Effects of Low-Molecular-Weight Fucoidan and High Stability Fucoxanthin on Glucose Homeostasis, Lipid Metabolism, and Liver Function in a Mouse Model of Type II Diabetes
Abstract
:1. Introduction
2. Results and Discussion
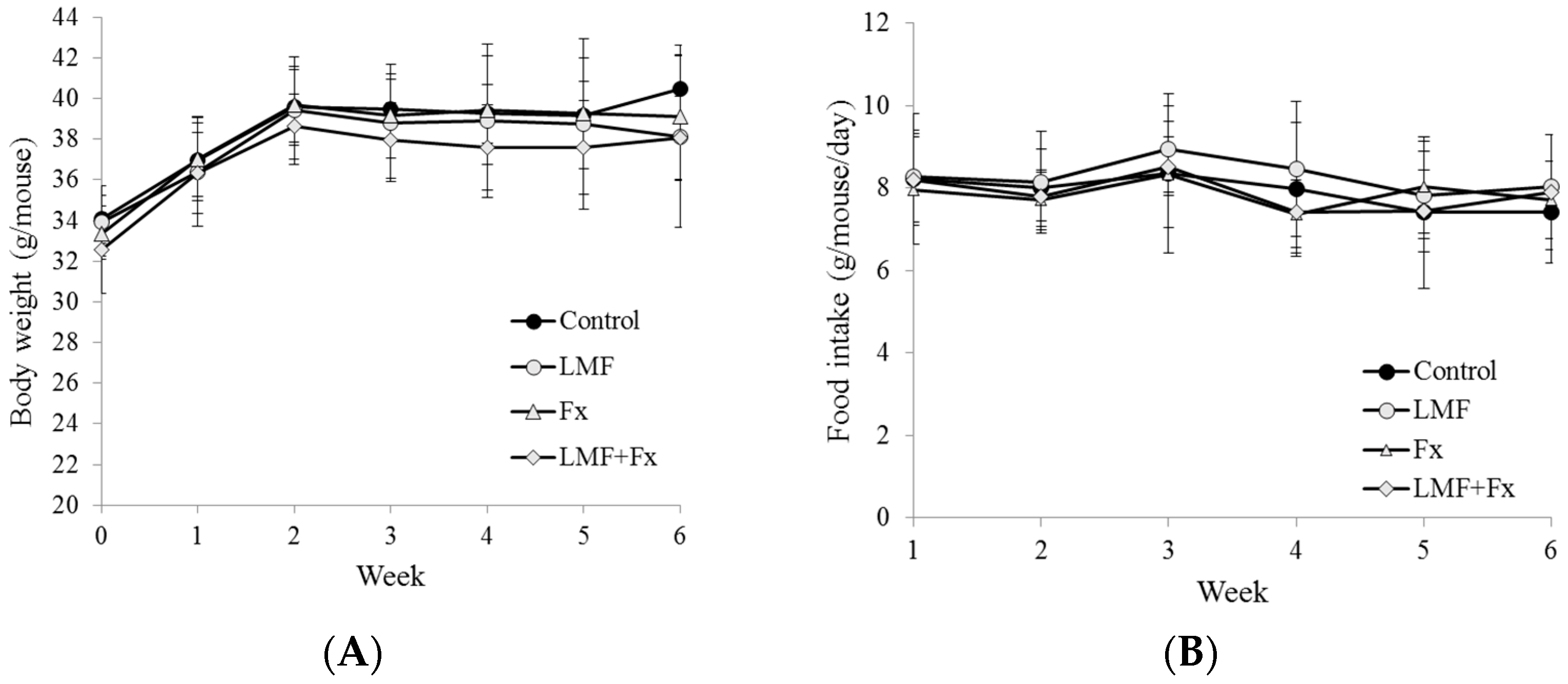
2.1. Effect on the Body Weight and Food Intake
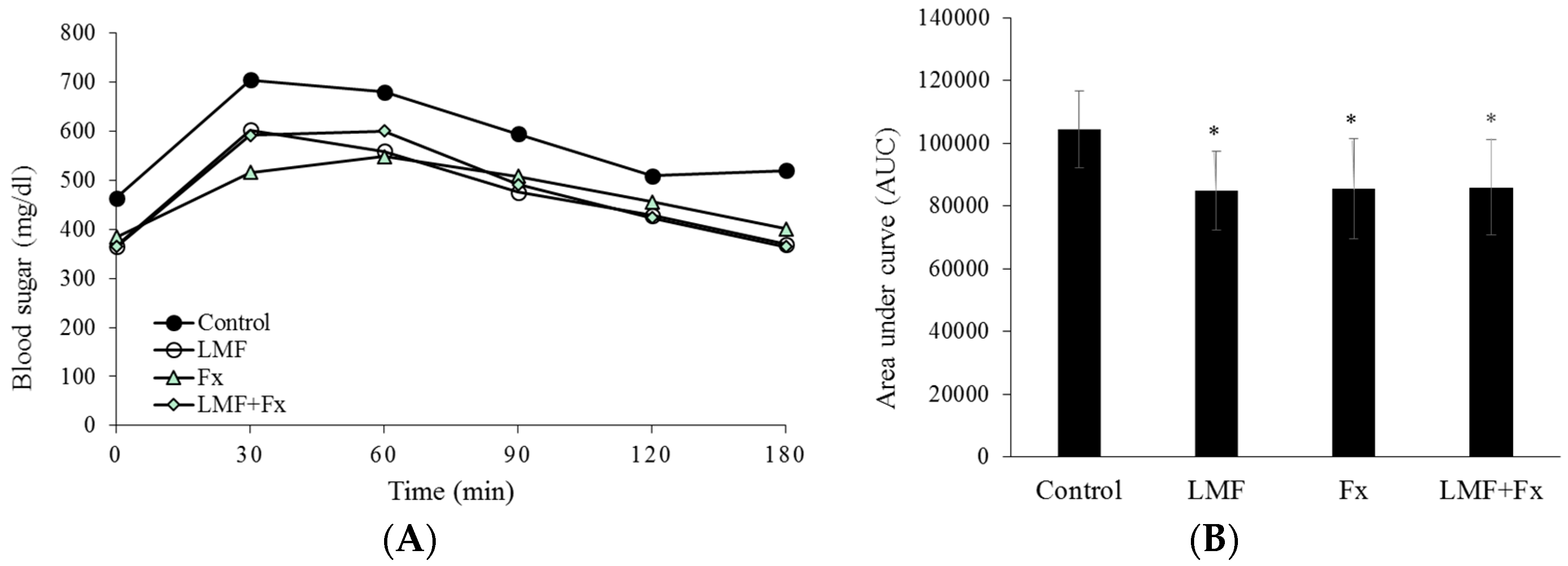
2.2. Reduction in Insulin Resistance
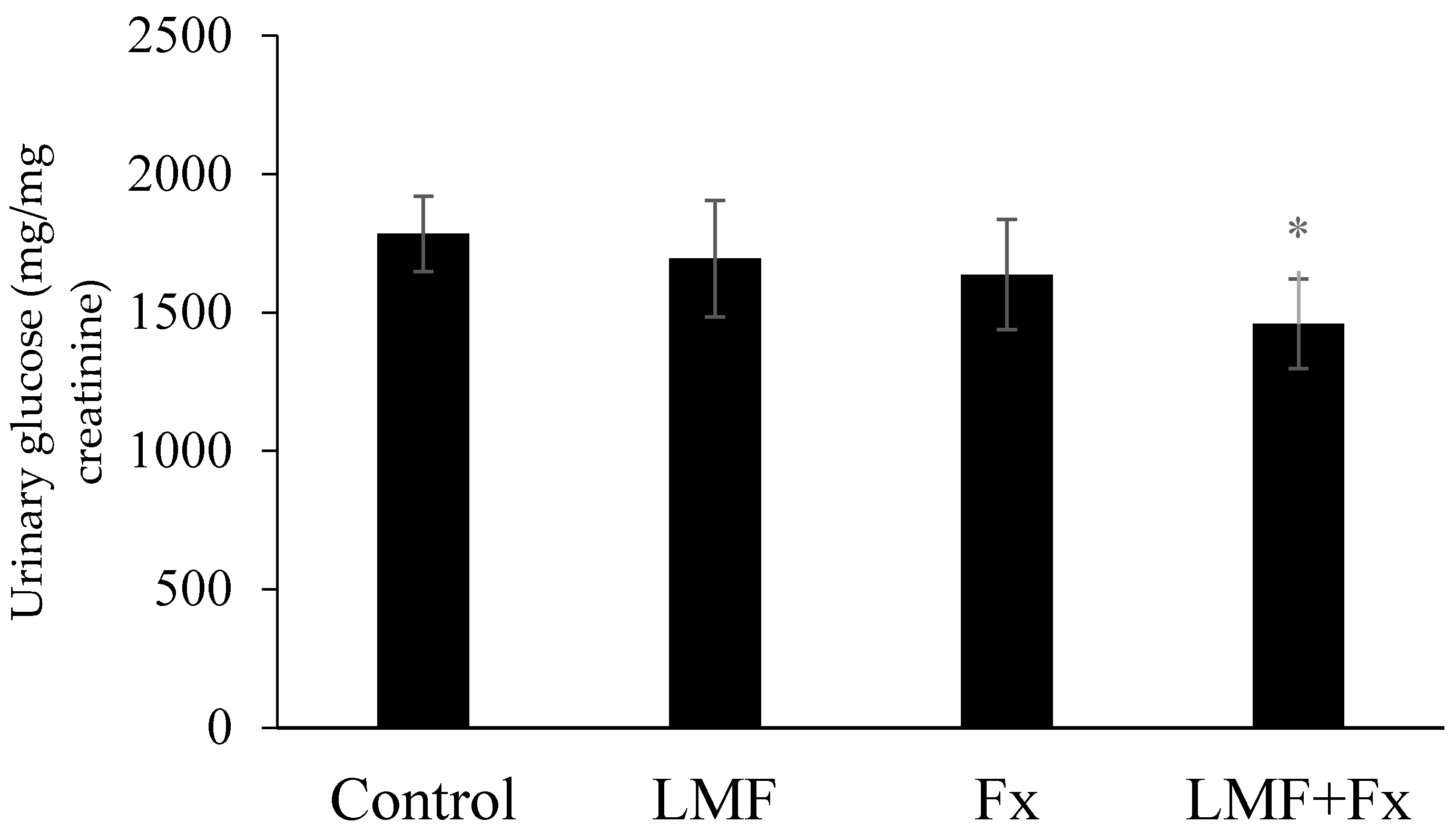
2.3. Reduction in Urinary Glucose Level
2.4. Effects on Hepatic Glycogen Levels and Hepatic Functions
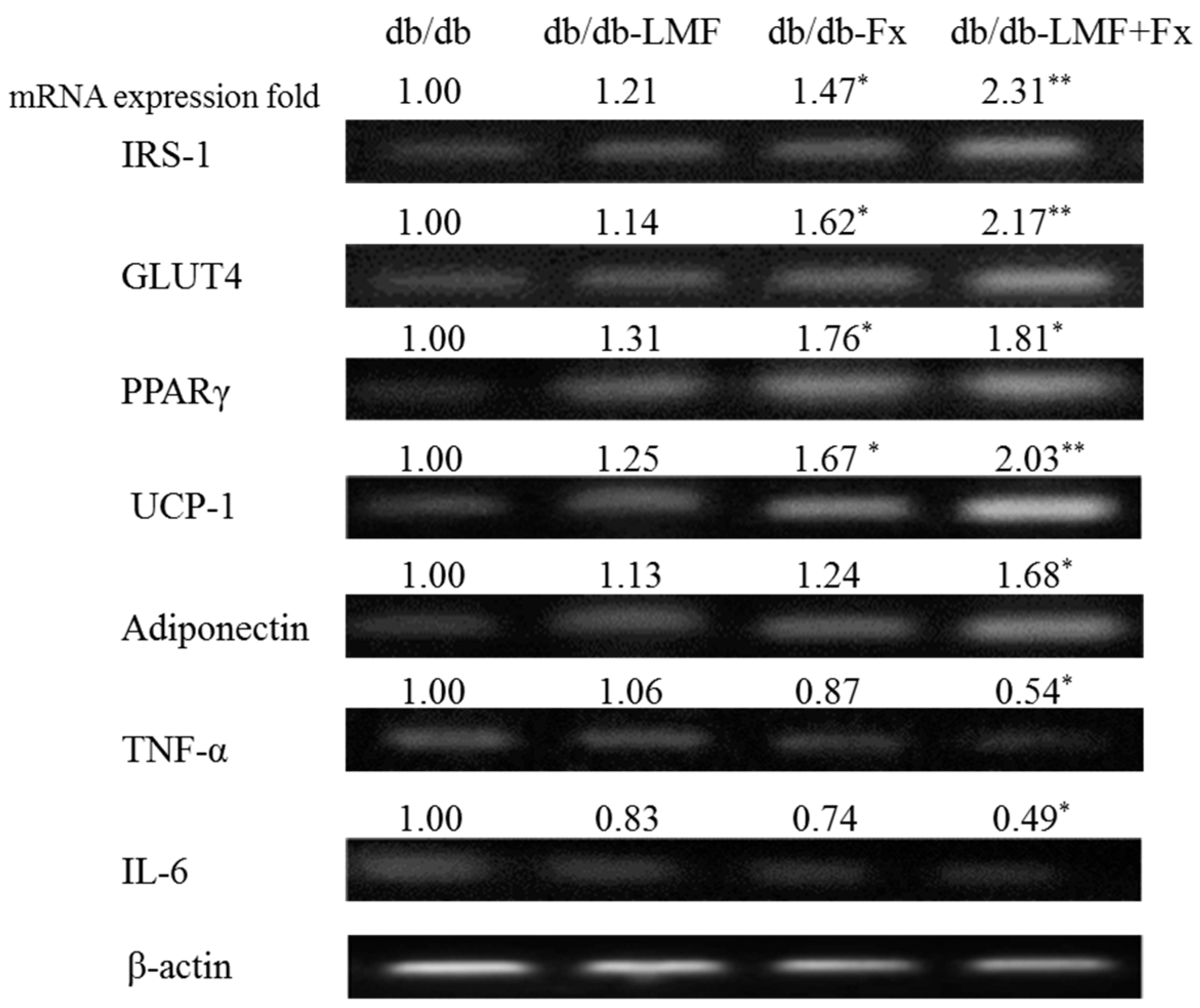
2.5. Effect on mRNA Expression Related to Glucose and Lipid Metabolism in the Adipose Tissue
3. Materials and Methods
3.1. Preparation of Low Molecular Weight Fucoidan (LMF) and High Stability Fucoxanthin (Fx)
3.2. Animal and Diets
3.3. Intraperitoneal Glucose Tolerance Analysis
3.4. Fasting Blood Sugar, Serum Insulin, and Adiponectin Analysis
3.5. Homeostatic Index of Insulin Resistance (HOMA-IR) and Homeostatic Index of β-Cell Function (HOMA-β)
3.6. Hepatic Enzymes Analysis
3.7. Hepatic Glycogen Analysis
3.8. Urinary Glucose Analysis
3.9. mRNA Analysis
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rossetti, L.; Giaccari, A.; DeFronzo, R.A. Glucose toxicity. Diabetes Care 1990, 13, 610–630. [Google Scholar] [CrossRef] [PubMed]
- Ritz, E.; Rychlik, I.; Locatelli, F.; Halimi, S. End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am. J. Kidney Dis. 1999, 34, 795–808. [Google Scholar] [CrossRef]
- Baron, A.D. Postprandial hyperglycaemia and alpha-glucosidase inhibitors. Diabetes Res. Clin. Pract. 1998, 40, S51–S55. [Google Scholar] [CrossRef]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Lehrke, M.; Hendler, R.E.; Shulman, G.I. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005, 54, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Goyal, B.R.; Mehta, A.A. Diabetic cardiomyopathy: Pathophysiological mechanisms and cardiac dysfuntion. Hum. Exp. Toxicol. 2013, 32, 571–590. [Google Scholar] [CrossRef] [PubMed]
