Marine Fish Proteins and Peptides for Cosmeceuticals: A Review
Abstract
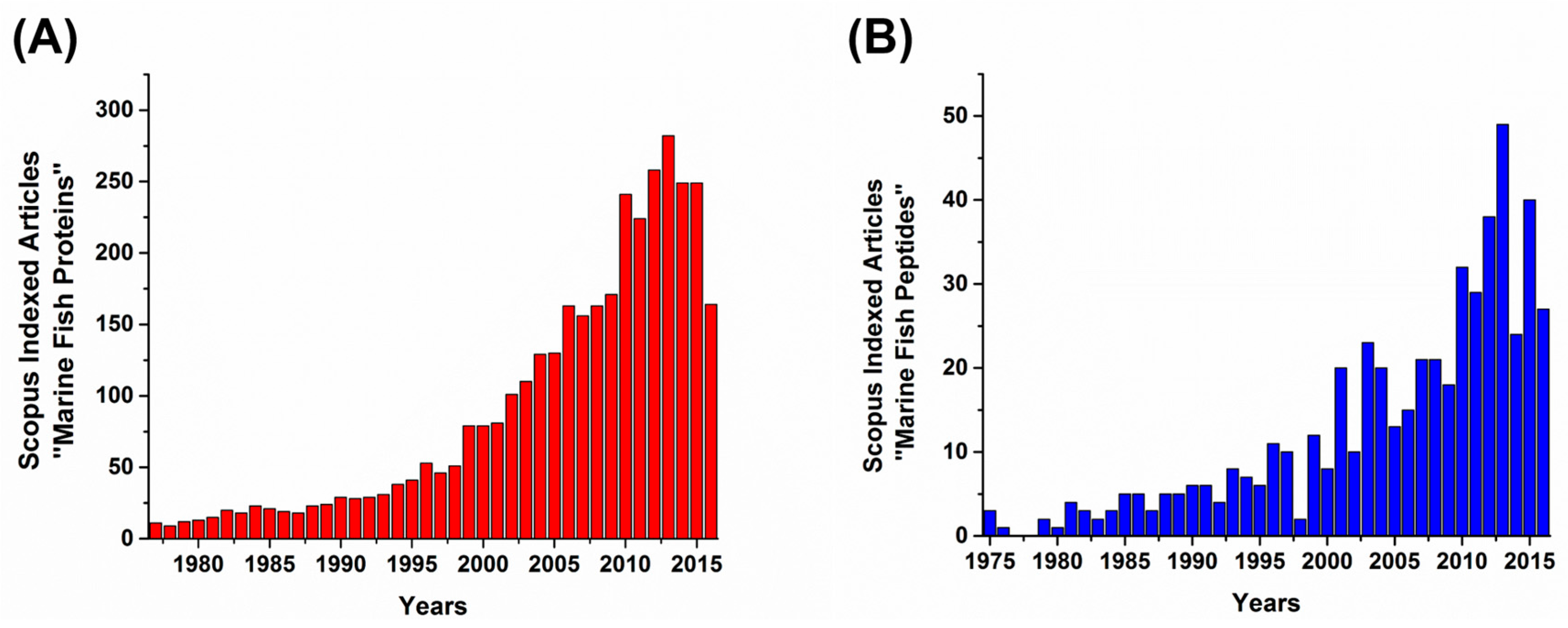
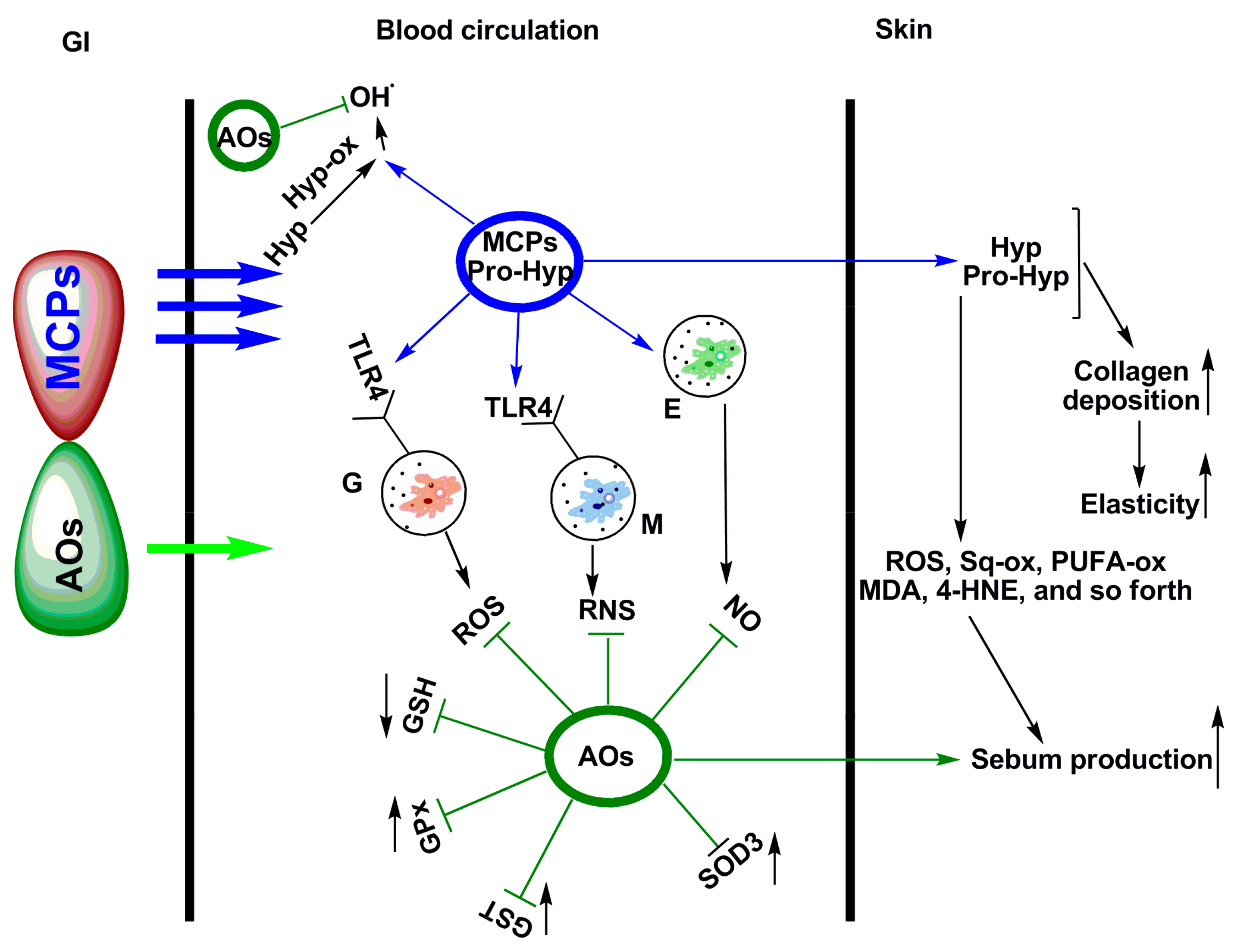
:1. Introduction
2. Marine Fish Proteins and Peptides
3. Marine Fish-Derived Collagen
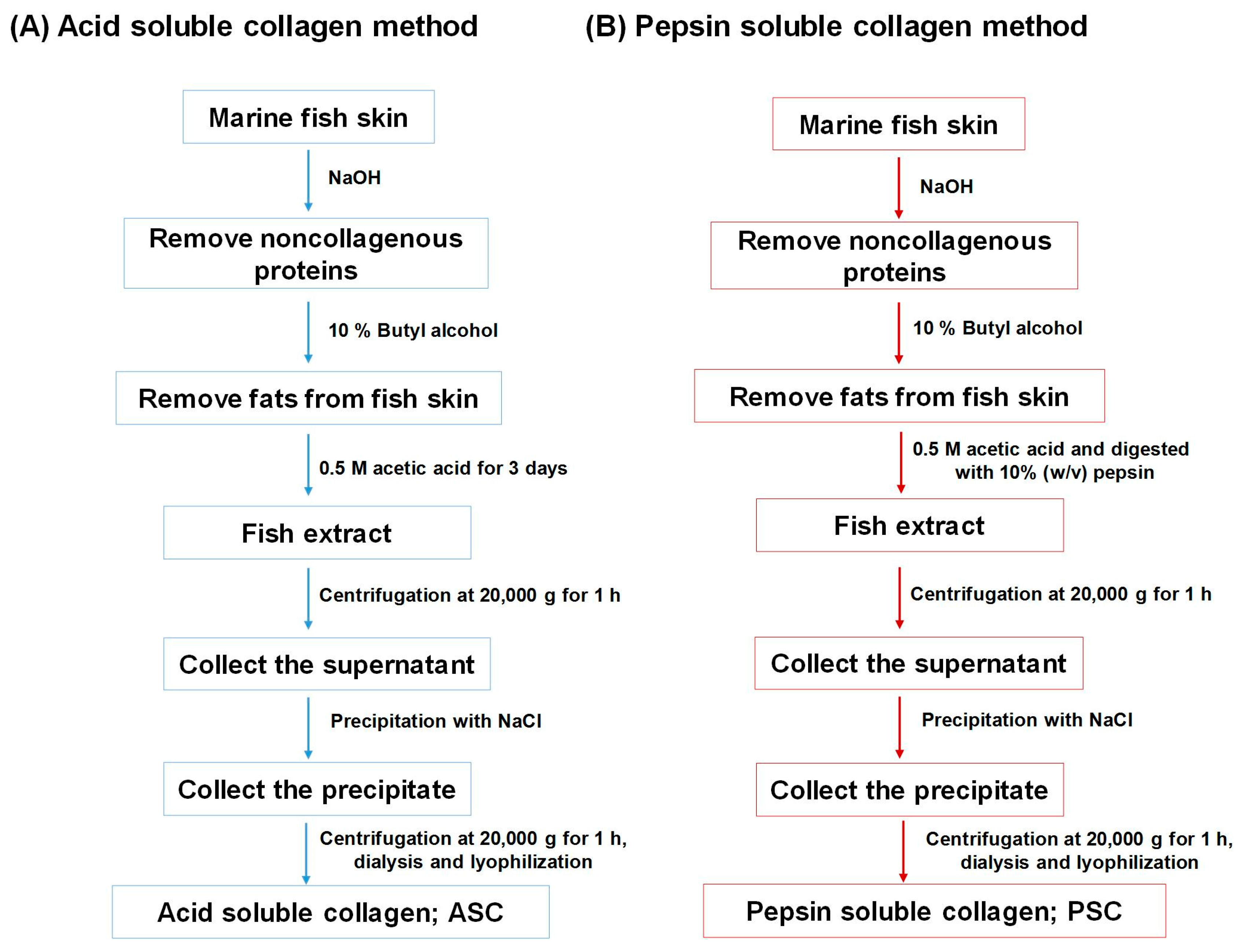
3.1. Isolation of Marine Fish-Derived Collagen
3.2. Marine Fish-Derived Collagen in Cosmeceuticals
3.3. Marine Fish-Derived Collagen in Wound Healing and Tissue Engineering
4. Marine Fish-Derived Peptides
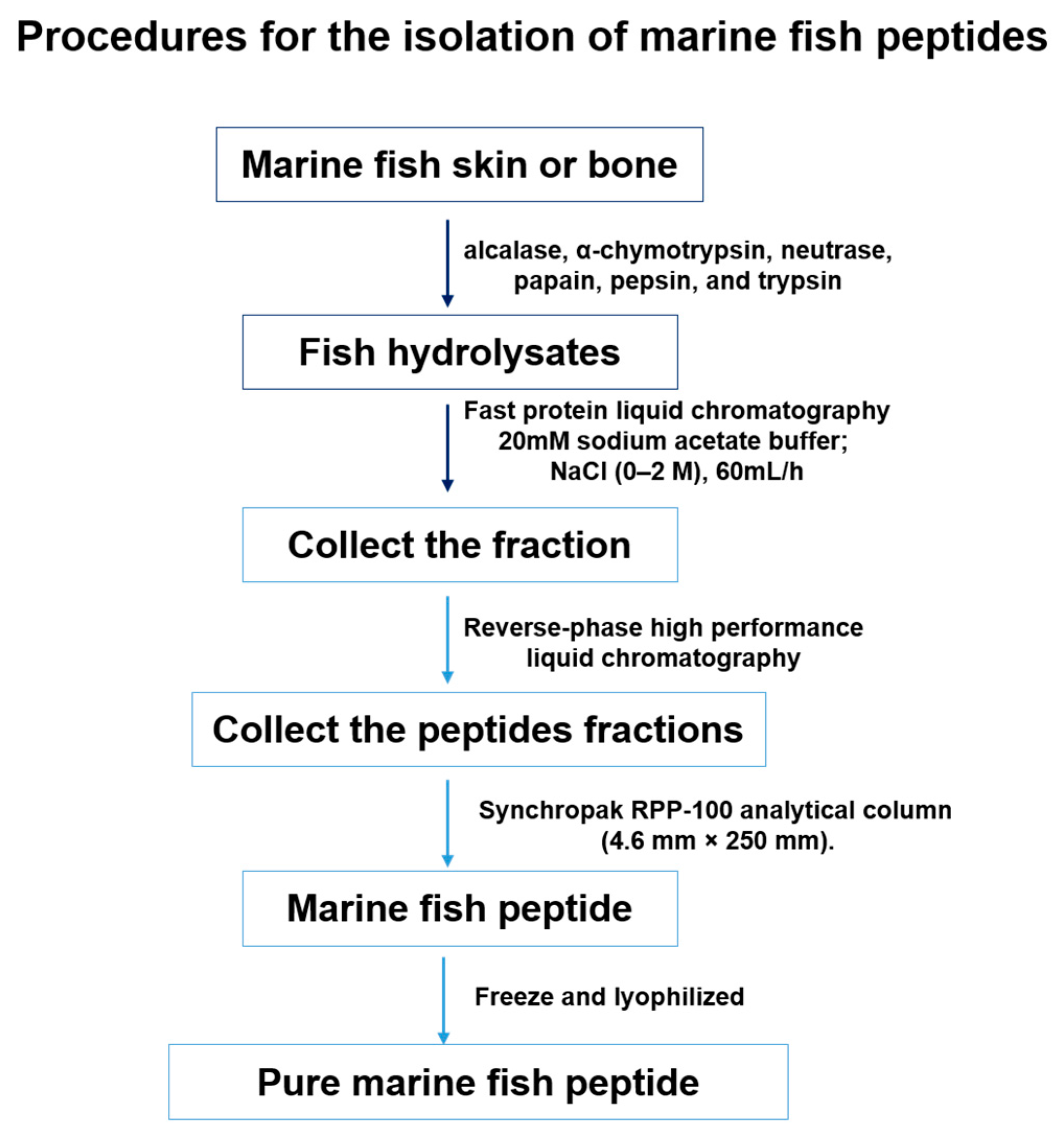
4.1. Isolation of Marine Fish Peptides
4.2. Biological Activities of Marine Fish Peptides as Cosmeceuticals
4.3. Antioxidant Fish Peptides
4.4. Antimicrobial Fish Peptides
4.5. Matrix Metalloproteinases Inhibiting Fish Peptides
5. Photo-Protective and Anti-Photoaging Activity of Fish Peptides and Fish Protein Hydrolysates
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Venkatesan, J. Introduction to marine biotechnology. In Springer Handbook of Marine Biotechnology; Kim, S.-K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–10. [Google Scholar]
- Najafian, L.; Babji, A.S. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Senevirathne, M.; Kim, S.-K. Utilization of seafood processing by-products: Medicinal applications. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Academic Press: Waltham, MA, USA, 2012; Volume 65, pp. 495–512. [Google Scholar]
- Rustad, T. Physical and chemical properties of protein seafood by-products. In Maximising the Value of Marine By-Products; Shahidi, F., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2007; pp. 3–21. [Google Scholar]
- Nilsang, S.; Lertsiri, S.; Suphantharika, M.; Assavanig, A. Optimization of enzymatic hydrolysis of fish soluble concentrate by commercial proteases. J. Food Eng. 2005, 70, 571–578. [Google Scholar] [CrossRef]
- Allard, R.; Malak, N.A.; Huc, A. Collagen Product Containing Collagen of Marine Origin with a Low Odor and Preferably with Improved Mechanical Properties, and Its Use in the Form of Cosmetic or Pharmaceutical Compositions or Products. U.S. Patent 6,660,280, 9 December 2003. [Google Scholar]
- Shahidi, F.; Kamil, Y.J. Enzymes from fish and aquatic invertebrates and their application in the food industry. Trends Food Sci. Technol. 2001, 12, 435–464. [Google Scholar] [CrossRef]
