Promoting Wound Healing Using Low Molecular Weight Fucoidan in a Full-Thickness Dermal Excision Rat Model
Abstract
:1. Introduction
2. Results
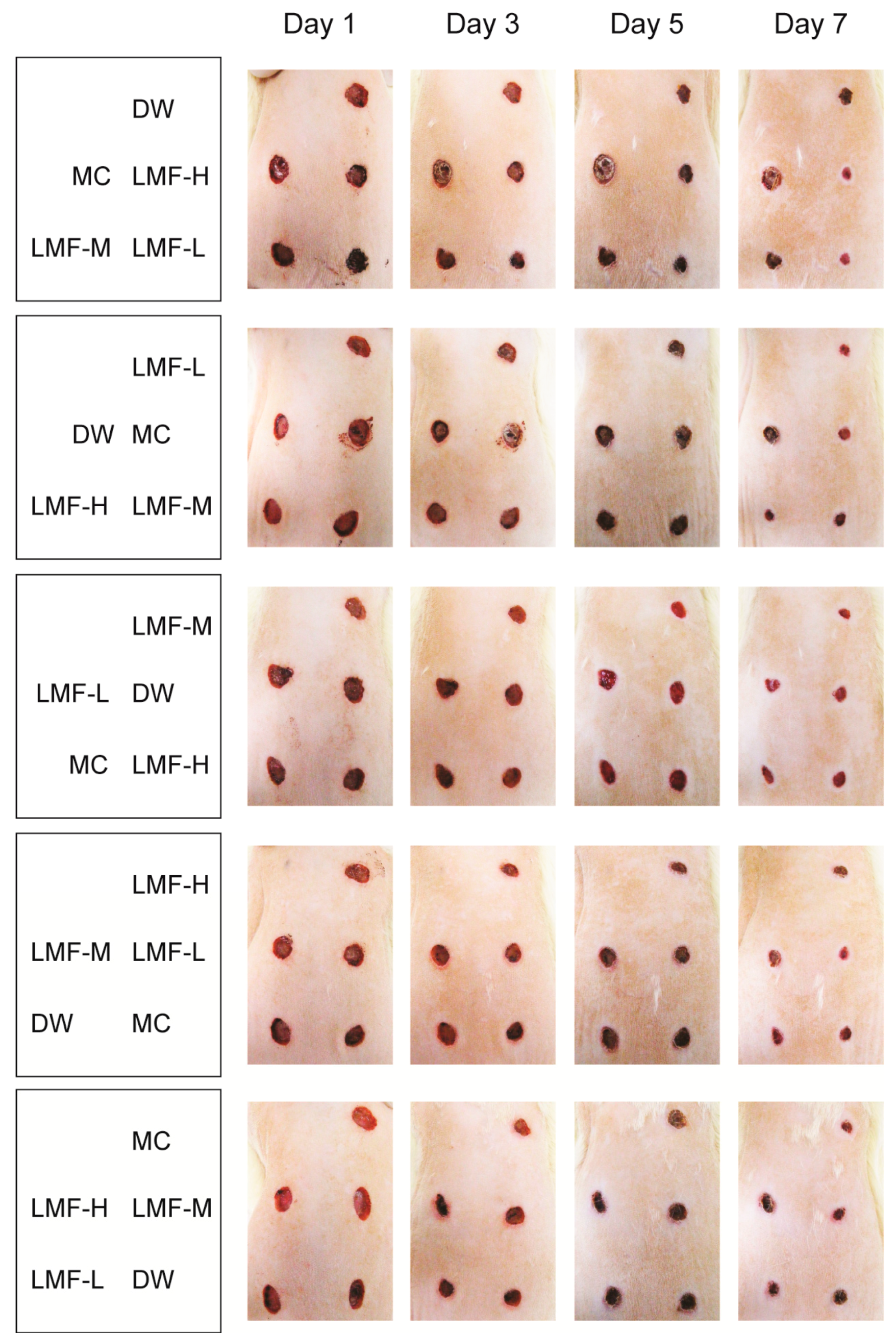
2.1. Promotion of Wound Contraction
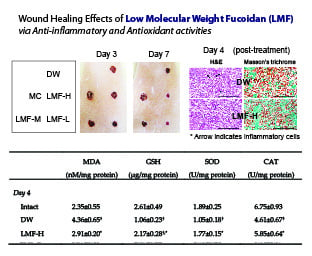
2.2. Wound Healing Effects on Day 4 Post-Treatment
2.3. Wound Healing Effects on Day 7 Post-Treatment
2.4. Effects on the Expression of Growth Factors in Dermal Wounds
2.5. Effects on Antioxidant Activities in Dermal Wounds
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Dermal Wound Induction and Treatment
4.4. Assessment of the Wound Area
4.5. Biochemical Analyses for Antioxidant Activities
4.6. Histopathology
4.7. Immunohistochemistry
4.8. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wild, T.; Rahbarnia, A.; Kellner, M.; Sobotka, L.; Eberlein, T. Basics in nutrition and wound healing. Nutrition 2010, 26, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [PubMed]
- Harding, K.G.; Morris, H.L.; Patel, G.K. Science, medicine and the future: Healing chronic wounds. BMJ 2002, 324, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- McDonough, A.K.; Curtis, J.R.; Saag, K.G. The epidemiology of glucocorticoid-associated adverse events. Curr. Opin. Rheumatol. 2008, 20, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Abood, W.N.; Al-Henhena, N.A.; Najim Abood, A.; Al-Obaidi, M.M.; Ismail, S.; Abdulla, M.A.; Al Bartan, R. Wound-healing potential of the fruit extract of Phaleria macrocarpa. Bosn. J. Basic Med. Sci. 2015, 15, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.P.; Kumar, M.; Tripathi, Y.B. Efficacy of Jasminum grandiflorum L. leaf extract on dermal wound healing in rats. Int. Wound J. 2013, 10, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, S.; Singhai, A.K. Wound healing effect of flavonoid rich fraction and luteolin isolated from Martynia annua Linn. on streptozotocin induced diabetic rats. Asian Pac. J. Trop. Med. 2013, 6, 253–259. [Google Scholar] [CrossRef]
