Chemistry and Selective Tumor Cell Growth Inhibitory Activity of Polyketides from the South China Sea Sponge Plakortis sp.
Abstract
:1. Introduction
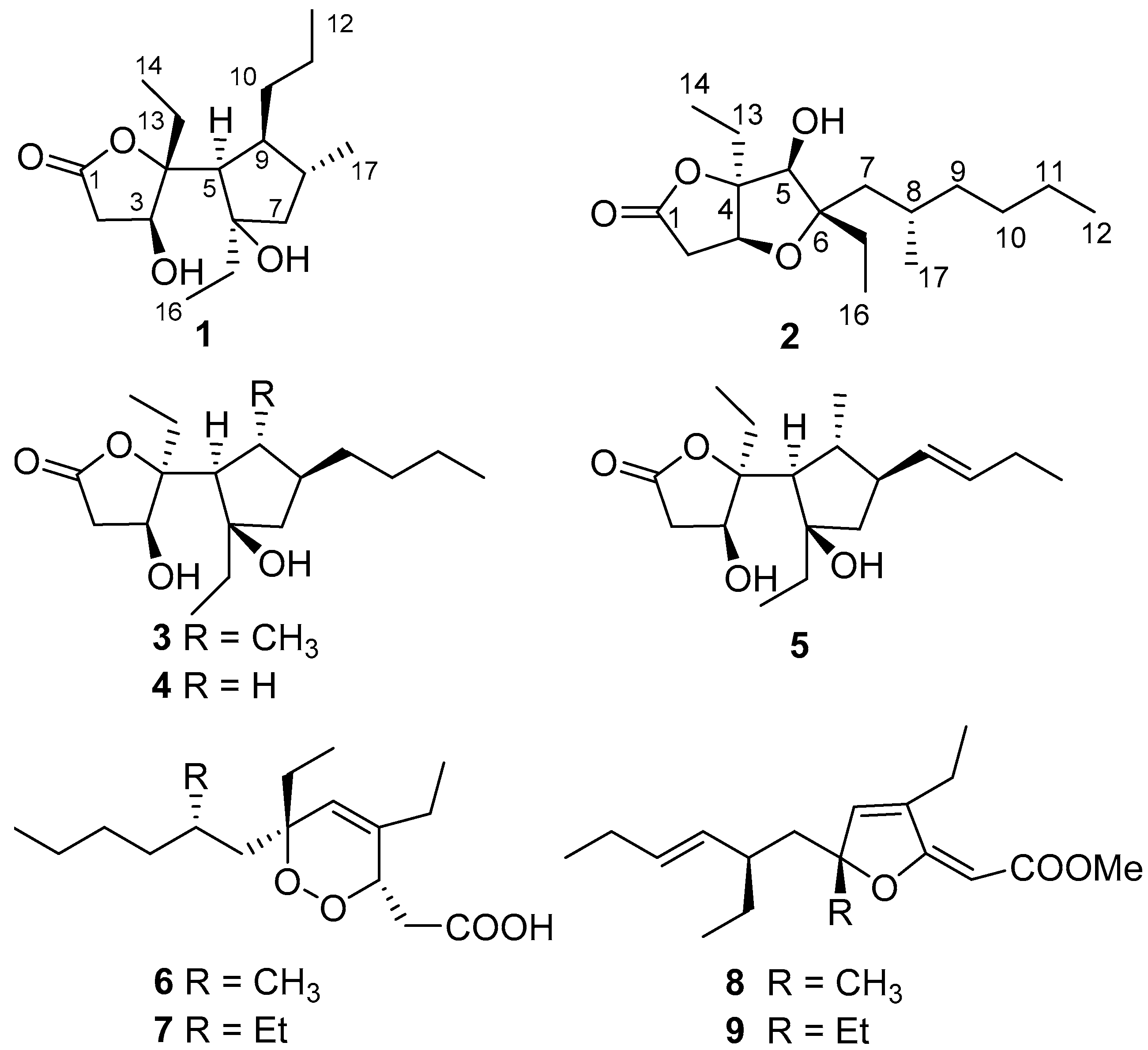
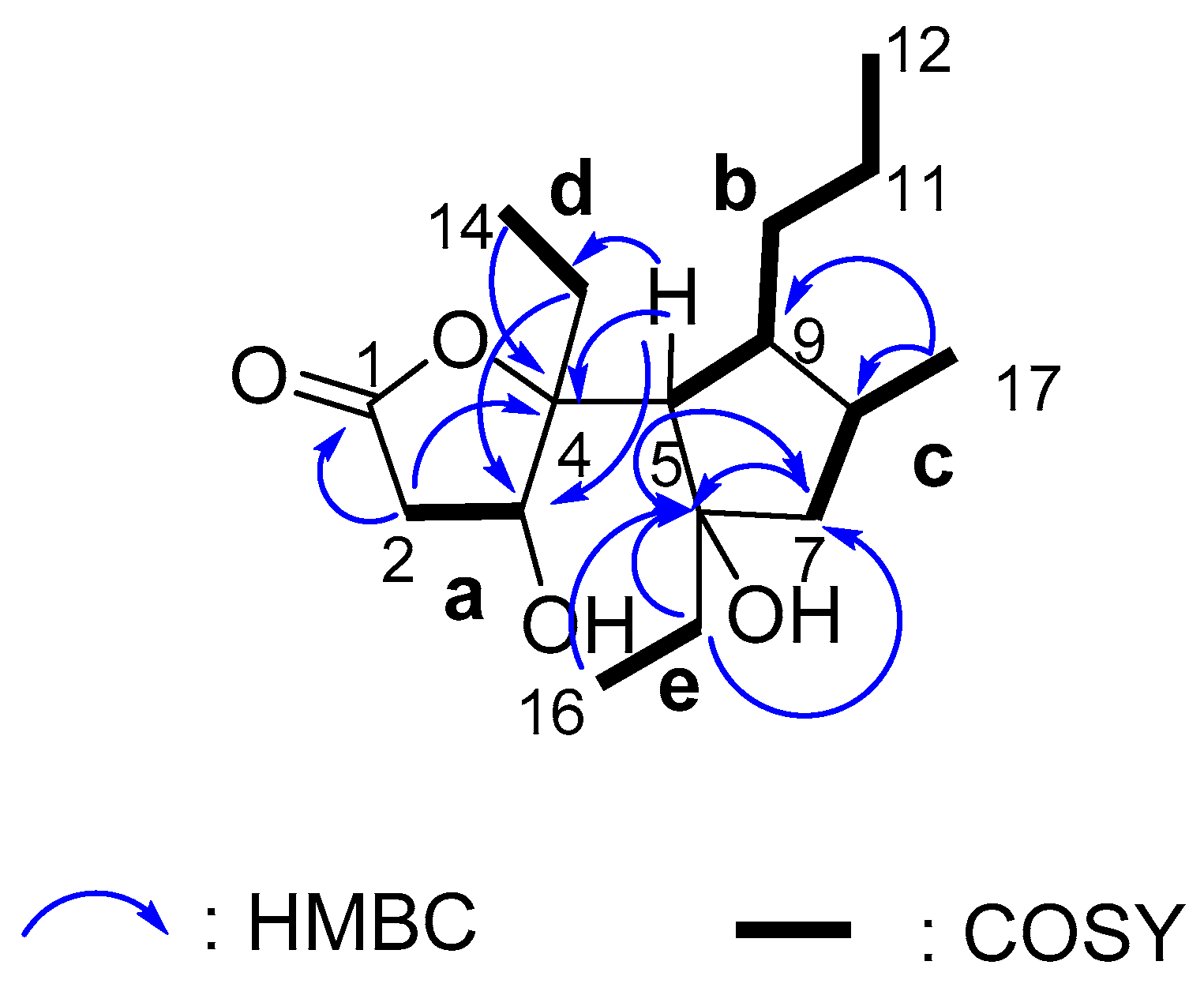
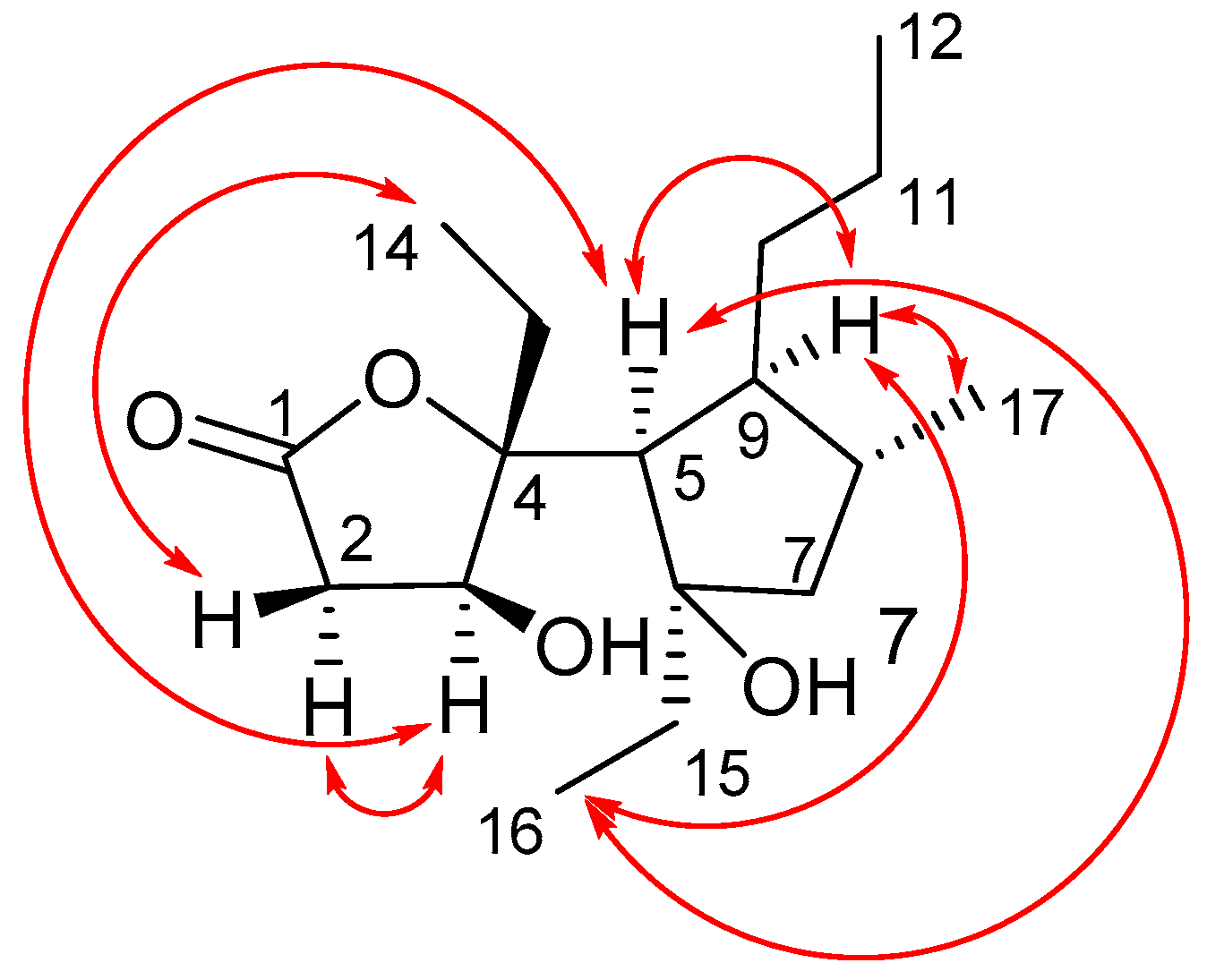
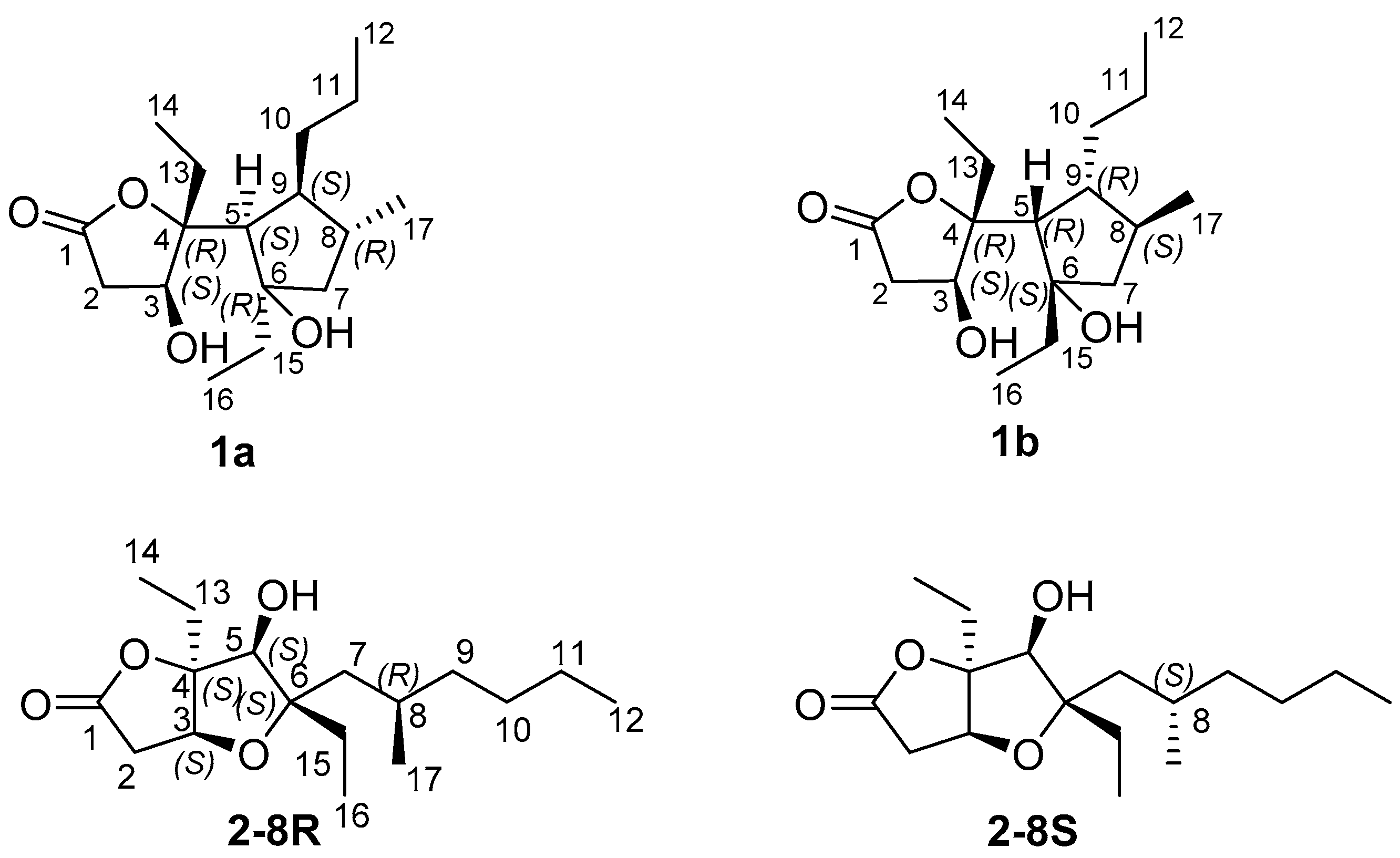
2. Results and Discussion
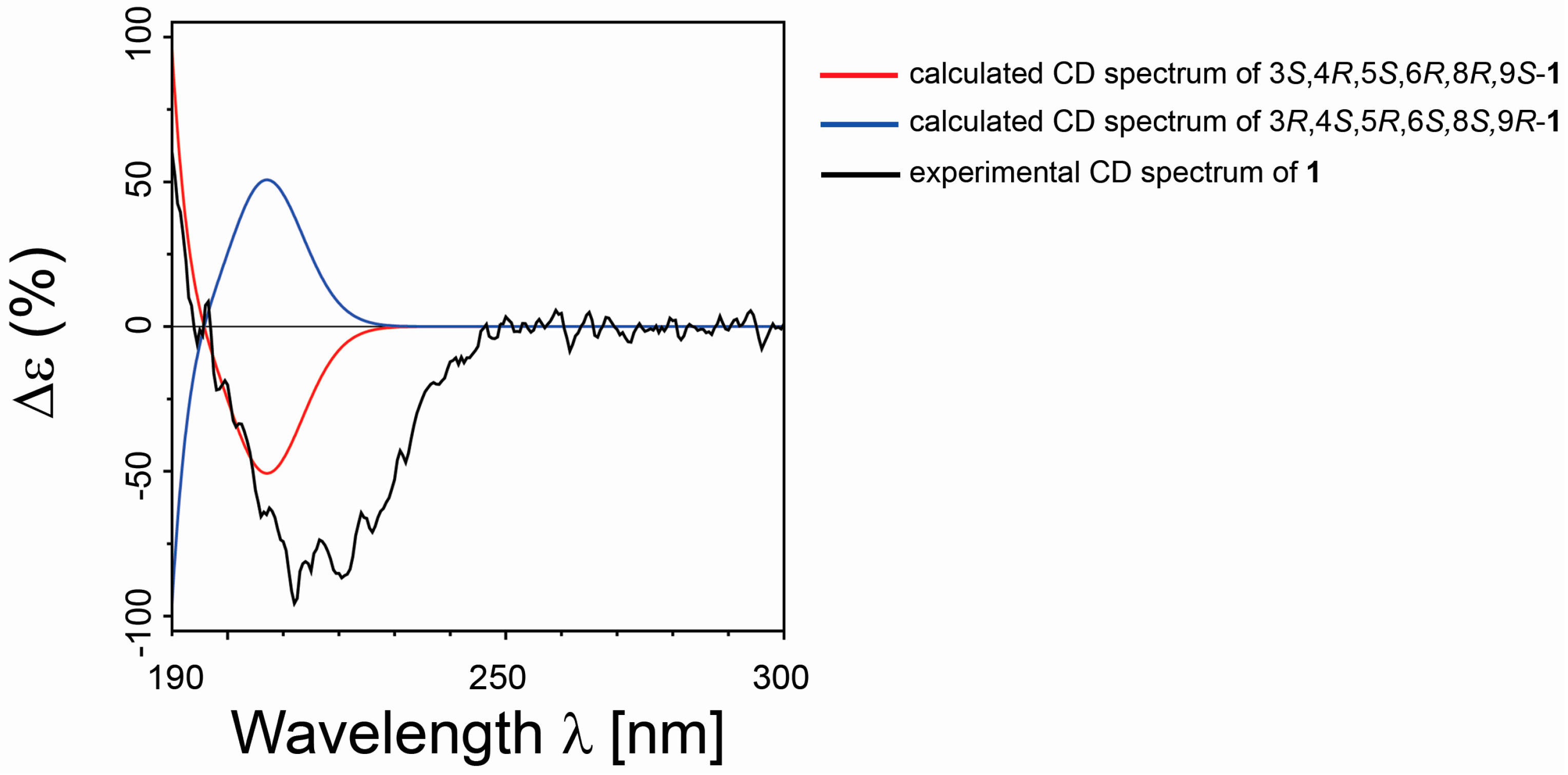
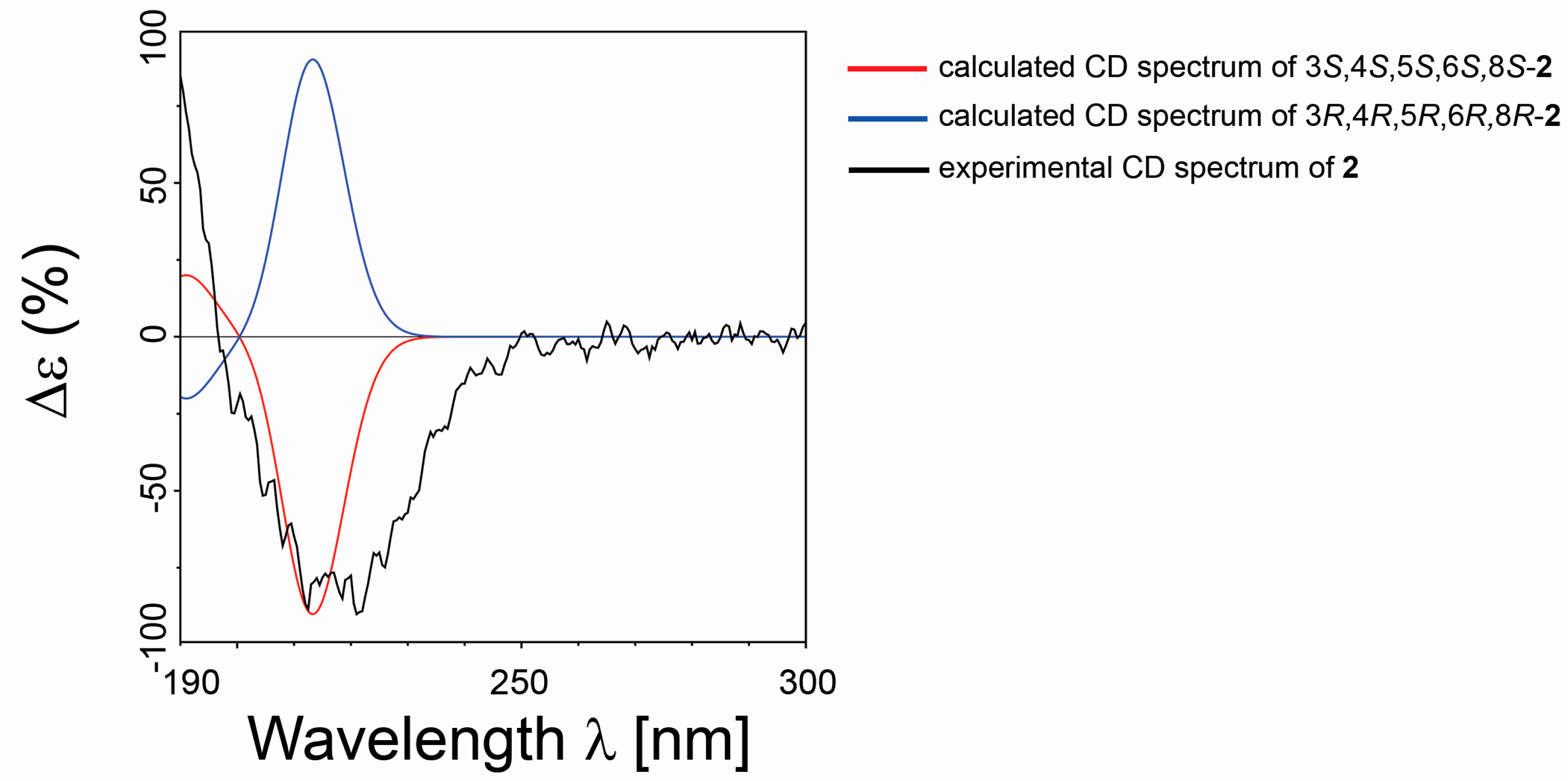
2.1. Isolation and Stereostructural Determination
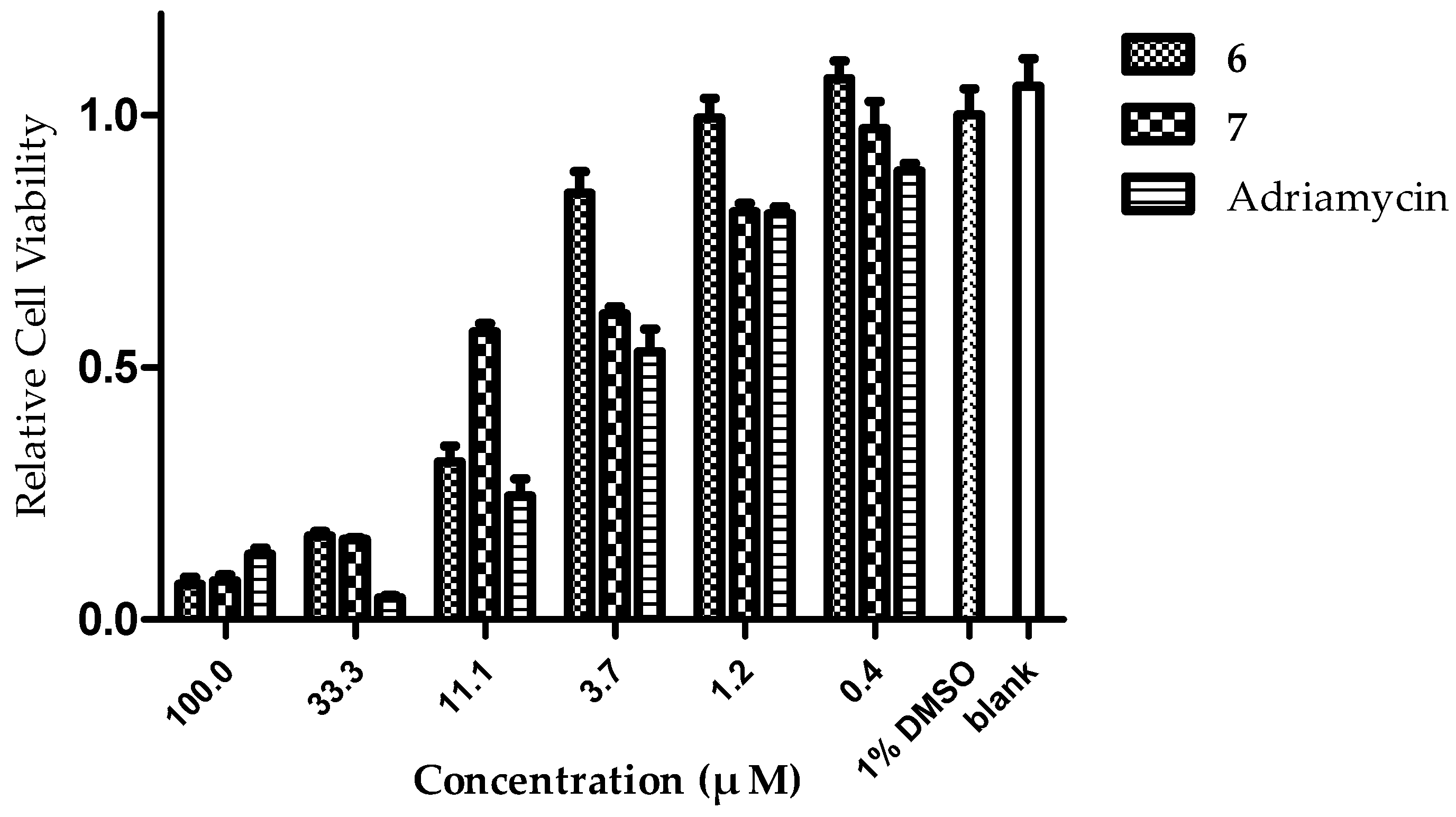
2.2. In Vitro Evaluation of Cytotoxic Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
Simplextone E (1):
3.4. Computational Details
3.5. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rahm, F.; Hayes, P.Y.; Kitching, W. Metabolites from marine sponges of the genus Plakortis. Heterocycles 2004, 64, 523–575. [Google Scholar] [CrossRef]
- Higgs, M.D.; Faulkner, D.J. Plakortin, an antibiotic from Plakortis halichondrioides. J. Org. Chem. 1978, 43, 3454–3457. [Google Scholar] [CrossRef]
- Santos, E.A.; Quintela, A.L.; Ferreira, E.G.; Sousa, T.S.; Fd, P.; Hajdu, E.; Carvalho, M.S.; Salani, S.; Rocha, D.D.; Wilke, D.V.; et al. Cytotoxic plakortides from the Brazilian marine sponge Plakortis angulospiculatus. J. Nat. Prod. 2015, 78, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.J.; Davis, R.A.; Sykes, M.; Avery, V.M.; Camp, D.; Quinn, R.J. Antitrypanosomal cyclic polyketide peroxides from the Australian marine sponge Plakortis sp. J. Nat. Prod. 2010, 73, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Jamison, M.T.; Dalisay, D.S.; Molinski, T.F. Peroxide natural products from Plakortis Zyggompha and the sponge association Plakortis halichondrioides-Xestospongia deweerdtae: Antifungal activity against Cryptococcus gattii. J. Nat. Prod. 2016, 79, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Manzo, E.; Ciavatta, M.L.; Melck, D.; Schupp, P.; de, V.N.; Gavagnin, M. Aromatic