- Hoyer, B.; Bernhardt, A.; Heinemann, S.; Stachel, I.; Meyer, M.; Gelinsky, M. Biomimetically mineralized salmon collagen scaffolds for application in bone tissue engineering. Biomacromolecules 2012, 13, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Yamada, S.; Guchi, K.Y.; Koyama, Z.; Ikeda, T. Chitosan and fish collagen as biomaterials for regenerative medicine. In Advances in Food and Nutrition Research; Kim, S.K., Ed.; Academic Press: Waltham, MA, USA, 2012; Volume 65, pp. 107–120. [Google Scholar]
- Lauritano, C.; Ianora, A. Marine organisms with anti-diabetes properties. Mar. Drugs 2016, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, V. Cosmeceuticals from marine fish and shellfish. In Marine Cosmeceuticals: Trends and Prospects; Kim, S.-K., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 211–232. [Google Scholar]
- Senevirathne, M.; Kim, S.-K. Development of bioactive peptides from fish proteins and their health promoting ability. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Academic Press: Waltham, MA, USA, 2012; Volume 65, pp. 235–248. [Google Scholar]
- Ngo, D.H.; Vo, T.S.; Ngo, D.N.; Wijesekara, I.; Kim, S.K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.-K. Bioactive peptide of marine origin for the prevention and treatment of non-communicable diseases. Mar. Drugs 2017, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Senaratne, L.; Park, P.-J.; Kim, S.-K. Isolation and characterization of collagen from brown backed toadfish (Lagocephalus gloveri) skin. Bioresour. Technol. 2006, 97, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Adhikari, B.; Dhara, S. Isolation and characterization of fish scale collagen of higher thermal stability. Bioresour. Technol. 2010, 101, 3737–3742. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-S.; Venkatesan, J.; Kim, S.-K. Isolation and characterization of collagen from marine fish (Thunnus obesus). Biotechnol. Bioprocess Eng. 2013, 18, 1185–1191. [Google Scholar] [CrossRef]
- Xu, Y.; Han, X.; Li, Y. Effect of marine collagen peptides on long bone development in growing rats. J. Sci. Food Agric. 2010, 90, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Swatschek, D.; Schatton, W.; Kellermann, J.; Müller, W.E.; Kreuter, J. Marine sponge collagen: Isolation, characterization and effects on the skin parameters surface-pH, moisture and sebum. Eur. J. Pharm. Biopharm. 2002, 53, 107–113. [Google Scholar] [CrossRef]