- Rajasekaran, N.; Nithya, M.; Rose, C.; Chandra, T. The effect of finger millet feeding on the early responses during the process of wound healing in diabetic rats. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2004, 1689, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Tew, K.D.; Ba, G.N.; Mathe, G. Polyphenols: Do they play a role in the prevention of human pathologies? Biomed. Pharmacother. 2002, 56, 200–207. [Google Scholar] [CrossRef]
- Che, C.T.; Wang, Z.J.; Chow, M.S.; Lam, C.W. Herb-herb combination for therapeutic enhancement and advancement: Theory, practice and future perspectives. Molecules 2013, 18, 5125–5141. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Joo, H.G. Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol. Lett. 2008, 115, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Aida, W.M.W.; Maskat, M.Y.; Mamot, S.; Ropien, J.; Mohd, D.M. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocoll. 2014, 42, 280–288. [Google Scholar] [CrossRef]
- Synytsya, A.; Bleha, R.; Synytsya, A.; Pohl, R.; Hayashi, K.; Yoshinaga, K.; Nakano, T.; Hayashi, T. Mekabu fucoidan: Structural complexity and defensive effects against avian influenza A viruses. Carbohydr. Polym. 2014, 111, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Chandia, N.P.; Matsuhiro, B. Characterization of a fucoidan from Lessonia vadosa (Phaeophyta) and its anticoagulant and elicitor properties. Int. J. Biol. Macromol. 2008, 42, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ko, C.I.; Ahn, G.; You, S.; Kim, J.S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.J. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Matou, S.; Helley, D.; Chabut, D.; Bros, A.; Fischer, A.M. Effect of fucoidan on fibroblast growth factor-2-induced angiogenesis in vitro. Thromb. Res. 2002, 106, 213–221. [Google Scholar] [CrossRef]