- Raz, I. Guideline approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care 2013, 36, S139–S144. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the study of diabetes. Diabetes Care 2009, 32, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Eidi, A.; Eidi, M.; Esmaeili, E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine 2006, 13, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, M.V.; Singh, S.; Chhipa, R.R.; Bhat, M.K. The hypoglycaemic activity of fenugreek seed extract is mediated through the stimulation of an insulin signalling pathway. Br. J. Pharmacol. 2005, 146, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Hsieh, C.L.; Wang, H.Y.; Chen, H.Y. Inhibitory effects of guava (Psidium guajava L.) leaf extracts and its active compounds on the glycation process of protein. Food Chem. 2009, 113, 78–84. [Google Scholar] [CrossRef]
- Wu, C.H.; Lin, H.T.; Wu, G.J.; Wang, S.H.; Tsai, G.J. Effects of cultural medium and conditions on the proliferation and hypoglycemic activity of Saccharomyces pastorianus no. 54. J. Biosci. Bioeng. 2011, 112, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Jain, S.; Sinha, P.R. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition 2007, 23, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Su, C.F.; Liu, I.M.; Cheng, J.T. Improvement of insulin resistance by Hon-Chi in fructose-rich chow-fed rats. Food Chem. 2007, 104, 45–52. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, X.; Hu, Y.S.; Wu, Y.; Wang, Q.Z.; Li, N.N.; Guo, Q.C.; Dong, X.C. Evaluation of in vivo antioxidant activities of Ganoderma lucidum polysaccharides in STZ-diabetic rats. Food Chem. 2009, 115, 32–36. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Yang, H.; Jeon, Y.J.; Lee, C.J.; Jin, Y.H.; Baek, N.I.; Kim, D.; Kang, S.M.; Yoon, M.; Yong, H.; et al. Phlorotannins of the edible brown seaweed Ecklonia cava Kjellman induce sleep via positive allosteric modulation of gamma-aminobutyric acid type A-benzodiazepine receptor: A novel neurological activity of seaweed polyphenols. Food Chem. 2012, 132, 1133–1142. [Google Scholar] [CrossRef]