cyclic peroxides and related keto-compounds from the Plakortis sp. Component of a sponge consortium. J. Nat. Prod. 2009, 72, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Freyer, A.J.; Bean, M.F.; Carte, B.K.; Westley, J.W.; Johnson, R.K. The plakortones, novel bicyclic lactones from the sponge Plakortis halichondrioides: Activators of cardiac SR-Ca2+-pumping ATPase. Tetrahedron 1996, 52, 377–394. [Google Scholar] [CrossRef]
- Cafieri, F.; Fattorusso, E.; Taglialatela-Scafati, O.; Di, R.M.; Ianaro, A. Metabolites from the sponge Plakortis simplex. II. Isolation of four bioactive lactone compounds and of a novel related amino acid. Tetrahedron 1999, 55, 13831–13840. [Google Scholar] [CrossRef]
- Gochfeld, D.J.; Hamann, M.T. Isolation and biological evaluation of filiformin, plakortide F, and plakortone G from the Caribbean sponge Plakortis sp. J. Nat. Prod. 2001, 64, 1477–1479. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.W.L.; De Voss, J.J.; Hooper, J.N.A.; Garson, M.J. Configurational assignment of cyclic peroxy metabolites provides an insight into their biosynthesis: Isolation of plakortolides, seco-plakortolides, and plakortones from the Australian marine sponge Plakinastrella clathrata. J. Nat. Prod. 2011, 74, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Song, Y.L.; Zhang, H.J.; Yang, F.; Yu, H.B.; Jiao, W.-H.; Piao, S.J.; Chen, W.S.; Lin, H.W. Simplextones A and B, unusual polyketides from the marine sponge Plakortis simplex. Org. Lett. 2011, 13, 3154–3157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, X.; Li, J.; Li, P.; de Voogd, N.J.; Ni, X.; Jin, X.; Yao, X.; Li, P.; Li, G. Cytotoxic polyketide derivatives from the South China Sea sponge Plakortis simplex. J. Nat. Prod. 2013, 76, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Chianese, G.; Yu, H.B.; Yang, F.; Sirignano, C.; Luciano, P.; Han, B.N.; Khan, S.; Lin, H.W.; Taglialatela-Scafati, O. PPAR modulating polyketides from a Chinese Plakortis simplex and clues on the origin of their chemodiversity. J. Org. Chem. 2016, 81, 5135–5143. [Google Scholar] [CrossRef] [PubMed]