- McCaffrey, T.A.; Falcone, D.J.; Vicente, D.; Du, B.; Consigli, S.; Borth, W. Protection of transforming growth factor-beta 1 activity by heparin and fucoidan. J. Cell. Physiol. 1994, 159, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Colliec, S.; Fischer, A.M.; Tapon-Bretaudiere, J.; Boisson, C.; Durand, P.; Jozefonvicz, J. Anticoagulant properties of a fucoidan fraction. Thromb. Res. 1991, 64, 143–154. [Google Scholar] [CrossRef]
- Freguin-Bouilland, C.; Alkhatib, B.; David, N.; Lallemand, F.; Henry, J.P.; Godin, M.; Thuillez, C.; Plissonnier, D. Low molecular weight fucoidan prevents neointimal hyperplasia after aortic allografting. Transplantation 2007, 83, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Deux, J.F.; Meddahi-Pelle, A.; Le Blanche, A.F.; Feldman, L.J.; Colliec-Jouault, S.; Bree, F.; Boudghene, F.; Michel, J.B.; Letourneur, D. Low molecular weight fucoidan prevents neointimal hyperplasia in rabbit iliac artery in-stent restenosis model. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Luyt, C.E.; Meddahi-Pelle, A.; Ho-Tin-Noe, B.; Colliec-Jouault, S.; Guezennec, J.; Louedec, L.; Prats, H.; Jacob, M.P.; Osborne-Pellegrin, M.; Letourneur, D.; et al. Low-molecular-weight fucoidan promotes therapeutic revascularization in a rat model of critical hindlimb ischemia. J. Pharmacol. Exp. Ther. 2003, 305, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Ku, S.K.; Han, J.S. Genotoxicity testing of low molecular weight fucoidan from brown seaweeds. Food Chem. Toxicol. 2012, 50, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Ku, S.K.; Kim, H.J.; Han, J.S. Low molecular weight fucoidan ameliorating the chronic cisplatin-induced delayed gastrointestinal motility in rats. Food Chem. Toxicol. 2012, 50, 4468–4478. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Lee, O.H.; Lee, B.Y. Genotoxicity studies on fucoidan from Sporophyll of Undaria pinnatifida. Food Chem. Toxicol. 