- Audibert, L.; Fauchon, M.; Blanc, N.; Hauchard, D.; Gall, E.A. Phenolic compounds in the brown seaweed Ascophyllum nodosum: Distribution and radical-scavenging activities. Phytochem. Anal. 2010, 21, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.Y.; Kim, G.Y.; Choi, I.W.; Kim, N.D.; Nam, T.J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-kappaB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.T.V.; Lu, W.J.; Tsai, G.J.; Chou, C.T.; Hsiao, H.I.; Hwang, P.A. Enhanced anti-inflammatory activity of brown seaweed Laminaria japonica by fermentation using Bacillus subtilis. Process Biochem. 2016, 51, 1945–1953. [Google Scholar] [CrossRef]
- Huang, L.; Wen, K.; Gao, X.; Liu, Y. Hypolipidemic effect of fucoidan from Laminaria japonica in hyperlipidemic rats. Pharm. Biol. 2010, 48, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Lin, T.Y.; Wu, Y.C.; Tsao, S.M.; Hwang, P.A.; Shih, Y.W.; Hsu, J. Fucoidan inhibition of lung cancer in vivo and in vitro: Role of the Smurf2-dependent ubiquitin proteasome pathway in TGFbeta receptor degradation. Oncotarget 2014, 5, 7870–7885. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Hung, Y.L.; Phan, N.N.; Hieu, B.T.; Chang, P.M.; Li, K.L.; Lin, Y.C. The in vitro and in vivo effects of the low molecular weight fucoidan on the bone osteogenic differentiation properties. Cytotechnology 2016, 68, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.T.; Kim, Y.D.; Jung, Y.M.; Park, D.C.; Lee, D.S.; Ku, S.K.; Li, X.; Lu, Y.; Chao, G.H.; Kim, K.J.; et al. Low molecular weight fucoidan improves endoplasmic reticulum stress-reduced insulin sensitivity through AMP-activated protein kinase activation in L6 myotubes and restores lipid homeostasis in a mouse model of type 2 diabetes. Mol. Pharmacol. 2013, 84, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Murakami-Funayama, K.; Miyashita, K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol. Med. Rep. 2009, 2, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Hung, Y.L.; Tsai, Y.K.; Chien, S.Y.; Kong, Z.L. The brown seaweed Sargassum hemiphyllum exhibits alpha-amylase and alpha-glucosidase inhibitory activity and enhances insulin release in vitro. Cytotechnology 2015, 67, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Shimomura, I.; Kihara, S.; Funahashi, T. Importance of adipocytokines in obesity-related diseases. Horm. Res. 2003, 60 (Suppl. 