- Kossuga, M.H.; Nascimento, A.M.; Reimao, J.Q.; Tempone, A.G.; Taniwaki, N.N.; Veloso, K.; Ferreira, A.G.; Cavalcanti, B.C.; Pessoa, C.; Moraes, M.O.; et al. Antiparasitic, antineuroinflammatory, and cytotoxic polyketides from the marine sponge Plakortis angulospiculatus collected in Brazil. J. Nat. Prod. 2008, 71, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Epifanio, R.d.A.; Pinheiro, L.S.; Alves, N.C. Polyketides from the marine sponge Plakortis angulospiculatus. J. Braz. Chem. Soc. 2005, 16, 1367–1371. [Google Scholar] [CrossRef]
- Compagnone, R.S.; Pina, I.C.; Rangel, H.R.; Dagger, F.; Suarez, A.I.; Reddy, M.V.R.; Faulkner, D.J. Antileishmanial cyclic peroxides from the Palauan sponge Plakortis aff. angulospiculatus. Tetrahedron 1998, 54, 3057–3068. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y.; Bao, J.; He, F.; Xu, X.; Qi, S. Phylogenetic survey and antimicrobial activity of culturable microorganisms associated with the South China Sea black coral Antipathes dichotoma. FEMS Microbiol. Lett. 2012, 336, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, E.; Taglialatela-Scafati, O.; Di, R.M.; Ianaro, A. Metabolites from the sponge Plakortis simplex. Part 3: Isolation and stereostructure of novel bioactive cycloperoxides and diol analogues. Tetrahedron 2000, 56, 7959–7967. [Google Scholar] [CrossRef]
- Lim, C.W.; Kim, Y.K.; Jang, M.S.; Park, J.I.; Park, H.Y. Coupling of ent-cyclic peroxide and ircinol A, two biologically active natural marine products. J. Fish. Sci. Technol. 2006, 9, 175–178. [Google Scholar] [CrossRef]