2010, 48, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Somboonwong, J.; Kankaisre, M.; Tantisira, B.; Tantisira, M.H. Wound healing activities of different extracts of Centella asiatica in incision and burn wound models: An experimental animal study. BMC Complement. Altern. Med. 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, S.; Sokolova, R.; Kim, S.M.; Um, B.H.; Isakov, V.; Zvyagintseva, T. Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: Structural characteristics and anticancer activity. Appl. Biochem. Biotechnol. 2011, 164, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, R.; Rerek, M.; Wood, E.J. Fucoidan modulates the effect of transforming growth factor (TGF)-beta1 on fibroblast proliferation and wound repopulation in in vitro models of dermal wound repair. Biol. Pharm. Bull. 2004, 27, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.D.; Hatipoglu, F.; Cevher, E.; Ogurtan, Z.; Bas, A.L.; Akbuga, J. Chitosan film containing fucoidan as a wound dressing for dermal burn healing: Preparation and in vitro/in vivo evaluation. AAPS PharmSciTech 2007, 8. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.D.; Cevher, E.; Hatipoglu, F.; Ogurtan, Z.; Bas, A.L.; Akbuga, J. The use of fucosphere in the treatment of dermal burns in rabbits. Eur. J. Pharm. Biopharm. 2008, 69, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Jang, K.H.; Park, S.C.; Jin, H.K. Promotion of dermal wound healing by polysaccharides isolated from Phellinus gilvus in rats. J. Vet. Med. Sci. 2005, 67, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Froget, S.; Barthelemy, E.; Guillot, F.; Soler, C.; Coudert, M.C.; Benbunan, M.; Dosquet, C. Wound healing mediator production by human dermal fibroblasts grown within a collagen-GAG matrix for skin repair in humans. Eur. Cytokine Netw. 2003, 14, 60–64. [Google Scholar] [PubMed]
