyloid beta-induced memory deficit: Modification of autophagy or apoptosis markers. Pharmacol. Biochem. Behav. 2015, 139 Pt A, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Pieri, M.; Amadoro, G.; Carunchio, I.; Ciotti, M.T.; Quaresima, S.; Florenzano, F.; Calissano, P.; Possenti, R.; Zona, C.; Severini, C. SP protects cerebellar granule cells against beta-amyloid-induced apoptosis by down-regulation and reduced activity of Kv4 potassium channels. Neuropharmacology 2010, 58, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.; Yoshida, H.; Tanji, K.; Matsumiya, T.; Xing, F.; Hayakari, R.; Wang, L.; Tsuruga, K.; Tanaka, H.; Mimura, J.; et al. Carnosic acid attenuates apoptosis induced by amyloid-beta 1–42 or 1–43 in SH-SY5Y human neuroblastoma cells. Neurosci. Res. 2015, 94, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sharoar, M.G.; Islam, M.I.; Shahnawaz, M.; Shin, S.Y.; Park, I.S. Amyloid beta binds procaspase-9 to inhibit assembly of Apaf-1 apoptosome and intrinsic apoptosis pathway. Biochim. Biophys. Acta 2014, 1843, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Feart, C.; Samieri, C.; Alles, B.; Barberger-Gateau, P. Potential benefits of adherence to the Mediterranean diet on cognitive health. Proc. Nutr. Soc. 2013, 72, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.; Lee, H.K.; Querfurth, H.W. Oleate prevents palmitate-induced mitochondrial dysfunction, insulin resistance and inflammatory signaling in neuronal cells. Biochim. Biophys. Acta 2014, 1843, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Gerhold, K.; Mayers, J.R.; Wiest, M.M.; Watkins, S.M.; Hotamisligil, G.S. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008, 134, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, H.; Xu, H.; Halim, V.; Zhang, W.; Wang, H.; Ong, K.T.; Woo, S.L.; Walzem, R.L.; Mashek, D.G.; et al. Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice. PLoS ONE 2012, 7, e39286. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.K.; Trepanier, M.O.; Bazinet, R.P. n-3 Polyunsaturated fatty acids in animal models with neuroinflammation. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.; Poppe, S.; Bondan, E. Neuroprotective Properties of the Marine Carotenoid Astaxanthin and Omega-3 Fatty Acids, and Perspectives for the Natural Combination of Both in Krill Oil. Nutrients 2014, 6, 1293–1317. [Google Scholar] [CrossRef] [PubMed]
- Uygur, R.; Aktas, C.; Tulubas, F.; Uygur, E.; Kanter, M.; Erboga, M.; Caglar, V.; Topcu, B.; Ozen, O.A. Protective effects of fish omega-3 fatty acids on doxorubicin-induced testicular apoptosis and oxidative damage in rats. Andrologia 2014, 46, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Luchtman, D.W.; Meng, Q.; Wang, X.; Shao, D.; Song, C. Omega-3 fatty acid eicosapentaenoic acid attenuates MPP+-induced neurodegeneration in fully differentiated human SH-SY5Y and primary mesencephalic cells. J. Neurochem. 2013, 124, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Kou, W.; Luchtman, D.; Song, C. Eicosapentaenoic acid (EPA) increases cell viability and expression of neurotrophin receptors in retinoic acid and brain-derived neurotrophic factor differentiated SH-SY5Y cells. Eur. J. Nutr. 2008, 47, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Horrobin, D. Omega-3 fatty acid ethyl-eicosapentaenoate, but not soybean oil, attenuates memory impairment induced by central IL-1beta administration. J. Lipid Res. 2004, 45, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Q.; Dang, R.L.; Tang, M.M.; Cai, H.L.; Li, H.D.; Liao, D.H.; He, X.; Cao, L.J.; Xue, Y.; Jiang, P. Long Chain Omega-3 Polyunsaturated Fatty Acid Supplementation Alleviates Doxorubicin-Induced Depressive-Like Behaviors and Neurotoxicity in Rats: Involvement of Oxidative Stress and Neuroinflammation. Nutrients 2016, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Green, K.N.; Martinez-Coria, H.; Khashwji, H.; Hall, E.B.; Yurko-Mauro, K.A.; Ellis, L.; LaFerla, F.M. Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J. Neurosci. 2007, 27, 4385–4395. [Google Scholar] [CrossRef] [PubMed]
- Begum, G.; Yan, H.Q.; Li, L.; Singh, A.; Dixon, C.E.; Sun, D. Docosahexaenoic acid reduces ER stress and abnormal protein accumulation and improves neuronal function following traumatic brain injury. J. Neurosci. 2014, 34, 3743–3755. [Google Scholar] [CrossRef] [PubMed]
- Plante, M.; Bailey, B.; Acworth, I. Analysis of Lipids by RP-HPLC Using the Corona Analysis of Lipids by RP-HPLC Using the Corona Ultra; ESA-A Dionex Company: Chelmsford, MA, USA, 2009. [Google Scholar]
- Delobette, S.; Privat, A.; Maurice, T. In vitro aggregation facilities beta-amyloid peptide-(25-35)-induced amnesia in the rat. Eur. J. Pharmacol. 1997, 319, 1–4. [Google Scholar] [CrossRef]
| FAME | Intensity (%) | Stdev (n = 4, %) |
|---|---|---|
| 14:0 | 2.10 | 0.25 |
| 16:0 | 6.56 | 0.58 |
| 18:0 | 0.70 | 0.08 |
| 16:1n-7 | 14.75 | 0.21 |
| 18:1n-5 | 0.77 | 0.16 |
| 18:1n-7 | 7.07 | 0.31 |
| 18:1n-9 | 20.65 | 2.62 |
| 20:1n-7 | 1.08 | 0.03 |
| 20:1n-9 | 7.97 | 0.97 |
| 20:1n-11 | 1.95 | 0.01 |
| 22:1n-7 | 11.04 | 0.24 |
| 22:1n-9 | 1.91 | 0.78 |
| 18:2n-6 | 0.86 | 0.10 |
| 20:4n-6 | 0.99 | 0.12 |
| 20:5n-3 | 8.45 | 0.83 |
| 22:6n-3 | 6.54 | 1.02 |
| Total | 93.39 | |
| Saturated | 9.36 | |
| Monounsaturated | 67.19 | |
| Polyunsaturated | 16.84 | |
| Omega-3 | 14.99 | |
| Omega-6 | 1.85 | |
| Omega-7 | 33.94 | |
| Omega-9 | 30.53 |
| Primer Names | Sequences |
|---|---|
| hActin F | GATGAGATTGGCATGGCTTT |
| hActin R | CACCTTCACCGTTCCAGTTT |
| hBAX F | GGGGACGAACTGGACAGTAA |
| hBAX R | CAGTTGAAGTTGCCGTCAGA |
| hBCL 2 F | TCTAGGGGAGGTGGTAGGCT |
| hBCL 2 R | CTGAGCAAGTCAGAGACCCC |
| hCaspase 3 F | GACTCTAGACGGCATCCAGC |
| hCaspase 3 R | TGACAGCCAGTGAGACTTGG |
| hNGF F | ACCTTTCTCAGTAGCGGCAA |
| hNGF R | TGTGTCACCTTGTCAGGGAA |
| hTNF-α F | AGGTTTGGCCTCACAAGGAC |
| hTNF-α R | GCGGTAGGGACAGTTCACAG |
| hBDNF F | GGAGACACATCCAGCAAT |
| hBDNF R | ACAAGAACGAACACAACAG |
| hTrkB F | CTATGCTGTGGTGGTGATT |
| hTrkB R | CCGAAGAAGATGGAGTGTTA |
| hTrkA F | TACAGCACCGACTATTACC |
| hTrkA R | ATGATGGCGTAGACCTCT |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Jiao, G.; Song, C.; Gu, S.; Brown, R.E.; Zhang, J.; Zhang, P.; Gagnon, J.; Locke, S.; Stefanova, R.; et al. An Extract from Shrimp Processing By-Products Protects SH-SY5Y Cells from Neurotoxicity Induced by Aβ25–35. Mar. Drugs 2017, 15, 83. https://doi.org/10.3390/md15030083
Zhang Y, Jiao G, Song C, Gu S, Brown RE, Zhang J, Zhang P, Gagnon J, Locke S, Stefanova R, et al. An Extract from Shrimp Processing By-Products Protects SH-SY5Y Cells from Neurotoxicity Induced by Aβ25–35. Marine Drugs. 2017; 15(3):83. https://doi.org/10.3390/md15030083
Chicago/Turabian StyleZhang, Yongping, Guangling Jiao, Cai Song, Shelly Gu, Richard E. Brown, Junzeng Zhang, Pingcheng Zhang, Jacques Gagnon, Steven Locke, Roumiana Stefanova, and et al. 2017. "An Extract from Shrimp Processing By-Products Protects SH-SY5Y Cells from Neurotoxicity Induced by Aβ25–35" Marine Drugs 15, no. 3: 83. https://doi.org/10.3390/md15030083