- Campagnuolo, C.; Fattorusso, E.; Romano, A.; Taglialatela-Scafati, O.; Basilico, N.; Parapini, S.; Taramelli, D. Antimalarial polyketide cycloperoxides from the marine sponge Plakortis simplex. Eur. J. Org. Chem. 2005, 5077–5083. [Google Scholar] [CrossRef]
- Holla, H.; Labaied, M.; Pham, N.; Jenkins, I.D.; Stuart, K.; Quinn, R.J. Synthesis of antitrypanosomal 1,2-dioxane derivatives based on a natural product scaffold. Bioorg. Med. Chem. Lett. 2011, 21, 4793–4797. [Google Scholar] [CrossRef] [PubMed]
- Chianese, G.; Scala, F.; Calcinai, B.; Cerrano, C.; Dien, H.A.; Kaiser, M.; Tasdemir, D.; Taglialatela-Scafati, O. Natural and semisynthetic analogues of manadoperoxide B reveal new structural requirements for trypanocidal activity. Mar. Drugs 2013, 11, 3297–3308. [Google Scholar] [CrossRef] [PubMed]
- Arzt, S.; Bourcet, E.; Muller, T.; Brase, S. Enantioselective total synthesis of plakotenin, a cytotoxic metabolite from Plakortis sp. Org. Biomol. Chem. 2010, 8, 3300–3306. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, C.; Campiani, G.; Catalanotti, B.; Persico, M.; Basilico, N.; Parapini, S.; Taramelli, D.; Campagnuolo, C.; Fattorusso, E.; Romano, A.; et al. Endoperoxide derivatives from marine organisms: 1,2-dioxanes of the plakortin family as novel antimalarial agents. J. Med. Chem. 2006, 49, 7088–7094. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Steliou, K. Synthetic studies toward bioactive cyclic peroxides from the marine sponge Plakortis angulospiculatus. Org. Lett. 2002, 4, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Ruider, S.A.; Carreira, E.M. A unified strategy to Plakortin pentalenes: Total syntheses of (±)-gracilioethers E and F. Org. Lett. 2016, 18, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, H.; Sato, S.; Tokudome, K.; Yamada, T. Stereoselective formation of tetrahydrofuran rings via [3 + 2] annulation: Total synthesis of plakortone L. Org. Lett. 2014, 16, 3384–3387. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.G.; Wu, X.W.; Lee, H.K.; Peng, X.S.; Wong, H.N.C. Total synthesis of plakortone B. Chem-Eur. J. 2010, 16, 6933–6941. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Isoda, Y.; Nishimoto, M.; Narazaki, M.; Oka, H.; Kuboki, A.; Ohira, S. Total synthesis and absolute stereochemistry of plakortone E. Tetrahedron Lett. 2006, 47, 2287–2290. [Google Scholar] [CrossRef]
- Cafieri, F.; Fattorusso, E.; Taglialatela-Scafati, O.; Ianaro, A. Metabolites from the sponge Plakortis simplex. Determination of absolute stereochemistry of plakortin. Isolation and stereostructure of three plakortin related compounds. Tetrahedron 1999, 55, 7045–7056. [Google Scholar] [CrossRef]
- Hayes, P.Y.; Kitching, W. Total synthesis and absolute stereochemistry of plakortone D. J. Am. Chem. Soc. 2002, 124, 9718–9719. [Google Scholar] [CrossRef] [PubMed]
- Kowashi, S.; Ogamino, T.; Kamei, J.; Ishikawa, Y.; Nishiyama, S. The first total synthesis and absolute stereochemistry of plakortone G from the Jamaican sponge Plakortis sp. Tetrahedron Lett. 2004, 45, 4393–4396. [Google Scholar] [CrossRef]
- Di, M.S.; Zampella, A.; D’Auria, M.V.; Festa, C.; De, M.S.; Riccio, R.; Butts, C.P.; Bifulco, G. Plakilactones G and H from a marine sponge. Stereochemical determination of highly flexible systems by quantitative NMR-derived interproton distances combined with quantum mechanical calculations of (13)C chemical shifts. Beilstein J. Org. Chem. 2013, 9, 2940–2949. [Google Scholar]
- Bifulco, G.; Dambruoso, P.; Gomez-Paloma, L.; Riccio, R. Determination of relative configuration in organic compounds by NMR spectroscopy and computational methods. Chem. Rev. 2007, 107, 3744–3779. [Google Scholar] [CrossRef] [PubMed]
- Micco, S.D.; Chini, M.G.; Riccio, R.; Bifulco, G. Quantum mechanical calculation of NMR parameters in the stereostructural determination of natural products. Eur. J. Org. Chem. 2010, 2010, 1411–1434. [Google Scholar] [CrossRef]