- Peluso, G.; Petillo, O.; Ranieri, M.; Santin, M.; Ambrosio, L.; Calabro, D.; Avallone, B.; Balsamo, G. Chitosan-mediated stimulation of macrophage function. Biomaterials 1994, 15, 1215–1220. [Google Scholar] [CrossRef]
- Yanagisawa-Miwa, A.; Uchida, Y.; Nakamura, F.; Tomaru, T.; Kido, H.; Kamijo, T.; Sugimoto, T.; Kaji, K.; Utsuyama, M.; Kurashima, C.; et al. Salvage of infarcted myocardium by angiogenic action of basic fibroblast growth factor. Science 1992, 257, 1401–1403. [Google Scholar] [CrossRef] [PubMed]
- Salvucci, O.; Yao, L.; Villalba, S.; Sajewicz, A.; Pittaluga, S.; Tosato, G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood 2002, 99, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, U.; Bertsch, T.; Dvortsak, E.; Liebetrau, C.; Lang, S.; Liebe, V.; Huhle, G.; Borggrefe, M.; Brueckmann, M. Matrix-metalloproteinases and their inhibitors are elevated in severe sepsis: Prognostic value of TIMP-1 in severe sepsis. Scand. J. Infect. Dis. 2006, 38, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Idrovo, J.P.; Yang, W.L.; Jacob, A.; Ajakaiye, M.A.; Cheyuo, C.; Wang, Z.; Prince, J.M.; Nicastro, J.; Coppa, G.F.; Wang, P. Combination of adrenomedullin with its binding protein accelerates cutaneous wound healing. PLoS ONE 2015, 10, e0120225. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, E.A.; Lortat-Jacob, H.; Priestley, G.V.; Nakamoto, B.; Papayannopoulou, T. Sulfated polysaccharides increase plasma levels of SDF-1 in monkeys and mice: Involvement in mobilization of stem/progenitor cells. Blood 2002, 99, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.S.; Fidelis, G.P.; Telles, C.B.; Dantas-Santos, N.; Camara, R.B.; Cordeiro, S.L.; Costa, M.S.; Almeida-Lima, J.; Melo-Silveira, R.F.; Oliveira, R.M.; et al. Antioxidant and antiproliferative activities of heterofucans from the seaweed Sargassum filipendula. Mar. Drugs 2011, 9, 952–966. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Zhang, J.; Li, P. Synthesized phosphorylated and aminated derivatives of fucoidan and their potential antioxidant activity in vitro. Int. J. Biol. Macromol. 2009, 44, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Atiba, A.; Ueno, H.; Uzuka, Y. The effect of aloe vera oral administration on cutaneous wound healing in type 2 diabetic rats. J. Vet. Med. Sci. 2011, 73, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Vodovotz, Y.; Bogdan, C. Control of nitric oxide synthase expression by transforming growth factor-beta: Implications for homeostasis. Prog. Growth Factor Res. 1994, 5, 341–351. [Google Scholar] [CrossRef]
- Murphy, P.S.; Evans, G.R. Advances in wound healing: A review of current wound healing products. Plast. Surg. Int. 2012, 2012, 190436. [Google Scholar] [CrossRef] [PubMed]
- Schwentker, A.; Vodovotz, Y.; Weller, R.; Billiar, T.R. Nitric oxide and wound repair: Role of cytokines? Nitric Oxide 2002, 7, 1–10. [Google Scholar] [CrossRef]
- Senni, K.; Gueniche, F.; Foucault-Bertaud, A.; Igondjo-Tchen, S.; Fioretti, F.; Colliec-Jouault, S.; Durand, P.; Guezennec, J.; Godeau, G.; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006, 445, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.R.; Kim, J.O.; Lee, J.H.; Kim, Y.I.; Kim, J.H.; Chang, S.W.; Jin, S.G.; Kim, J.A.; Lyoo, W.S.; Han, S.S.; et al. Gentamicin-loaded wound dressing with polyvinyl alcohol/dextran hydrogel: Gel characterization and in vivo healing evaluation. AAPS PharmSciTech 2010, 11, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lim, S.J.; Oh, D.H.; Ku, S.K.; Li, D.X.; Yong, C.S.; Choi, H.G. Wound healing evaluation of sodium fucidate-loaded polyvinylalcohol/sodium carboxymethylcellulose-based wound dressing. Arch. Pharm. Res. 2010, 33, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Park, S.J.; Lee, Y.J.; Lee, J.E.; Song, C.H.; Choi, S.H.; Ku, S.K.; Kang, S.J. Inhibition of UVB-induced skin damage by exopolymers from Aureobasidium pullulans SM-2001 in hairless mice. Basic Clin. Pharmacol. Toxicol. 2015, 116, 73–86. [Google Scholar] [CrossRef] [PubMed]