- Cho, J.K.; Jin, Y.G.; Rha, S.J.; Kim, S.J.; Hwang, J.H. Biochemical characteristics of four marine fish skins in Korea. Food Chem. 2014, 159, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Haug, I.J.; Draget, K.I.; Smidsrød, O. Physical and rheological properties of fish gelatin compared to mammalian gelatin. Food Hydrocoll. 2004, 18, 203–213. [Google Scholar] [CrossRef]
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A. Marine collagen: An emerging player in biomedical applications. J. Food Sci. Technol. 2015, 52, 4703–4707. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, N.; Jeya Shakila, R.; Sukumar, D.; Jeyasekaran, G. A Skin, bone and muscle collagen extraction from the trash fish, leather jacket (Odonus niger) and their characterization. J. Food Sci. Technol. 2013, 50, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Araki, Y.; Suzuki, N. Collagen of the skin of ocellate puffer fish (Takifugu rubripes). Food Chem. 2002, 78, 173–177. [Google Scholar] [CrossRef]
- Shanmugam, V.; Ramasamy, P.; Subhapradha, N.; Sudharsan, S.; Seedevi, P.; Moovendhan, M.; Krishnamoorthy, J.; Shanmugam, A.; Srinivasan, A. Extraction, structural and physical characterization of type I collagen from the outer skin of Sepiella inermis (Orbigny, 1848). Afr. J. Biotechnol. 2012, 11, 14326–14337. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Isolation and characterisation of acid and pepsin-solubilised collagens from the skin of Brownstripe red snapper (Lutjanus vitta). Food Chem. 2005, 93, 475–484. [Google Scholar] [CrossRef]
- Kumar, N.S.S.; Nazeer, R.A. Wound healing properties of collagen from the bone of two marine fishes. Int. J. Pept. Res. Ther. 2012, 18, 185–192. [Google Scholar] [CrossRef]
- Veeruraj, A.; Arumugam, M.; Balasubramanian, T. Isolation and characterization of thermostable collagen from the marine eel-fish (Evenchelys macrura). Process Biochem. 2013, 48, 1592–1602. [Google Scholar] [CrossRef]
- Veeruraj, A.; Arumugam, M.; Ajithkumar, T.; Balasubramanian, T. Isolation and characterization of drug delivering potential of type-I collagen from eel fish Evenchelys macrura. J. Mater. Sci. Mater. Med. 2012, 23, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Minh Thuy, L.T.; Okazaki, E.; Osako, K. Isolation and characterization of acid-soluble collagen from the scales of marine