- Gong, J.; Sun, P.; Jiang, N.; Riccio, R.; Lauro, G.; Bifulco, G.; Li, T.J.; Gerwick, W.H.; Zhang, W. New steroids with a rearranged skeleton as (h)p300 inhibitors from the sponge Theonella swinhoei. Org. Lett. 2014, 16, 2224–2227. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Yu, Q.; Li, J.; Riccio, R.; Lauro, G.; Bifulco, G.; Kurtan, T.; Mandi, A.; Tang, H.; Li, T.J.; et al. Bissubvilides A and B, cembrane-capnosane heterodimers from the soft coral Sarcophyton subviride. J. Nat. Prod. 2016, 79, 2552–2558. [Google Scholar] [CrossRef] [PubMed]
- Takada, N.; Watanabe, M.; Yamada, A.; Suenaga, K.; Yamada, K.; Ueda, K.; Uemura, D. Isolation and structures of haterumadioxins A and B, cytotoxic endoperoxides from the Okinawan sponge Plakortis lita. J. Nat. Prod. 2001, 64, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Capon, R.J.; Singh, S.; Ali, S.; Sotheeswaran, S. Spongosoritin A: A new polyketide from a Fijian marine sponge, Spongosorites sp. Aust. J. Chem. 2005, 58, 18–20. [Google Scholar] [CrossRef]
- Stierle, D.B.; Faulkner, D.J. Metabolites of three marine sponges of the genus Plakortis. J. Org. Chem. 1980, 45, 3396–3401. [Google Scholar] [CrossRef]
- Schmidt, E.W.; Faulkner, D.J. Absolute configuration of methyl (2z,6r,8r,9e)-3,6-epoxy-4,6,8-triethyl-2,4,9-dodecatrienoate from the sponge Plakortis halichondrioides. Tetrahedron Lett. 1996, 37, 6681–6684. [Google Scholar] [CrossRef]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef] [PubMed]
- Nadmid, S.; Plaza, A.; Lauro, G.; Garcia, R.; Bifulco, G.; Müller, R. Hyalachelins A–C, unusual siderophores isolated from the terrestrial myxobacterium Hyalangium minutum. Org. Lett. 2014, 16, 4130–4133. [Google Scholar] [CrossRef] [PubMed]
- Maestro, version 10.2; Schrödinger LLC: New York, NY, USA, 2015.