3), 56–59. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Minehira, K. New data and new concepts on the role of the liver in glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Cotrozzi, G.; Casini Raggi, V.; Relli, P.; Buzzelli, G. Role of the liver in the regulation of glucose metabolism in diabetes and chronic liver disease. Ann. Ital. Med. Int. 1997, 12, 84–91. [Google Scholar] [PubMed]
- Tolman, K.G.; Fonseca, V.; Dalpiaz, A.; Tan, M.H. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care 2007, 30, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Charlat, O.; Tartaglia, L.A.; Woolf, E.A.; Weng, X.; Ellis, S.J.; Lakey, N.D.; Culpepper, J.; Moore, K.J.; Breitbart, R.E.; et al. Evidence that the diabetes gene encodes the leptin receptor: Identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996, 84, 491–495. [Google Scholar] [CrossRef]
- Cantello, B.C.C.; Cawthorne, M.A.; Haigh, D.; Hindley, R.M.; Smith, S.A.; Thurlby, P. The synthesis of BRL 49653—A novel and potent antihyperglycemic agent. Bioorg. Med. Chem. Lett. 1997, 4, 1181–1184. [Google Scholar] [CrossRef]
- Lee, S.H.; Min, K.H.; Han, J.S.; Lee, D.H.; Park, D.B.; Jung, W.K.; Park, P.J.; Jeon, B.T.; Kim, S.K.; Jeon, Y.J. Effects of brown alga, Ecklonia cava on glucose and lipid metabolism in C57BL/KsJ-db/db mice, a model of type 2 diabetes mellitus. Food Chem. Toxicol. 2012, 50, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.K.; Inzucchi, S.E. Investigational anti-hyperglycemic agents: The future of type 2 diabetes therapy? Endocrine 2013, 44, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Yoon, K.Y.; Lee, B.Y. Fucoidan regulate blood glucose homeostasis in C57BL/KSJ m+/+db and C57BL/KSJ db/db mice. Fitoterapia 2012, 83, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Han, B.R.; Oh, Y.S.; Ahn, K.H.; Kim, H.Y.; Hong, S.C.; Oh, M.J.; Kim, H.J.; Kim, Y.T.; Lee, K.W.; Kim, S.H. Clinical implication of 2nd trimester glycosuria. Korean J. Perinatol. 2010, 21, 258–265. [Google Scholar]
