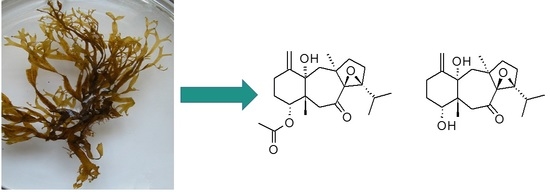
Two New Oxodolastane Diterpenes from the Jamaican Macroalga Canistrocarpus cervicornis
Abstract
:1. Introduction
2. Results and Discussion
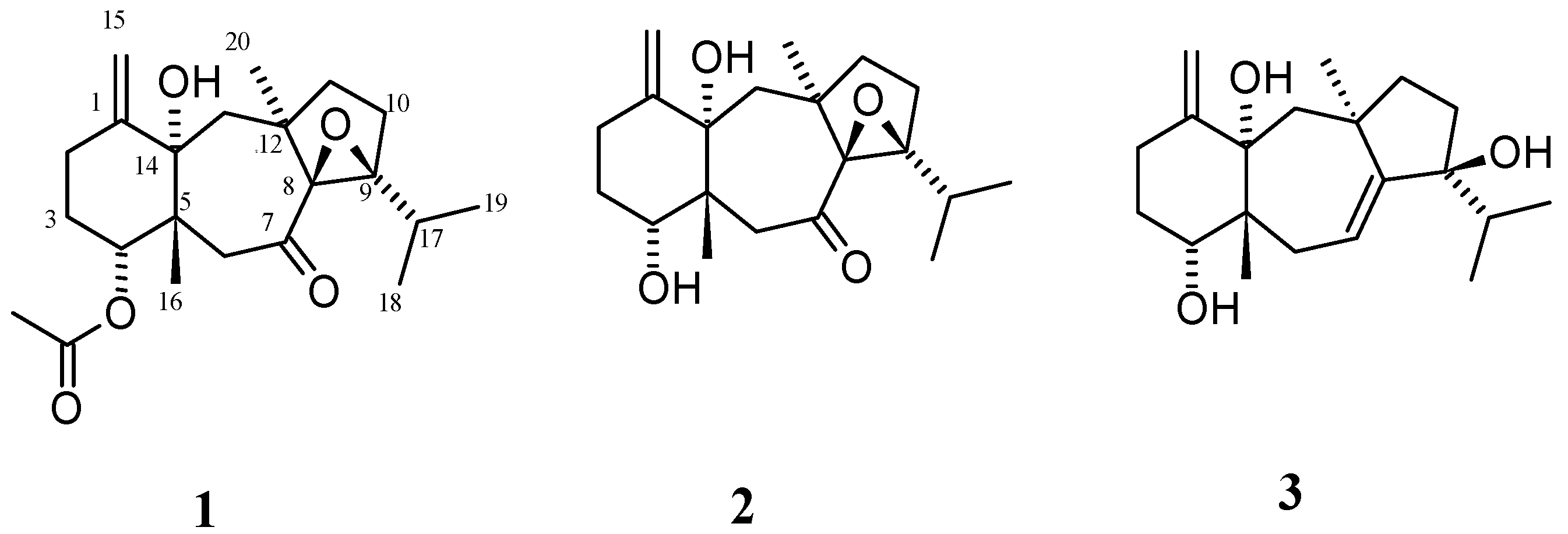
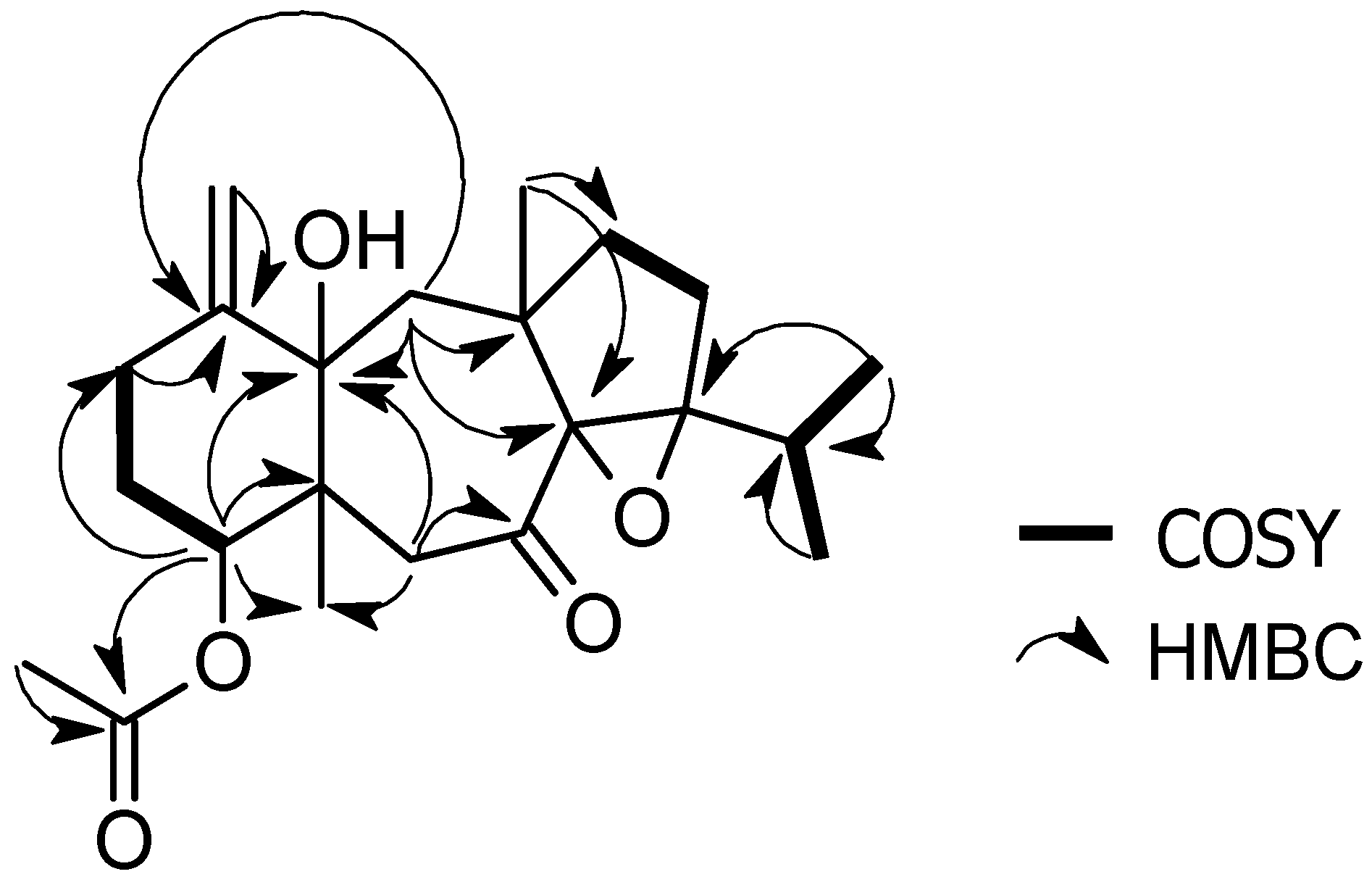
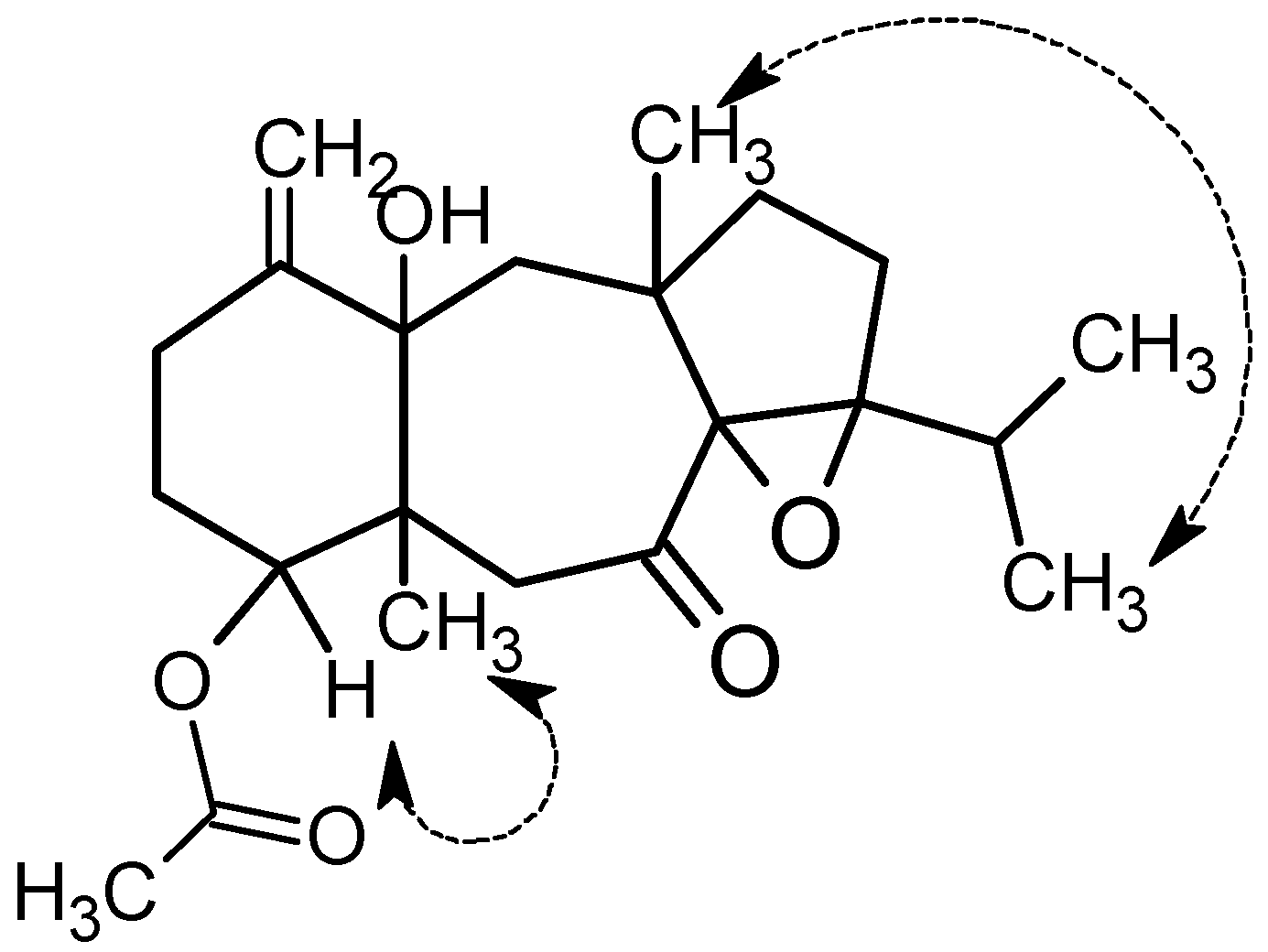
2.1. Structural Elucidation
2.2. Cytotoxicity Evaluation against PC3 and HT29 Cancer Cell Lines
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Algal Sample Collection
3.3. Extraction, Isolation, and Structural Elucidation of Compounds
3.3.1. 4R-Acetoxy-8S,9S-epoxy-14S-hydroxy-7-oxodolastane (1)
3.3.2. 4R-Hydroxy-8S,9S-epoxy-14S-hydroxy-7-oxodolastane (2)
3.3.3. (4R,9S,14S)-4,9,14-Trihydroxydolast-1(15),7-diene (3)
3.4. Cytotoxic Evaluation of Compounds
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Malve, H.O. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Media Centre: Cancer Fact Sheet, February 2017. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/ (accessed on 2 May 2017).