fishes from Japan and Vietnam. Food Chem. 2014, 149, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Duan, R.; Huang, L.; Song, Y.; Regenstein, J.M. Characterisation of acid-soluble and pepsin-solubilised collagen from jellyfish (Cyanea nozakii Kishinouye). Food Chem. 2014, 150, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Muthumari, K.; Anand, M.; Maruthupandy, M. Collagen extract from marine finfish scales as a potential mosquito larvicide. Protein J. 2016, 35, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 2005, 89, 363–372. [Google Scholar] [CrossRef]
- Nalinanon, S.; Benjakul, S.; Visessanguan, W.; Kishimura, H. Use of pepsin for collagen extraction from the skin of bigeye snapper (Priacanthus tayenus). Food Chem. 2007, 104, 593–601. [Google Scholar] [CrossRef]
- Benjakul, S.; Thiansilakul, Y.; Visessanguan, W.; Roytrakul, S.; Kishimura, H.; Prodpran, T.; Meesane, J. Extraction and characterisation of pepsin-solubilised collagens from the skin of bigeye snapper (Priacanthus tayenus and Priacanthus macracanthus). J. Sci. Food Agric. 2010, 90, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Matmaroh, K.; Benjakul, S.; Prodpran, T.; Encarnacion, A.B.; Kishimura, H. Characteristics of acid soluble collagen and pepsin soluble collagen from scale of spotted golden goatfish (Parupeneus heptacanthus). Food Chem. 2011, 129, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, W.; Li, G. Isolation and characterisation of collagens from the skin of largefin longbarbel catfish (Mystus macropterus). Food Chem. 2009, 115, 826–831. [Google Scholar] [CrossRef]
- Khan, S.B.; Qian, Z.-J.; Ryu, B.; Kim, S.-K. Isolation and biochemical characterization of collagens from seaweed pipefish, Syngnathus schlegeli. Biotechnol. Bioprocess Eng. 2009, 14, 436–442. [Google Scholar] [CrossRef]
- Nagai, T.; Ogawa, T.; Nakamura, T.; Ito, T.; Nakagawa, H.; Fujiki, K.; Nakao, M.; Yano, T. Collagen of edible jellyfish exumbrella. J. Sci. Food Agric. 1999, 79, 855–858. [Google Scholar] [CrossRef]
- Barzideh, Z.; Latiff, A.A.; Gan, C.Y.; Benjakul, S.; Karim, A.A. Isolation and characterisation of collagen from the ribbon jellyfish (Chrysaora sp.). Int. J. Food Sci. Technol. 2014, 49, 1490–1499. [Google Scholar] [CrossRef]
- Available online: https://www.justvitamins.co.uk/blog/bovine-collagen-vs-marine-collagen/ (accessed on 11 May 2017).