- Ben Rebah, F.; Smaoui, S.; Frikha, F.; Gargouri, Y.; Miled, N. Inhibitory effects of Tunisian marine algal extracts on digestive lipases. Appl. Biochem. Biotechnol. 2008, 151, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Chamundeswaramma, K.V.; Das, P.; Rathnamma, V.V.; Philip, G.H. Activity levels of aspartate transaminase (AAT) and alaninetransaminase (ALAT) in freshwater fish Labeo rohita exposed to deltamethrin. Ecol. Environ. Conserv. 2010, 16, 145–148. [Google Scholar]
- Basu, A.; Basu, R.; Shah, P.; Vella, A.; Johnson, C.M.; Jensen, M.; Nair, K.S.; Schwenk, W.F.; Rizza, R.A. Type 2 diabetes impairs splanchnic uptake of glucose but does not alter intestinal glucose absorption during enteral glucose feeding: Additional evidence for a defect in hepatic glucokinase activity. Diabetes 2001, 50, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Mori, D.M.; Baviera, A.M.; Ramalho, L.T.D.; Vendramini, R.C.; Brunetti, I.L.; Pepato, M.T. Temporal response pattern of biochemical analytes in experimental diabetes. Biotechnol. Appl. Biochem. 2003, 38, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Heeba, G.H.; Morsy, M.A. Fucoidan ameliorates steatohepatitis and insulin resistance by suppressing oxidative stress and inflammatory cytokines in experimental non-alcoholic fatty liver disease. Environ. Toxicol. Pharm. 2015, 40, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D. Obesity: Metabolically healthy obesity and NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Chen, X.; Chan, C.Y.; Li, D.; Yuan, C.; Yu, F.; Lin, M.C.; Yew, D.T.; Kung, H.F.; Lai, L. Two-dimensional differential gel electrophoresis/analysis of diethylnitrosamine induced rat hepatocellular carcinoma. Int. J. Cancer 2008, 122, 2682–2688. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, W.; Wang, B.; Zhu, H.; Ye, L.; Feng, M. Antioxidant effect of apolipoprotein A-I on high-fat diet-induced non-alcoholic fatty liver disease in rabbits. Acta Biochim. Biophys. Sin. 2013, 45, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.W.; Xia, G.H.; Wang, J.F.; Wang, Y.M.; Li, Z.J.; Xue, C.H. Fucoidan from sea cucumber protects against high-fat high-sucrose diet-induced hyperglycaemia and insulin resistance in mice. J. Funct. Foods 2014, 10, 128–138. [Google Scholar] [CrossRef]