- Bianco, E.M.; Francisco, T.M.; Pinheiro, C.B.; Vasconcellos Azeredo, R.B.; Teixera, V.L.; Pereira, R.C. 10β-Acetoxy-8α,9α-epoxy-14β-hydroxy-7-oxodolastane—A new diterpene isolated from the Brazilian brown macroalga Canistrocarpus cervicornis. Helv. Chim. Acta 2015, 98, 785–794. [Google Scholar] [CrossRef]
- Santos, A.O.; Britta, E.A.; Bianco, E.M.; Ueda-Nakamura, T.; Filho, B.P.D.; Pereira, R.C.; Nakamura, C.V. 4-Acetoxydolastane diterpene from the Brazilian brown alga Canistrocarpus cervicornis as antileishmanial Agent. Mar. Drugs 2011, 9, 2369–2383. [Google Scholar] [CrossRef] [PubMed]
- De Andrade Moura, L.; Bianco, E.M.; Pereira, R.C.; Teixeira, V.L.; Fuly, A.L. Anticoagulation, and antiplatelet effects of dolastane diterpene isolated from the marine brown alga Canistrocarpus cervicornis. J. Thromb. Thrombolysis 2011, 31, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Domingos, T.H.S.; Vallim, M.A.; Cavalcanti, D.N.; Sanchez, E.F.; Teixeira, V.L.; Fuly, A.L. Effect of diterpenes isolated of the marine alga Canistrocarpus cervicornis against some toxic effects of the venom of the Bothrops jararaca snake. Molecules 2015, 20, 3515–3526. [Google Scholar] [CrossRef] [PubMed]
- Miceli, L.A.; Souza, A.M.T.; de Rodrigues, C.R.; Paixão, I.C.N.P.; Teixeira, V.L.; Castro, H.C. HIV-1 reverse transcriptase: A potential target for marine products. Braz. J. Pharmacogn. 2012, 22, 881–888. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Martin, J.D.; Norte, M.; Rivera, P. Structure and absolute configuration of Dictyota sp. diterpenes. Tetrahedron 1983, 39, 3355–3357. [Google Scholar] [CrossRef]
- Bianco, E.M.; Francisco, T.M.; Pinheiro, C.B.; Vasconcellos Azeredo, R.B.; Teixera, V.L.; Pereira, R.C. 4α-Acetoxyamijidictyol—A new antifeeding dolastane diterpene from the Brazilian brown alga Canistrocarpus cervicornis. Chem. Biodivers. 2015, 12, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, V.L.; Tomassini, T.; Fleury, B.G.; Kelecom, A. Dolastane and secodolastane diterpenes from the marine brown alga, Dictyota cervicornis. J. Nat. Prod. 1986, 49, 570–575. [Google Scholar] [CrossRef]
- Ioannou, E.; Vagias, C.; Roussis, V. Isolation and structure elucidation of three dolastane from brown alga Dilophus spiralis. Mar. Drugs 2013, 11, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhao, M.; Sun, Z.; Yuan, W.; Zhang, S.; Xiang, Z.; Cai, Y.; Dong, J.; Huang, K.; Yan, P. Diterpenes from a Chinese collection of the brown alga Dictyota plectens. J. Nat. Prod. 2014, 77, 2685–2693. [Google Scholar] [CrossRef] [PubMed]
- Vallim, M.A.; Paula, J.C.D.; Pereira, R.C.; Teixeira, V.L. The diterpene from the Dictyotacean marine brown algae in the Tropical Atlantic American Region. Biochem. Syst. Ecol. 2005, 33, 1–16. [Google Scholar] [CrossRef]
- Bianco, E.M.; Rogers, R.; Teixeira, V.L.; Pereira, R.C. Antifoulant diterpenes produced by the brown seaweed Canistrocarpus cervicornis. J. Appl. Phycol. 2009, 21, 341–346. [Google Scholar] [CrossRef]
- Kelecom, A.; Teixera, V.L. Dolastane diterpenes from the marine brown alga Dictyota cervicornis. Phytochemistry 1988, 27, 2097–2909. [Google Scholar] [CrossRef]