- MacroModel, version 10.2; Schrödinger LLC: New York, NY, USA, 2013.
- Jorgensen, W.L.; Tiradorives, J. The OPLS potential functions for proteins-energy minimizations for crystals of cyclic-peptides and crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpeCdis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Das, S.G.; Srinivasan, B.; Hermanson, D.L.; Bleeker, N.P.; Doshi, J.M.; Tang, R.; Beck, W.T.; Xing, C. Structure-activity relationship and molecular mechanisms of ethyl 2-amino-6-(3,5-dimethoxyphenyl)-4-(2-ethoxy-2-oxoethyl)-4h-chromene-3-carboxylate (cxl017) and its analogues. J. Med. Chem. 2011, 54, 5937–5948. [Google Scholar] [CrossRef] [PubMed]
- Schirmeister, T.; Oli, S.; Wu, H.; Sala, G.D.; Costantino, V.; Seo, E.-J.; Efferth, T. Cytotoxicity of endoperoxides from the Caribbean sponge Plakortis halichondrioides towards sensitive and multidrug-resistant leukemia cells: Acids vs. esters activity evaluation. Mar. Drugs 2017, 15, 63. [Google Scholar] [CrossRef] [PubMed]

| Position | δC | δH | COSY | HMBC (H→C) |
|---|---|---|---|---|
| 1 | 175.0, C | |||
| 2α 2β | 38.1, CH2 | 2.98 dd (18.4, 9.3) 2.56 dd (18.4, 7.0) | 1, 4 1 | |
| 3 | 72.5, CH | 4.90 m | 2α, 2β | |
| 4 | 94.4, C | |||
| 5 | 55.5, CH | 2.25 d (7.8) | 9 | 3, 4, 6, 7, 8, 13 |
| 6 | 84.3, C | |||
| 7α 7β | 48.9, CH2 | 1.43 m 1.93 m | ||
| 8 | 36.4, CH | 2.00 m | 7β | |
| 9 | 50.2, CH | 1.58 m | 10a | |
| 10a 10b | 33.5, CH2 | 1.69 m 1.46 m | 11 | 8, 9 8, 9 |
| 11 | 22.3, CH2 | 1.38 m | 12 | 9 |
| 12 | 14.3, CH3 | 0.92 ov | 10a, 10b | |
| 13a 13b | 26.6, CH2 | 2.08 m 1.87 m | 4 3, 4 | |
| 14 | 8.3, CH3 | 1.05 t (7.3) | 13 | 4 |
| 15a 15b | 36.4, CH2 | 1.71 m 1.56 m | 6, 7 6, 7 | |
| 16 | 8.5, CH3 | 0.96 ov | 15 | 6 |
| 17 | 22.0, CH3 | 0.99 d (6.5) | 8 | 7, 8, 9 |
| MAE Values (ppm) | DP4+ Probability | |||||
|---|---|---|---|---|---|---|
| Isomer | Number of Conformers | 13C MAE | 1H MAE | 13C Data | 1H Data | All Data |