- Henry, R.R. Glucose control and insulin resistance in non-insulin-dependent diabetes mellitus. Ann. Intern. Med. 1996, 124, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Veilleux, A.; Blouin, K.; Rheaume, C.; Daris, M.; Marette, A.; Tchernof, A. Glucose transporter 4 and insulin receptor substrate-1 messenger RNA expression in omental and subcutaneous adipose tissue in women. Metabolism. 2009, 58, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.; Jansson, P.A.; Nagaev, I.; Wenthzel, A.M.; Smith, U. Insulin resistance with low cellular IRS-1 expression is also associated with low GLUT4 expression and impaired insulin-stimulated glucose transport. FASEB J. 2001, 15, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Graves, R.A.; Budavari, A.I.; Erdjument-Bromage, H.; Lui, M.; Hu, E.; Tempst, P.; Spiegelman, B.M. Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors, PPAR gamma and RXR alpha. Nucleic Acids Res. 1994, 22, 5628–5634. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, T.; Rakugi, H.; Ikegami, H.; Mikami, H.; Masuo, K. Enhancement of insulin sensitivity by troglitazone lowers blood pressure in diabetic hypertensives. Am. J. Hypertens. 1995, 8, 316–320. [Google Scholar] [CrossRef]
- Kelly, L.J.; Vicario, P.P.; Thompson, G.M.; Candelore, M.R.; Doebber, T.W.; Ventre, J.; Wu, M.S.; Meurer, R.; Forrest, M.J.; Conner, M.W.; et al. Peroxisome proliferator-activated receptors gamma and alpha mediate in vivo regulation of uncoupling protein (UCP-1, UCP-2, UCP-3) gene expression. Endocrinology 1998, 139, 4920–4927. [Google Scholar] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-Ay mice. J. Agric. Food Chem. 2007, 55, 7701–7706. [Google Scholar] [CrossRef] [PubMed]