- Crews, P.; Klein, T.E.; Hogue, E.R.; Meyers, B.L. Tricyclic diterpene from the brown marine algae Dictyota divaricate and Dictyota linearis. J. Org. Chem. 1982, 47, 811–815. [Google Scholar] [CrossRef]
- Tawfik, E.; Ahamed, M.; Almalik, A.; Alfaqeeh, M.; Alshamsan, A. Prolonged exposure of colon cancer cells to 5-fluorouracil nanoparticles improves its anticancer activity. Saudi Pharm. J. 2017, 25, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.K.; Liang, C.Z.; Huang, J. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Blackledge, G.R. High-dose bicalutamide monotherapy for the treatment of prostate cancer. Urology 1996, 47, 44–47. [Google Scholar] [CrossRef]
- Quero, L.; Giocanti, N.; Hennequin, C.; Favaudon, V. Antagonistic interaction between bicalutamide (Casodex) and radiation in androgen-positive prostate cancer LNCaP cells. Prostate 2010, 70, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Focaccetti, C.; Bruno, A.; Magnani, E.; Bartolini, D.; Principi, E.; Dallaglio, K.; Bucci, E.O.; Finzi, G.; Sess, F.; Noonan, D.M.; et al. Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ROS production in endothelial cells and cardiomyocytes. PLoS ONE 2015, 10, e0115686. [Google Scholar] [CrossRef] [PubMed]
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH | δC | δH | |
| 1 | 149.6 | - | 149.6 | - |
| 2 | 26.3 | 2.66 (t, 12.2) | 25.7 | 2.88 (d, 13.5) |
| 2.13 (m) | 2.10 (m) | |||
| 3 | 27.10 | 1.92 (d, 14.0) | 28.5 | 1.78–1.82 (m) |
| 1.83 (m) | 1.96 (t, 14.0) | |||
| 4 | 81.7 | 4.81 (br s) | 78.7 | 3.53 (br s) |
| 5 | 43.7 | - | 41.2 | - |
| 6 | 50.7 | 3.77 (d, 15.1) | 50.7 | 4.05 (d, 15.3) |
| 2.23 (15.1) | 2.45 (d, 15.3) | |||
| 7 | 207.7 | - | 207.8 | - |
| 8 | 72.0 | - | 70.7 | - |
| 9 | 82.0 | - | 80.7 | - |
| 10 | 21.7 | 1.81 (m) | 21.8 | 1.82 (m) |
| 11 | 36.7 | 1.31 (m) | 36.8 | 1.21 (m) |
| 12 | 40.7 | - | 40.8 | - |
| 13 | 41.2 | 1.92 (d,14.0) | 42.6 | 2.24 (d,14.9) |
| 2.14 (m) | 1.78 (m) | |||
| 14 | 78.2 | - | 78.6 | - |
| 15 | 110.7 | 4.98 (br s) | 109.3 | 4.91 (br s) |
| 5.01 (br s) | 4.95 (br s) | |||
| 16 | 19.5 | 1.07 (s) | 18.3 | 0.96 (d, 6.5) |
| 17 | 27.2 | 2.74 (sept.,6.6) | 27.0 | 2.79 (sept., 6.6) |
| 18 | 18.9 | 1.07 (s) | 17.7 | 1.07 (s) |
| 19 | 19.3 | 0.96 (d, 6.5) | 18.1 | 0.96 (d, 6.5) |
| 20 | 22.9 | 1.37 (s) | 24.9 | 1.41 (s) |
| MeCO | 169.0 | - | - | - |
| MeCO | 21.3 | 2.16 (s) | - | - |
| 4-OH | - | - | - | 3.49 (m) |
| 14-OH | 3.70 (s) | - | - | 3.43 (s) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, S.; Murray, J.; Delgoda, R.; Gallimore, W. Two New Oxodolastane Diterpenes from the Jamaican Macroalga Canistrocarpus cervicornis. Mar. Drugs 2017, 15, 150. https://doi.org/10.3390/md15060150
Campbell S, Murray J, Delgoda R, Gallimore W. Two New Oxodolastane Diterpenes from the Jamaican Macroalga Canistrocarpus cervicornis. Marine Drugs. 2017; 15(6):150. https://doi.org/10.3390/md15060150
Chicago/Turabian StyleCampbell, Sanjay, JeAnn Murray, Rupika Delgoda, and Winklet Gallimore. 2017. "Two New Oxodolastane Diterpenes from the Jamaican Macroalga Canistrocarpus cervicornis" Marine Drugs 15, no. 6: 150. https://doi.org/10.3390/md15060150