- Xhauflaire-Uhoda, E.; Fontaine, K.; Pierard, G. Kinetics of moisturizing and firming effects of cosmetic formulations. Int. J. Cosmet. Sci. 2008, 30, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Chandika, P.; Ko, S.-C.; Oh, G.-W.; Heo, S.-Y.; Nguyen, V.-T.; Jeon, Y.-J.; Lee, B.; Jang, C.H.; Kim, G.; Park, W.S. Fish collagen/alginate/chitooligosaccharides integrated scaffold for skin tissue regeneration application. Int. J. Biol. Macromol. 2015, 81, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Purohit, V.; Agarwal, M.; Singh, A.; Goel, R. In vivo healing potential of Aegle marmelos in excision, incision, and dead space wound models. Sci. World J. 2014, 2014, 740197. [Google Scholar] [CrossRef] [PubMed]
- Nithya, M.; Suguna, L.; Rose, C. The effect of nerve growth factor on the early responses during the process of wound healing. Biochim. Biophys. Acta Gen. Subj. 2003, 1620, 25–31. [Google Scholar] [CrossRef]
- Song, E.; Yeon Kim, S.; Chun, T.; Byun, H.J.; Lee, Y.M. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.; Jung, W.-K.; Kim, G. Fabrication, characterisation and biological activity of phlorotannin-conjugated PCL/β-TCP composite scaffolds for bone tissue regeneration. J. Mater. Chem. 2012, 22, 3568–3577. [Google Scholar] [CrossRef]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Nagai, N.; Murata, M.; Nishimura, D.; Sugi, M.; Munekata, M. Development of salmon milt DNA/salmon collagen composite for wound dressing. J. Mater. Sci. Mater. Med. 2008, 19, 3473–3479. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Yamamoto, K.; Ikeda, T.; Yanagiguchi, K.; Hayashi, Y. Potency of fish collagen as a scaffold for regenerative medicine. Biomed Res. Int. 2014, 2014, 302932. [Google Scholar] [CrossRef] [PubMed]
- Elango, J.; Zhang, J.; Bao, B.; Palaniyandi, K.; Wang, S.; Wenhui, W.; Robinson, J.S. Rheological, biocompatibility and osteogenesis assessment of fish collagen scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2016, 91, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.I.; Kim, S.Y.; Cho, S.K.; Chong, M.S.; Kim, K.S.; Kim, H.; Lee, S.B.; Lee, Y.M. Tissue-engineered vascular grafts composed of marine collagen and PLGA fibers using pulsatile perfusion bioreactors. Biomaterials 2007, 28, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.C.; Lai, Y.L.; Lee, S.Y.; Hung, S.L.; Chen, H.L. Osteoblastic response to collagen scaffolds varied in freezing temperature and glutaraldehyde crosslinking. J. Biomed. Mater. Res. 2007, 80, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Mullen, C.; Haugh, M.; Schaffler, M.; Majeska, R.; McNamara, L. Osteocyte differentiation is regulated by extracellular matrix stiffness and intercellular separation. J. Mech. Behav. Biomed. 2013, 28, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Byrne, E.M.; Farrell, E.; McMahon, L.A.; Haugh, M.G.; O’Brien, F.J.; Campbell, V.A.; Prendergast, P.J.; O’Connell, B.C. Gene expression by marrow stromal cells in a porous collagen–glycosaminoglycan scaffold is affected by pore size and mechanical stimulation. J. Mater. Sci. Mater. Med. 2008, 19, 3455–3463. [Google Scholar] [CrossRef] [PubMed]
- Keogh, M.B.; O’Brien, F.J.; Daly, J.S. A novel collagen scaffold supports human osteogenesis—Applications for bone tissue engineering. Cell Tissue Res. 2010, 340, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Chen, H.-M.; Shiau, C.-Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 2003, 36, 949–957. [Google Scholar] [CrossRef]
- Kim, S.-K. Marine Proteins and Peptides: Biological Activities and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Lintner, K.; Peschard, O. Biologically active peptides: From a laboratory bench curiosity to a functional skin care product. Int. J. Cosmet. Sci. 2000, 22, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Khora, S.S. Marine fish-derived bioactive peptides and proteins for human therapeutics. Int. J. Pharm. Pharm. Sci. 2013, 5, 31–37. [Google Scholar]
- Halim, N.; Yusof, H.; Sarbon, N. Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- Sila, A.; Hedhili, K.; Przybylski, R.; Ellouz-Chaabouni, S.; Dhulster, P.; Bougatef, A.; Nedjar-Arroume, N. Antibacterial activity of new peptides from barbel protein hydrolysates and mode of action via a membrane damage mechanism against Listeria monocytogenes. J. Funct. Foods 2014, 11, 322–329. [Google Scholar] [CrossRef]
- Kim, S.K. Marine cosmeceuticals. J. Cosmet. Dermatol. 2014, 13, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.V.; Kim, S.-K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Ravichandran, Y.D.; Khan, S.B.; Kim, Y.T. Prospective of the cosmeceuticals derived from marine organisms. Biotechnol. Bioprocess Eng. 2008, 13, 511–523. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef] [PubMed]
- Je, J.-Y.; Qian, Z.-J.; Byun, H.-G.; Kim, S.-K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Je, J.-Y.; Qian, Z.-J.; Lee, S.-H.; Byun, H.-G.; Kim, S.-K. Purification and antioxidant properties of bigeye tuna (Thunnus obesus) dark muscle peptide on free radical-mediated oxidative systems. J. Med. Food 2008, 11, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.-J.; Byun, H.-G.; Kim, S.-K. Improvement of functional properties of cod frame protein hydrolysates using ultrafiltration membranes. Process Biochem. 1999, 35, 471–478. [Google Scholar] [CrossRef]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Yoon, N.Y.; Eom, T.-K.; Kim, M.-M.; Kim, S.-K. Inhibitory effect of phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J. Agric. Food Chem. 2009, 57, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Schurink, M.; van Berkel, W.J.; Wichers, H.J.; Boeriu, C.G. Novel peptides with tyrosinase inhibitory activity. Peptides 2007, 28, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-M.; Van Ta, Q.; Mendis, E.; Rajapakse, N.; Jung, W.-K.; Byun, H.-G.; Jeon, Y.-J.; Kim, S.-K. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci. 2006, 79, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-A.; Kim, S.-K. Bioactive peptides from marine sources as potential anti-inflammatory therapeutics. Curr. Protein Pept. Sci. 2013, 14, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Winata, A.; Lorenz, K. Antioxidant potential of 5-n-pentadecylresorcinol. J. Food Process. Preserv. 1996, 20, 417–429. [Google Scholar] [CrossRef]