- Ricquier, D.; Bouillaud, F. Mitochondrial uncoupling proteins: From mitochondria to the regulation of energy balance. J. Physiol. 2000, 529, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, C.; Lorenz, K.; Braithwaite, S.S.; Colca, J.R.; Palazuk, B.J.; Hotamisligil, G.S.; Spiegelman, B.M. Altered gene expression for tumor necrosis factor-alpha and its receptors during drug and dietary modulation of insulin resistance. Endocrinology 1994, 134, 264–270. [Google Scholar] [PubMed]
- Hosokawa, M.; Miyashita, T.; Nishikawa, S.; Emi, S.; Tsukui, T.; Beppu, F.; Okada, T.; Miyashita, K. Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK-Ay mice. Arch. Biochem. Biophys. 2010, 504, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Drevon, C.A. Fatty acids and expression of adipokines. Biochim. Biophys. Acta-Mol. Basis Dis. 2005, 1740, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Stauber, J.L.; Jeffrey, S.W. Photosynthetic pigments in fifty-one species of marine diatoms. J. Phycol. 1988, 24, 158–172. [Google Scholar] [CrossRef]
- Hwang, P.A.; Yan, M.D.; Lin, H.T.V.; Li, K.L.; Lin, Y.C. Toxicological evaluation of low molecular weight fucoidan in vitro and in vivo. Mar. Drugs 2016, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Seifter, S.; Dayton, S.; Novic, B.; Muntwyler, E. The estimation of glycogen with the anthrone reagent. Arch. Biochem. Biophys. 1950, 25, 191–200. [Google Scholar]
| Experiments and Indices | Control | LMF | Fx | LMF + Fx |
|---|---|---|---|---|
| Fasting blood sugar (mg/dL) | 475.18 ± 73.04 | 391.12 ± 47.60 * | 392.85 ± 70.27 * | 381.56 ± 57.67 * |
| Serum insulin (μU/mL) | 109.95 ± 10.10 | 99.49 ± 30.22 | 98.55 ± 19.00 | 96.82 ± 16.46 |
| Serum adiponectin (μg/mL) | 12.25 ± 3.40 | 24.21 ± 2.87 * | 22.34 ± 3.71 * | 27.67 ± 1.98 * |
| HOMA-IR | 130.02 ± 26.39 | 95.59 ± 30.22 * | 99.46 ± 34.49 * | 93.88 ± 23.48 * |
| HOMA-β | 97.69 ± 14.28 | 113.30 ± 14.64 | 107.17 ± 13.15 | 108.02 ± 20.59 |
| Experiments | Control | LMF | Fx | LMF + Fx |
|---|---|---|---|---|
| Hepatic glycogen (μg/mg liver) | 101.34 ± 8.87 | 120.56 ± 9.90 * | 110.25 ± 10.34 | 118.45 ± 7.43 * |
| ALT (IU/L) | 62.78 ± 6.25 | 51.56 ± 7.01 * | 59.90 ± 6.78 | 50.89 ± 7.25 * |
| AST (IU/L) | 60.79 ± 2.91 | 50.05 ± 9.32 * | 56.44 ± 3.90 | 53.60 ± 6.01 * |
| SOD (U/mg protein) | 31.11 ± 4.02 | 40.84 ± 3.61 * | 38.24 ± 3.17 * | 39.92 ± 3.08 * |