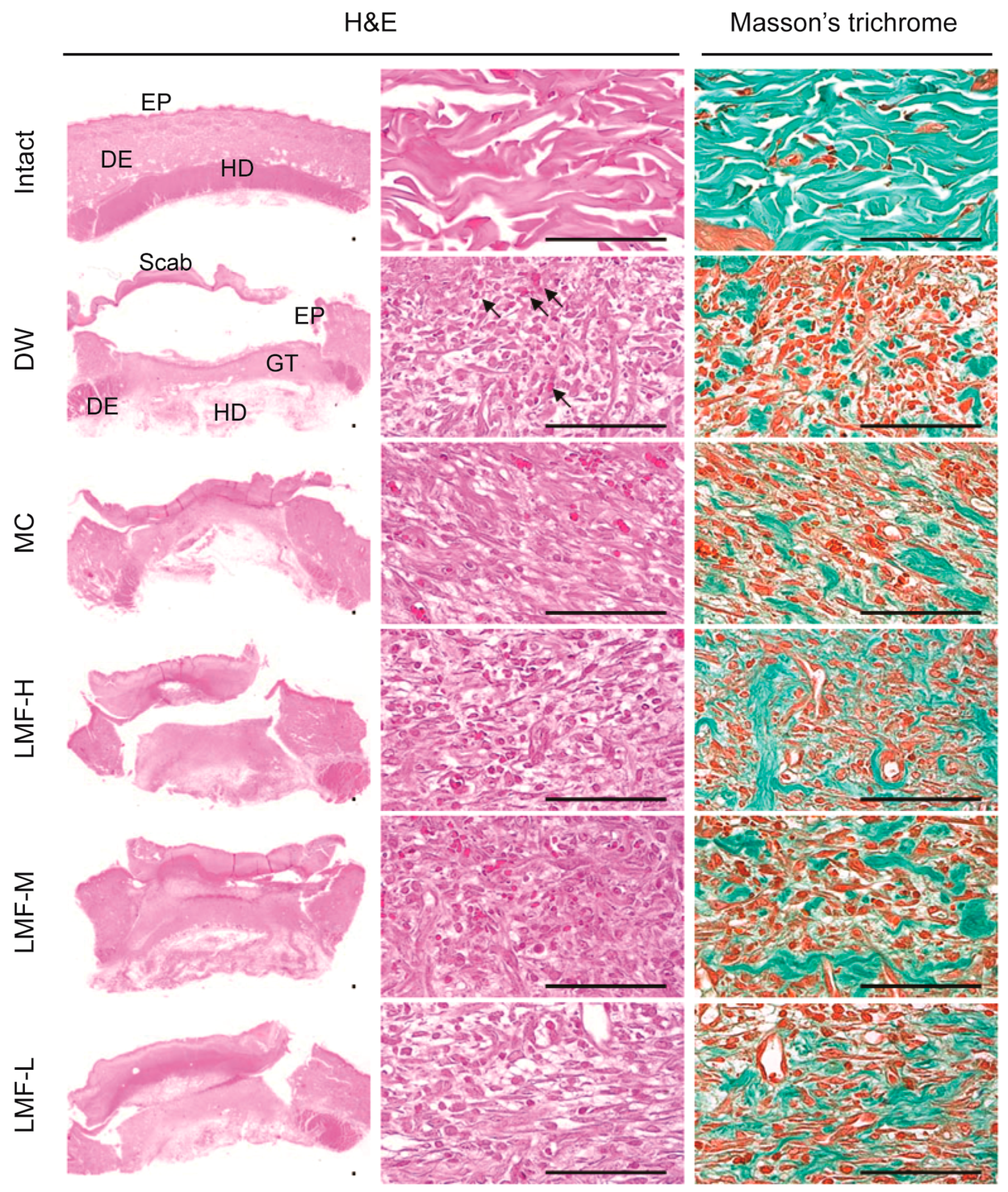


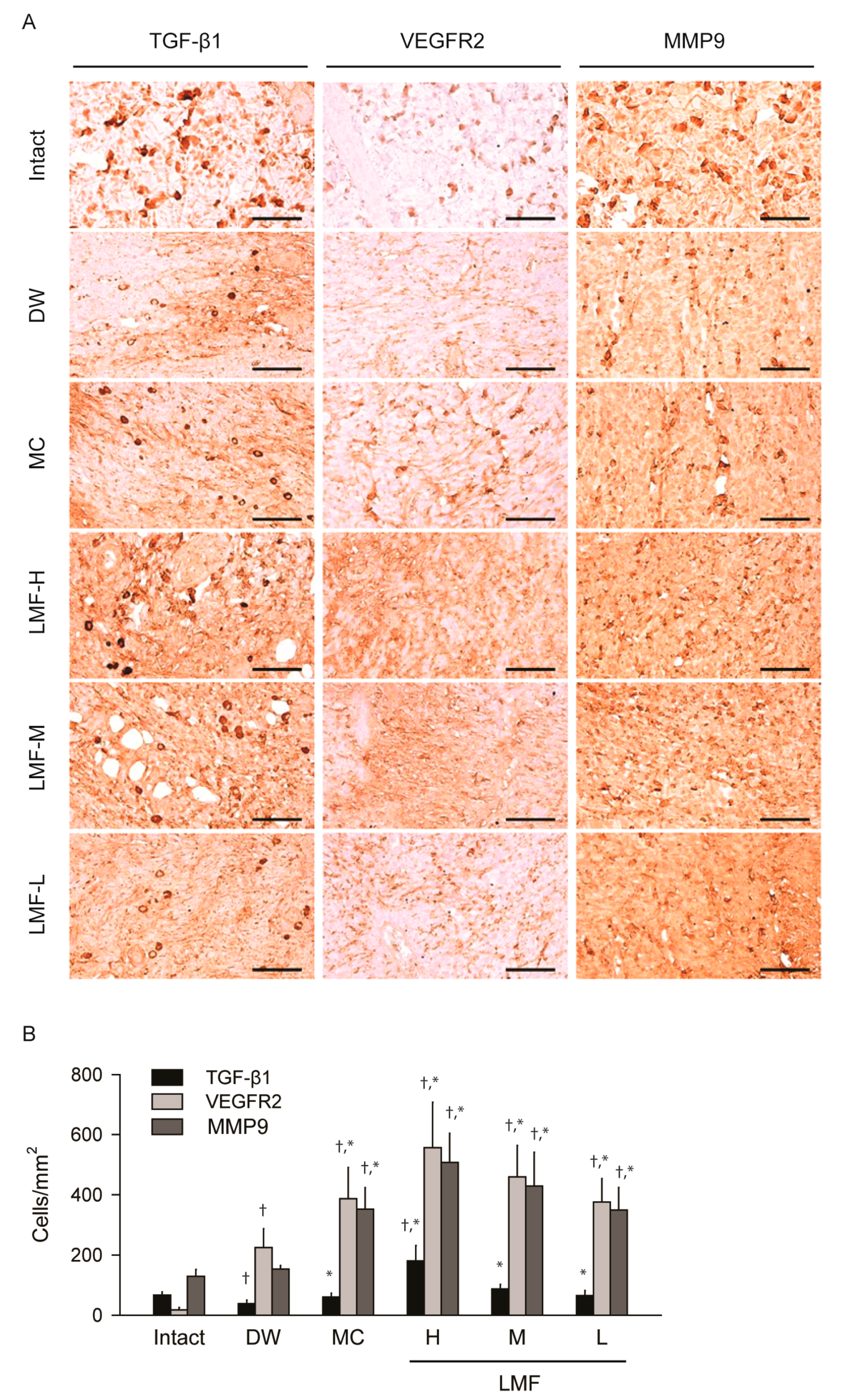
| Group | Defected Epithelium (mm) | Granulation Tissue of Dermal Wound or Intact Dermis | |||
|---|---|---|---|---|---|
| Area (mm2) | Microvessels (n/mm2) | IF Cells (n/mm2) | Collagen Tissue (mm2) | ||
| Intact | NA | NA | 4.30 ± 2.31 | 7.80 ± 2.78 | 65.01 ± 11.20 |
| DW | 5.89 ± 0.42 | 4.15 ± 0.13 | 56.30 ± 18.82 † | 291.10 ± 135.79 † | 21.76 ± 6.79 † |
| MC | 4.59 ± 0.75 * | 4.53 ± 0.32 | 159.10 ± 39.40 †,* | 176.20 ± 30.31 † | 37.72 ± 11.03 †,* |
| LMF-H | 4.28 ± 0.47 * | 5.37 ± 0.62 * | 228.30 ± 39.65 †,* | 84.80 ± 11.46 †,* | 43.68 ± 10.22 †,* |
| LMF-M | 4.28 ± 0.47 * | 5.20 ± 0.68 * | 169.70 ± 21.02 †,* | 130.30 ± 27.42 †,* | 40.45 ± 10.38 †,* |
| LMF-L | 5.14 ± 0.54 * | 5.08 ± 0.55 * | 134.70 ± 25.18 †,* | 171.70 ± 17.58 †,* | 37.93 ± 10.54 †,* |
| Group | Defected Epithelium (mm) | Granulation Tissue of Dermal Wound or Intact Dermis | |||
|---|---|---|---|---|---|
| Area (mm2) | Microvessels (n/mm2) | IF Cells (n/mm2) | Collagen Tissue (mm2) | ||
| Intact | NA | NA | 6.40 ± .95 | 7.90 ± 3.07 | 68.27 ± 10.24 |
| DW | 2.98 ± 0.55 | 5.77 ± 1.21 | 135.90 ± 23.84 † | 151.80 ± 46.24 † | 32.64 ± 10.36 † |
| MC | 1.94 ± 0.31 * | 3.76 ± 1.27 * | 187.40 ± 19.80 †,* | 85.50 ± 29.52 †,* | 45.25 ± 4.39 †,* |
| LMF-H | 0.44 ± 0.27 * | 2.32 ± 0.43 * | 225.60 ± 19.39 †,* | 27.00 ± 10.83 †,* | 55.45 ± 11.00 †,* |
| LMF-M | 1.21 ± 0.33 * | 2.56 ± 0.81 * | 209.30 ± 29.36 †,* | 49.10 ± 12.21 †,* | 51.16 ± 11.80 †,* |
| LMF-L | 1.79 ± 0.44 * | 3.82 ± 0.94 * | 187.20 ± 23.30 †,* | 87.40 ± 32.53 †,* | 45.06 ± 7.51 †,* |
| Group | MDA (nM/mg Protein) | GSH (μg/mg Protein) | SOD (U/mg Protein) | CAT (U/mg Protein) |