- Becker, G. Preserving food and health: Antioxidants make functional, nutritious preservatives. Food Process. 1993, 12, 54–56. [Google Scholar]
- Osawa, T.; Namiki, M. Natural antioxidants isolated from Eucalyptus leaf waxes. J. Agric. Food Chem. 1985, 33, 777–780. [Google Scholar] [CrossRef]
- Byun, H.-G.; Lee, J.K.; Park, H.G.; Jeon, J.-K.; Kim, S.-K. Antioxidant peptides isolated from the marine rotifer, Brachionus rotundiformis. Process Biochem. 2009, 44, 842–846. [Google Scholar] [CrossRef]
- Kim, S.-K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Je, J.-Y.; Cho, Y.-S. Antioxidant and anti-inflammatory peptide fraction from salmon byproduct protein hydrolysates by peptic hydrolysis. Food Res. Int. 2012, 49, 92–98. [Google Scholar] [CrossRef]
- Jiang, H.; Tong, T.; Sun, J.; Xu, Y.; Zhao, Z.; Liao, D. Purification and characterization of antioxidative peptides from round scad (Decapterus maruadsi) muscle protein hydrolysate. Food Chem. 2014, 154, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Je, J.-Y.; Kim, S.-K. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007, 18, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-Y.; Lee, J.-H.; Samarakoon, K.; Kim, J.-S.; Jeon, Y.-J. Purification and determination of two novel antioxidant peptides from flounder fish (Paralichthys olivaceus) using digestive proteases. Food Chem. Toxicol. 2013, 52, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.S.; Nazeer, R.; Jaiganesh, R. Purification and biochemical characterization of antioxidant peptide from horse mackerel (Magalaspis cordyla) viscera protein. Peptides 2011, 32, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.S.; Nazeer, R.; Jaiganesh, R. Purification and identification of antioxidant peptides from the skin protein hydrolysate of two marine fishes, horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber). Amino Acids 2012, 42, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Mendis, E.; Rajapakse, N.; Kim, S.-K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Nazeer, R.; Kumar, N.S.; Ganesh, R.J. In vitro and in vivo studies on the antioxidant activity of fish peptide isolated from the croaker (Otolithes ruber) muscle protein hydrolysate. Peptides 2012, 35, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.-H.; Qian, Z.-J.; Ryu, B.; Park, J.W.; Kim, S.-K. In vitro antioxidant activity of a peptide isolated from Nile tilapia (Oreochromis niloticus) scale gelatin in free radical-mediated oxidative systems. J. Funct. Foods 2010, 2, 107–117. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.; Li-Chan, E.C. Autolysis-assisted production of fish protein hydrolysates with antioxidant properties from Pacific hake (Merluccius productus). Food Chem. 2008, 107, 768–776. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X.; Zhuang, Y. Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides 2012, 38, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-S.; Jeon, J.-K.; Byun, H.-G. Characterization of a novel antioxidative peptide from the sand eel Hypoptychus dybowskii. Process Biochem. 2011, 46, 1207–1211. [Google Scholar] [CrossRef]
- Kong, Y.-Y.; Chen, S.-S.; Wei, J.-Q.; Chen, Y.-P.; Lan, W.-T.; Yang, Q.-W.; Huang, G.-R. Preparation of antioxidative peptides from spanish mackerel (Scomberomorus niphonius) processing byproducts by enzymatic hydrolysis. Biotechnology 2015, 14, 188–193. [Google Scholar]
- Jeevithan, E.; Bao, B.; Zhang, J.; Hong, S.; Wu, W. Purification, characterization and antioxidant properties of low molecular weight collagenous polypeptide (37 kDa) prepared from whale shark cartilage (Rhincodon typus). J. Food Sci. Technol. 2015, 52, 6312–6322. [Google Scholar] [CrossRef] [PubMed]
- Gajanan, P.G.; Elavarasan, K.; Shamasundar, B.A. Bioactive and functional properties of protein hydrolysates from fish frame processing waste using plant proteases. Environ. Sci. Pollut. Res. 2016, 23, 24901–24911. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Q.; Li, J.; Zhou, B. Peptides derived from Rhopilema esculentum hydrolysate exhibit angiotensin converting enzyme (ACE) inhibitory and antioxidant abilities. Molecules 2014, 19, 13587–13602. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Maeda, T.; Hasegawa, Y.; Tokunaga, T.; Ogawa, S.; Fukuda, K.; Nagatsuka, N.; Nagao, K.; Ueno, S. Antioxidant activity of the giant jellyfish Nemopilema nomurai measured by the oxygen radical absorbance capacity and hydroxyl radical averting capacity methods. Mol. Med. Rep. 2011, 4, 919–922. [Google Scholar] [PubMed]
- Samanta, J.K.M.P.K.; Khora, S. Antioxidant activity of fish protein hydrolysates from Sardinella longiceps. Int. J. Drug Dev. Res. 2014, 6, 137–145. [Google Scholar]
- Chi, C.-F.; Cao, Z.-H.; Wang, B.; Hu, F.-Y.; Li, Z.-R.; Zhang, B. Antioxidant and functional properties of collagen hydrolysates from spanish mackerel skin as influenced by average molecular weight. Molecules 2014, 19, 11211–11230. [Google Scholar] [CrossRef] [PubMed]
- Kangsanant, S.; Thongraung, C.; Jansakul, C.; Murkovic, M.; Seechamnanturakit, V. Purification and characterisation of antioxidant and nitric oxide inhibitory peptides from Tilapia (Oreochromis niloticus) protein hydrolysate. Int. J. Food Sci. Technol. 2015, 50, 660–665. [Google Scholar] [CrossRef]