| CAT (μmmol/min/mg protein) | 2.31 ± 0.71 | 3.12 ± 0.47 * | 3.29 ± 0.39 * | 3.34 ± 0.52 * |
| Gene Name | Oligonucleotide Sequences | PCR Conditions |
|---|---|---|
| IRS-1 | F 1: AAGGCCAGCACCTTACCTCG | 1 min of 60 °C annealing |
| R 2: AGCCATGGTGGCCCTGGGCAG | 1 min of 72 °C extension | |
| GLUT4 | F: ACATACCTGACAGGGCAAGG | 1 min of 59 °C annealing |
| R: CGCCCTTAGTTGGTCAGAAG | 1 min of 72 °C extension | |
| PPARγ | F: TGATATCGACCAGCTGAACC | 1 min of 58 °C annealing |
| R: GTCCTCCAGCTGTTCGCCA | 1 min of 72 °C extension | |
| UCP-1 | F: TATCATCACCTTCCCGCTG | 1 min of 58 °C annealing |
| R: GTCATATGTTACCAGCTCTG | 1 min of 72 °C extension | |
| Adiponectin | F: GACGTTACTACAACTGAAGAGC | 1 min of 57 °C annealing |
| R: CATTCTTTTCCTGATACTGGTC | 1 min of 72 °C extension | |
| TNF-α | F: GCCTCTTCTCATTCCTGCTTG | 1 min of 60 °C annealing |
| R: CTGATGAGAGGGAGGCCATT | 1 min of 72 °C extension | |
| IL-6 | F: ACGGCCTTCCCTACTTCACA | 1 min of 65 °C annealing |
| R: CATTTCCACGATTTCCCAGA | 1 min of 72 °C extension | |
| β-actin | F: GACTACCTCATGAAGATCCT | 1 min of 59 °C annealing |
| R: CCACATCTGCTGGAAGGTGG | 1 min of 72 °C extension |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-T.V.; Tsou, Y.-C.; Chen, Y.-T.; Lu, W.-J.; Hwang, P.-A. Effects of Low-Molecular-Weight Fucoidan and High Stability Fucoxanthin on Glucose Homeostasis, Lipid Metabolism, and Liver Function in a Mouse Model of Type II Diabetes. Mar. Drugs 2017, 15, 113. https://doi.org/10.3390/md15040113
Lin H-TV, Tsou Y-C, Chen Y-T, Lu W-J, Hwang P-A. Effects of Low-Molecular-Weight Fucoidan and High Stability Fucoxanthin on Glucose Homeostasis, Lipid Metabolism, and Liver Function in a Mouse Model of Type II Diabetes. Marine Drugs. 2017; 15(4):113. https://doi.org/10.3390/md15040113
Chicago/Turabian StyleLin, Hong-Ting Victor, Yu-Chi Tsou, Yu-Ting Chen, Wen-Jung Lu, and Pai-An Hwang. 2017. "Effects of Low-Molecular-Weight Fucoidan and High Stability Fucoxanthin on Glucose Homeostasis, Lipid Metabolism, and Liver Function in a Mouse Model of Type II Diabetes" Marine Drugs 15, no. 4: 113. https://doi.org/10.3390/md15040113