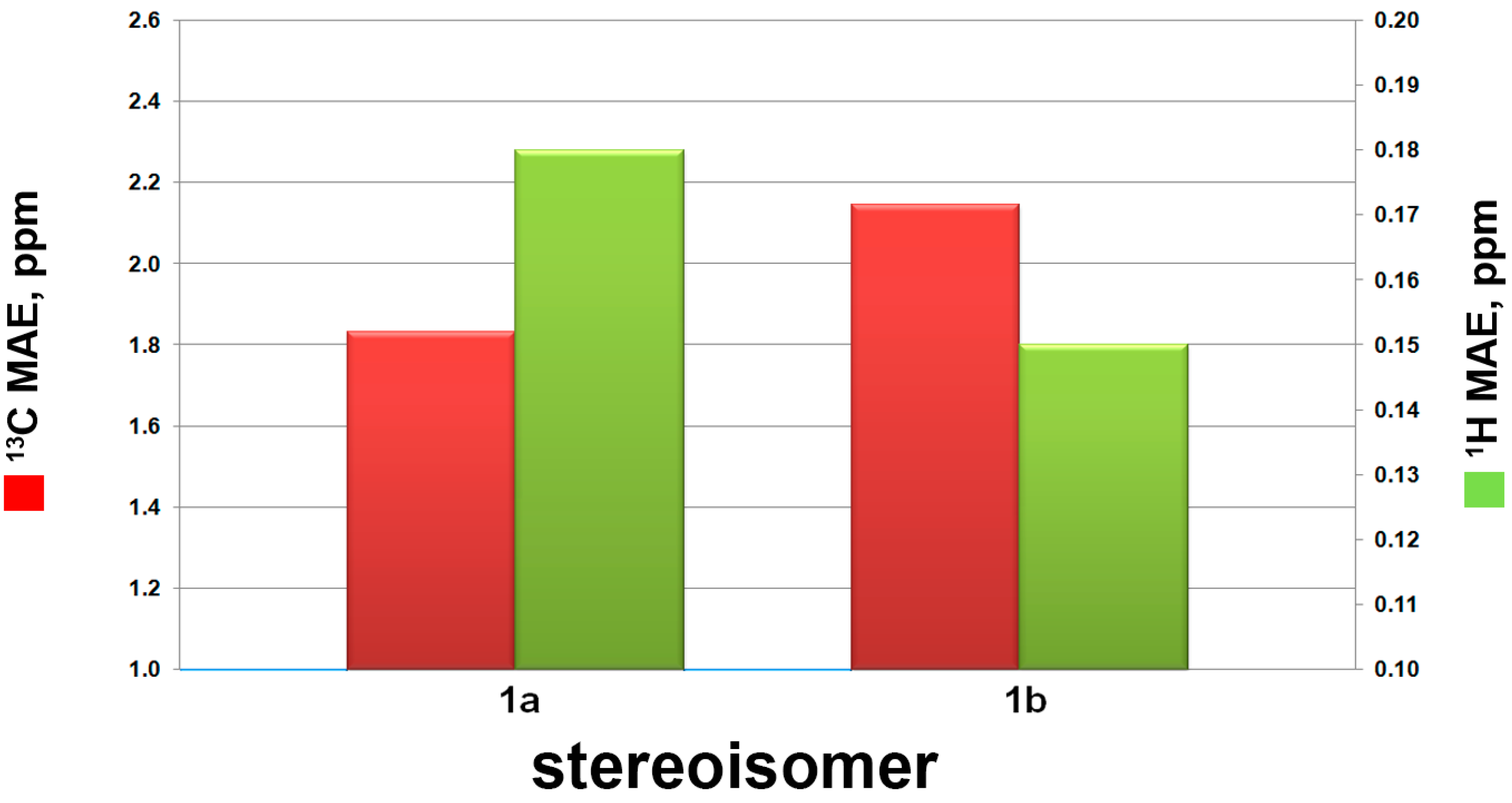
| 1a | 15 | 1.83 | 0.18 | 100.00% | 2.70% | 99.98% |
| 1b | 16 | 2.14 | 0.15 | 0.00% | 97.30% | 0.01% |
| MAE Values (ppm) | DP4+ Probability | |||||
|---|---|---|---|---|---|---|
| Isomer | Number of Conformers | 13C MAE | 1H MAE | 13C Data | 1H Data | All Data |
| 2-8R | 170 | 1.52 | 0.14 | 61.67% | 6.25% | 9.69% |
| 2-8S | 150 | 1.44 | 0.13 | 38.33% | 93.75% | 90.31% |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Adriamycin a | |
|---|---|---|---|---|---|---|---|---|---|---|
| HCT-116 | >50.0 | >50.0 | >50.0 | >50.0 | >50.0 | 8.3 ± 2.4 | 8.4 ± 2.3 | >50.0 | >50.0 | 4.1 |
| MCF-7 | >50.0 | >50.0 | 49.3 ± 3.5 | >50.0 | >50.0 | 13.2 ± 1.6 | >50.0 | >50.0 | >50.0 | 6.2 |
| K562 | >50.0 | >50.0 | >50.0 | - | >50.0 | 25.4 ± 5.4 | 30.3 ± 3.5 | >50.0 | - | 5.1 |
| SMMC-7721 | >50.0 | >50.0 | >50.0 | >50.0 | >50.0 | >50.0 | >50.0 | >50.0 | >50.0 | 5.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, C.; Riccio, R.; Lauro, G.; Bifulco, G.; Li, T.-J.; Tang, H.; Zhuang, C.-L.; Ma, H.; Sun, P.; et al. Chemistry and Selective Tumor Cell Growth Inhibitory Activity of Polyketides from the South China Sea Sponge Plakortis sp. Mar. Drugs 2017, 15, 129. https://doi.org/10.3390/md15050129
Li J, Li C, Riccio R, Lauro G, Bifulco G, Li T-J, Tang H, Zhuang C-L, Ma H, Sun P, et al. Chemistry and Selective Tumor Cell Growth Inhibitory Activity of Polyketides from the South China Sea Sponge Plakortis sp. Marine Drugs. 2017; 15(5):129. https://doi.org/10.3390/md15050129
Chicago/Turabian StyleLi, Jiao, Cui Li, Raffaele Riccio, Gianluigi Lauro, Giuseppe Bifulco, Tie-Jun Li, Hua Tang, Chun-Lin Zhuang, Hao Ma, Peng Sun, and et al. 2017. "Chemistry and Selective Tumor Cell Growth Inhibitory Activity of Polyketides from the South China Sea Sponge Plakortis sp." Marine Drugs 15, no. 5: 129. https://doi.org/10.3390/md15050129