- Bardan, A.; Nizet, V.; Gallo, R.L. Antimicrobial peptides and the skin. Expert Opin. Biol. Ther. 2004, 4, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Wei, R.-B.; Luo, H.-Y.; Wang, D.-F. Isolation and characterization of an antibacterial peptide fraction from the pepsin hydrolysate of half-fin anchovy (Setipinna taty). Molecules 2012, 17, 2980–2991. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Wei, R.; Zhang, B.; Wang, D. Optimization of the antibacterial activity of half-fin anchovy (Setipinna taty) hydrolysates. Food Bioprocess Technol. 2012, 5, 1979–1989. [Google Scholar] [CrossRef]
- Ennaas, N.; Hammami, R.; Beaulieu, L.; Fliss, I. Purification and characterization of four antibacterial peptides from protamex hydrolysate of Atlantic mackerel (Scomber scombrus) by-products. Biochem. Biophys. Res. Commun. 2015, 462, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Ennaas, N.; Hammami, R.; Beaulieu, L.; Fliss, I. Production of antibacterial fraction from Atlantic mackerel (Scomber scombrus) and its processing by-products using commercial enzymes. Food Bioprod. Process. 2015, 96, 145–153. [Google Scholar] [CrossRef]
- Ryu, B.; Qian, Z.J.; Kim, S.K. SHP-1, A novel peptide isolated from seahorse inhibits collagen release through the suppression of collagenases 1 and 3, nitric oxide products regulated by NF-κB/p38 kinase. Peptides 2010, 31, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Qian, Z.J.; Kim, S.K. Purification of a peptide from seahorse, that inhibits TPA-induced MMP, iNOS and COX-2 expression through MAPK and NF-κB activation, and induces human osteoblastic and chondrocytic differentiation. Chem. Biol. Interact. 2010, 184, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Guo, R.; Dai, Z.; Zhang, Y. Investigation of enzymatic hydrolysis conditions on the properties of protein hydrolysate from fish muscle (Collichthys niveatus) and evaluation of its functional properties. J. Agric. Food Chem. 2012, 60, 5192–5198. [Google Scholar] [CrossRef] [PubMed]
- Lødemel, J.B.; Egge-Jacobsen, W.; Olsen, R.L. Detection of TIMP-2-like protein in Atlantic cod (Gadus morhua) muscle using two-dimensional real-time reverse zymography. Biosci. Biotechnol. Biochem. 2004, 139, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.; Elmets, C.A.; Katiyar, S.K. Green tea and skin cancer: Photoimmunology, angiogenesis and DNA repair. J. Nutr. Biochem. 2007, 18, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Hou, H.; Zhao, X.; Zhang, Z.; Li, B. Effects of collagen and collagen hydrolysate from jellyfish (Rhopilema esculentum) on mice skin photoaging induced by UV irradiation. J. Food Sci. 2009, 74, H183–H188. [Google Scholar] [CrossRef] [PubMed]
- Diffey, B.L. Solar ultraviolet radiation effects on biological systems. Phys. Med. Biol. 1991, 36, 299–328. [Google Scholar] [CrossRef] [PubMed]
- Tanino, Y.; Budiyanto, A.; Ueda, M.; Nakada, A.; Nyou, W.T.; Yanagisawa, M.; Ichihashi, M.; Yamamoto, Y. Decrease of antioxidants and the formation of oxidized diacylglycerol in mouse skin caused by UV irradiation. J. Dermatol. Sci. Suppl. 2005, 1, S21–S28. [Google Scholar] [CrossRef]
- Han, Y.-T.; Han, Z.-W.; Yu, G.-Y.; Wang, Y.-J.; Cui, R.-Y.; Wang, C.-B. Inhibitory effect of polypeptide from Chlamys farreri on ultraviolet A-induced oxidative damage on human skin fibroblasts in vitro. Pharmacol. Res. 2004, 49, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, Z.; Liu, X.; Wang, Y. Effects of polypeptides from Chlamys farreri on the structure of skin and the content of antioxidants in hairless mice irradiated by ultraviolet B. China J. Lepr. Skin Dis. 2004, 20, 20–23. [Google Scholar]
- Wang, C.-B.; Ding, B.-X.; Guo, S.-B.; Wang, Y.-Z.; Han, Y.-T.; Wang, Y.-J. Protective effect of polypeptide from Chlamys farreri on mitochondria in human dermal fibroblasts irradiated by ultraviolet B. Acta Pharmacol. Sin. 2003, 24, 692–696. [Google Scholar] [PubMed]
- Leone, A.; Lecci, R.M.; Durante, M.; Meli, F.; Piraino, S. The bright side of gelatinous blooms: Nutraceutical value and antioxidant properties of three Mediterranean jellyfish (Scyphozoa). Mar. Drugs 2015, 13, 4654–4681. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhuang, Y.; Li, B. Effects of collagen and collagen hydrolysate from jellyfish umbrella on histological and immunity changes of mice photoaging. Nutrients 2013, 5, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Li, B.; Zhang, Z.; Xue, C.; Yu, G.; Wang, J.; Bao, Y.; Bu, L.; Sun, J.; Peng, Z.; et al. Moisture absorption and retention properties, and activity in alleviating skin photodamage of collagen polypeptide from marine fish skin. Food Chem. 2012, 135, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Hou, H. Protective effect of gelatin polypeptides from Pacific cod (Gadus macrocephalus) against UV irradiation-induced damages by inhibiting inflammation and improving transforming growth factor-β/Smad signaling pathway. J. Photochem. Photobiol. B Biol. 2016, 162, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, S.; Ozawa, Y.; Toda, T.; Watanabe, K.; Tometsuka, C.; Ogura, T.; Koyama, Y.I.; Shimizu, T. Collagen peptide and vitamin C additively attenuate age-related skin atrophy in Sod1-deficient mice. Biosci. Biotechnol. Biochem. 2014, 78, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Hou, H.; Lu, J.; Zhang, K.; Li, B. Protective effect of gelatin and gelatin hydrolysate from salmon skin on UV irradiation-induced photoaging of mice skin. J. Ocean Univ. China 2016, 15, 711–718. [Google Scholar] [CrossRef]
- Jimbo, N.; Kawada, C.; Nomura, Y. Optimization of dose of collagen hydrolysate to prevent UVB-irradiated skin damage. Biosci. Biotechnol. Biochem. 2016, 80, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, Y.; Zhuang, Y. Antiphotoaging effect and purification of an antioxidant peptide from tilapia (Oreochromis niloticus) gelatin peptides. J. Funct. Foods 2013, 5, 154–162. [Google Scholar] [CrossRef]