|---|---|---|---|---|
| Day 4 | ||||
| Intact | 2.35 ± 0.55 | 2.61 ± 0.49 | 1.89 ± 0.25 | 6.75 ± 0.93 |
| DW | 4.36 ± 0.65 † | 1.06 ± 0.23 † | 1.05 ± 0.18 † | 4.61 ± 0.67 † |
| MC | 3.13 ± 0.34 †,* | 1.88 ± 0.21 †,* | 1.54 ± 0.20 †,* | 5.43 ± 0.41 † |
| LMF-H | 2.91 ± 0.20 * | 2.17 ± 0.28 †,* | 1.77 ± 0.15 * | 5.85 ± 0.64 * |
| LMF-M | 3.10 ± 0.25 †,* | 1.90 ± 0.37 †,* | 1.53 ± 0.13 †,* | 5.60 ± 0.44 * |
| LMF-L | 3.40 ± 0.43 † | 1.69 ± 0.29 †,* | 1.40 ± 0.26 †,* | 5.26 ± 0.20 † |
| Day 7 | ||||
| Intact | 2.38 ± 0.52 | 2.67 ± 0.43 | 1.84 ± 0.24 | 6.89 ± 0.79 |
| DW | 4.88 ± 1.23 † | 1.17 ± 0.27 † | 1.11 ± 0.17 † | 4.89 ± 0.36 † |
| MC | 3.40 ± 0.50 †,* | 2.16 ± 0.21 * | 1.63 ± 0.16 †,* | 5.71 ± 0.64 †,* |
| LMF-H | 2.89 ± 0.48 * | 2.51 ± 0.30 * | 2.02 ± 0.17 †,* | 6.75 ± 0.64 * |
| LMF-M | 3.10 ± 0.29 * | 2.28 ± 0.21 * | 1.78 ± 0.15 * | 6.27 ± 0.40 †,* |
| LMF-L | 3.37 ± 0.45 † | 2.17 ± 0.22 * | 1.65 ± 0.24 †,* | 5.84 ± 0.57 †,* |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-H.; Choi, S.-H.; Park, S.-J.; Lee, Y.J.; Park, J.H.; Song, P.H.; Cho, C.-M.; Ku, S.-K.; Song, C.-H. Promoting Wound Healing Using Low Molecular Weight Fucoidan in a Full-Thickness Dermal Excision Rat Model. Mar. Drugs 2017, 15, 112. https://doi.org/10.3390/md15040112
Park J-H, Choi S-H, Park S-J, Lee YJ, Park JH, Song PH, Cho C-M, Ku S-K, Song C-H. Promoting Wound Healing Using Low Molecular Weight Fucoidan in a Full-Thickness Dermal Excision Rat Model. Marine Drugs. 2017; 15(4):112. https://doi.org/10.3390/md15040112
Chicago/Turabian StylePark, Jun-Hyeong, Seong-Hun Choi, Soo-Jin Park, Young Joon Lee, Jong Hyun Park, Phil Hyun Song, Chang-Mo Cho, Sae-Kwang Ku, and Chang-Hyun Song. 2017. "Promoting Wound Healing Using Low Molecular Weight Fucoidan in a Full-Thickness Dermal Excision Rat Model" Marine Drugs 15, no. 4: 112. https://doi.org/10.3390/md15040112