- Zhuang, Y.; Sun, L. Preparation of reactive oxygen scavenging peptides from tilapia (Oreochromis niloticus) skin gelatin: Optimization using response surface methodology. J. Food Sci. 2011, 76, C483–C489. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Mikhal’Chik, E.V.; Suprun, M.V.; Papacharalambous, M.; Truhanov, A.I.; Korkina, L.G. Skin antiageing and systemic Redox effects of supplementation with marine collagen peptides and plant-derived antioxidants: A single-blind case-control clinical study. Oxid. Med. Cell. Longev. 2016, 2016, 4389410. [Google Scholar] [CrossRef] [PubMed]
| Fish Species Name | Parts | Method | Yield (%) | Reference |
|---|---|---|---|---|
| Lagocephalus gloveri | Skin | PSC | 54.3 | [17] |
| Thunnus obesus | Bone | ASC and PSC | -- | [19] |
| Paralichthys olivaceus, Sebastes schlegeli, Lateolabrax maculatus, Pagrus major | Skin | ASC | -- | [22] |
| Takifugu rubripes | Skin | ASC and PSC | 10.7 and 44.7 | [27] |
| Sepiella inermis | Skin | ASC and PSC | 0.58 and 16.23 | [28] |
| Lutjanus vitta | Skin | ASC and PSC | 9.0 and 4.7 | [29] |
| Magalaspis cordyla | Bone | ASC and PSC | 30.5 and 27.6 | [30] |
| Otolithes ruber | Bone | ASC and PSC | 45.1 and 48.6 | [30] |
| Evenchelys macrura | Skin | ASC and PSC | 80 and 7.1 | [31,32] |
| Saurida spp., Trachurus japonicus, Mugil cephalis, Cypselurus melanurus, Dentex tumifrons | Scales | ASC | 0.13–1.5% | [33] |
| Cyanea nozakii Kishinouye | All parts | ASC and PSC | 13.0 and 5.5 | [34] |
| Sardinella longiceps | Scales | ASC and PSC | 1.25 and 3 | [35] |
| Priacanthus tayenus | Skin and bone | ASC | 10.94 and 1.59 (Skin and bone) | [36] |
| Priacanthus tayenus | Skin | PSC | -- | [37] |
| Priacanthus tayenus and Priacanthus macracanthus | Skin | PSC | -- | [38] |
| Parupeneus heptacanthus | Scale | ASC and PSC | 0.46 and 1.2 | [39] |
| Mystus macropterus | Skin | ASC and PSC | 16.8 and 28 | [40] |
| Syngnathus schlegeli | All parts | ASC and PSC | 5.5 and 33.2 | [41] |
| Jellyfish | All parts | PSC | 46.4 | [42] |
| Chrysaora sp. | All parts | PSC | 9–19 | [43] |
| Enzymes for Hydrolysis | Buffer | pH | Temperature (°C) |
|---|---|---|---|
| alcalase | 0.1 M Na2HPO4–NaH2PO4 | 7 | 50 |
| α-chymotrypsin | 0.1 M Na2HPO4–NaH2PO4 | 8 | 37 |
| papain | 0.1 M Na2HPO4–NaH2PO4 | 6 | 37 |
| pepsin | 0.1 M Glycine–HCl | 2 | 37 |
| neutrase | 0.1 M Na2HPO4–NaH2PO4 | 8 | 50 |
| trypsin | 0.1 M Na2HPO4–NaH2PO4 | 8 | 37 |
| Activity | Cosmeceutical Applications | Reference |
|---|---|---|
| Antioxidant | Anti-aging, photo-protective effects | [15] |
| Tyrosinase inhibitor | Whitening | [75] |
| MMP inhibitor | Anti-wrinkle | [76] |
| Anti-inflammatory | Skin soothing | [77] |
| Fish Species Name | Enzymes for Hydrolysis | Peptides (Amino Acid Sequence) | Reference |
|---|---|---|---|
| Scomber austriasicus | protease N | -- | [60] |
| Thunnus obesus | alcalase, α-chymotrypsin, neutrase, papain, pepsin, and trypsin | H-Leu-Asn-Leu-Pro-Thr-Ala-Val-Tyr-Met-Val-Thr-OH | [71] |
| Salmon | alcalase, flavourzyme, neutrase, pepsin, protamex, and trypsin | Peptides (unknown sequence, 1000–2000 Da) | [86] |
| Decapterus maruadsi | alcalase, neutral protease, papain, pepsin, and trypsin | His-Asp-His-Pro-Val-Cys and His-Glu-Lys-Val-Cys | [87] |
| Johnius belengerii | pepsin, trypsin, papain, α-chymotrypsin, alcalase, and neutrase | Glu-Ser-Thr-Val-Pro-Glu-Arg-Thr-His-Pro-Ala-Cys-Pro-Asp-Phe-Asn | [88] |
| Paralichthys olivaceus | papain, pepsin, trypsin, neutrase, alcalase, kojizyme, protamex, and α-chymotrypsin | Val-Cys-Ser-Val and Cys-Ala-Ala-Pro | [89] |
| Magalaspis cordyla | pepsin, trypsin, and α-chymotrypsin | Ala–Cys–Phe–Leu (518.5 Da), | [90] |
| Magalaspis cordyla | pepsin/trypsin, and α-chymotrypsin | Asn-His-Arg-Tyr-Asp-Arg (856 Da) | [91] |
| Otolithes ruber | pepsin/trypsin and α-chymotrypsin | Gly-Asn-Arg-Gly-Phe-Ala-Cys-Arg-His-Ala (1101.5 Da) | [91] |
| Johnius belengerii | trypsin, R-chymotrypsin, and pepsin | His-Gly-Pro-Leu-Gly-Pro-Leu | [92] |
| Otolithes ruber | pepsin, trypsin, and α-chymotrypsin | Lys-Thr-Phe-Cys-Gly-Arg-His | [93] |
| Oreochromis niloticus | alcalase, pronase E, pepsin, and trypsin | Asp-Pro-Ala-Leu-Ala-Thr-Glu-Pro-Asp-Pro-Met-Pro-Phe | [94] |
| Merluccius productus | Validase ® BNP (V) and Flavourzyme ® | -- | [95] |
| Oreochromis niloticus | properase E and multifect neutral | Glu-Gly-Leu (317.33 Da) and Tyr-Gly-Asp-Glu-Tyr | [96] |
| Hypoptychus dybowskii | alcalase, neutrase, α-chymotrypsin, papain, pepsin, and trypsin | Ile–Val–Gly–Gly–Phe–Pro–His–Tyr–Leu | [97] |
| Name of Fish Species | Enzymes for Hydrolysis | Microorganisms | Reference |
|---|---|---|---|
| Setipinna taty | pepsin | Escherichia coli | [107] |
| Setipinna taty | papain, pepsin, trypsin, alkaline protease, acidic protease, and flavoring protease | Escherichia coli, Pseudomonas fluorescens Proteus vulgaris, Bacillus megaterium Staphylococcus aureus, Bacillus subtilis, Bacillus megaterium, Sarcina lutea | [108] |
| Scomber scombrus | -- | Listeria innocua, Escherichia coli | [109] |
| Scomber scombrus | protamex, neutrase, papain, and flavourzyme. | Listeria innocua HPB13 and Escherichia coli | [110] |
| Name of Fish Species and Parts | Fish-Derived Proteins and Peptides | Enzymes for Hydrolysis | Reference |
|---|---|---|---|
| Jellyfish | Collagen | properase E | [123] |
| Cod skin | Collagen polypeptides | alkaline protease and pepsin | [124] |
| Cod skin | Gelatin hydrolysate | alkaline protease and trypsin | [125] |
| Salmon skin | Gelatin | alkaline protease and trypsin | [127] |
| Tilapia | Gelatin peptides | properase E | [129] |
| Pollachius virens, Hippoglossus hippoglossus, and Pleuronectes platessa | Marine collagen peptides | complex proteases | [131] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs 2017, 15, 143. https://doi.org/10.3390/md15050143
Venkatesan J, Anil S, Kim S-K, Shim MS. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Marine Drugs. 2017; 15(5):143. https://doi.org/10.3390/md15050143
Chicago/Turabian StyleVenkatesan, Jayachandran, Sukumaran Anil, Se-Kwon Kim, and Min Suk Shim. 2017. "Marine Fish Proteins and Peptides for Cosmeceuticals: A Review" Marine Drugs 15, no. 5: 143. https://doi.org/10.3390/md15050143







