Cytotoxic Effects of Sarcophyton sp. Soft Corals—Is There a Correlation to Their NMR Fingerprints?
Abstract
:1. Introduction
2. Results
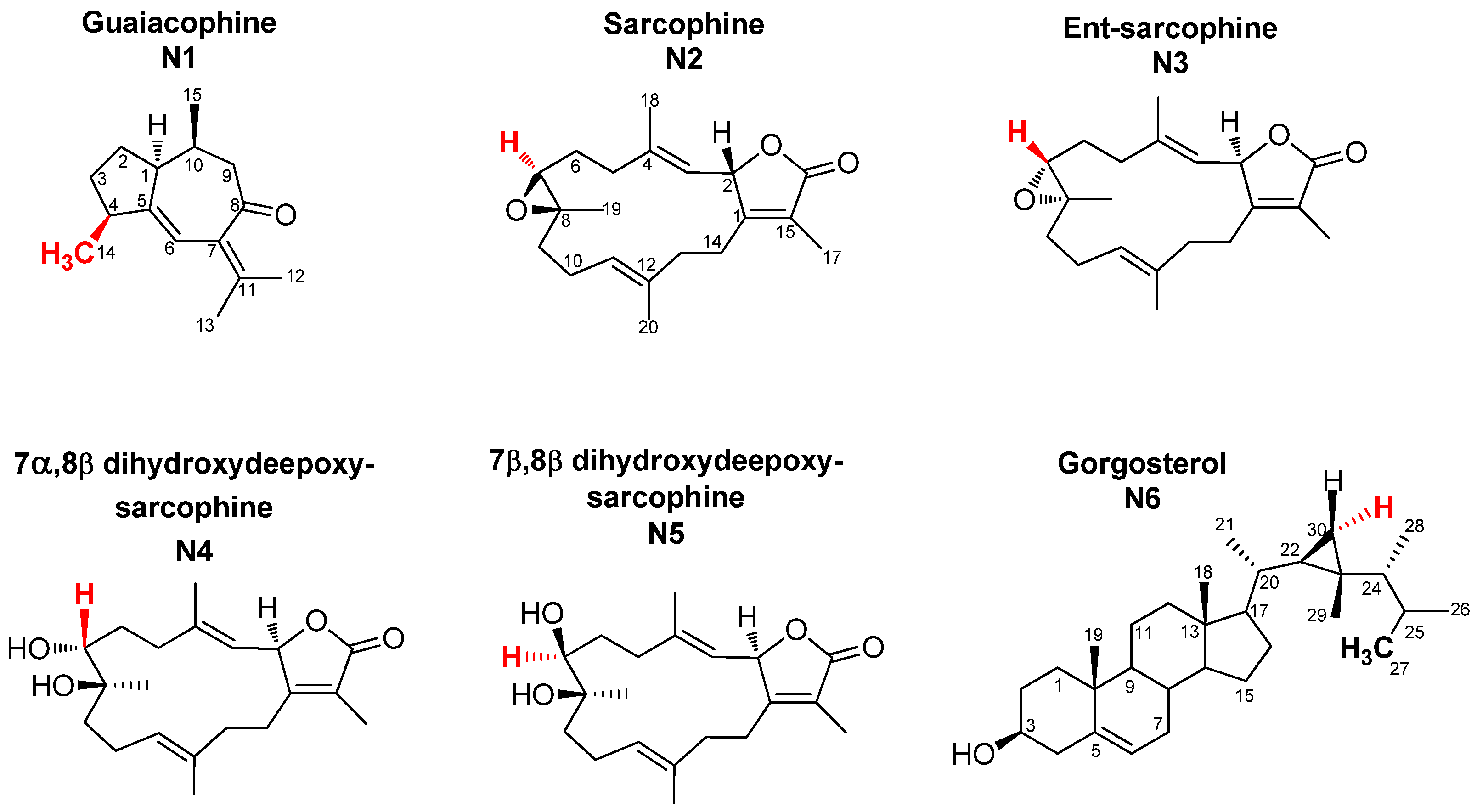
2.1. Selection and Quantification of Terpene/Sterol NMR Signals
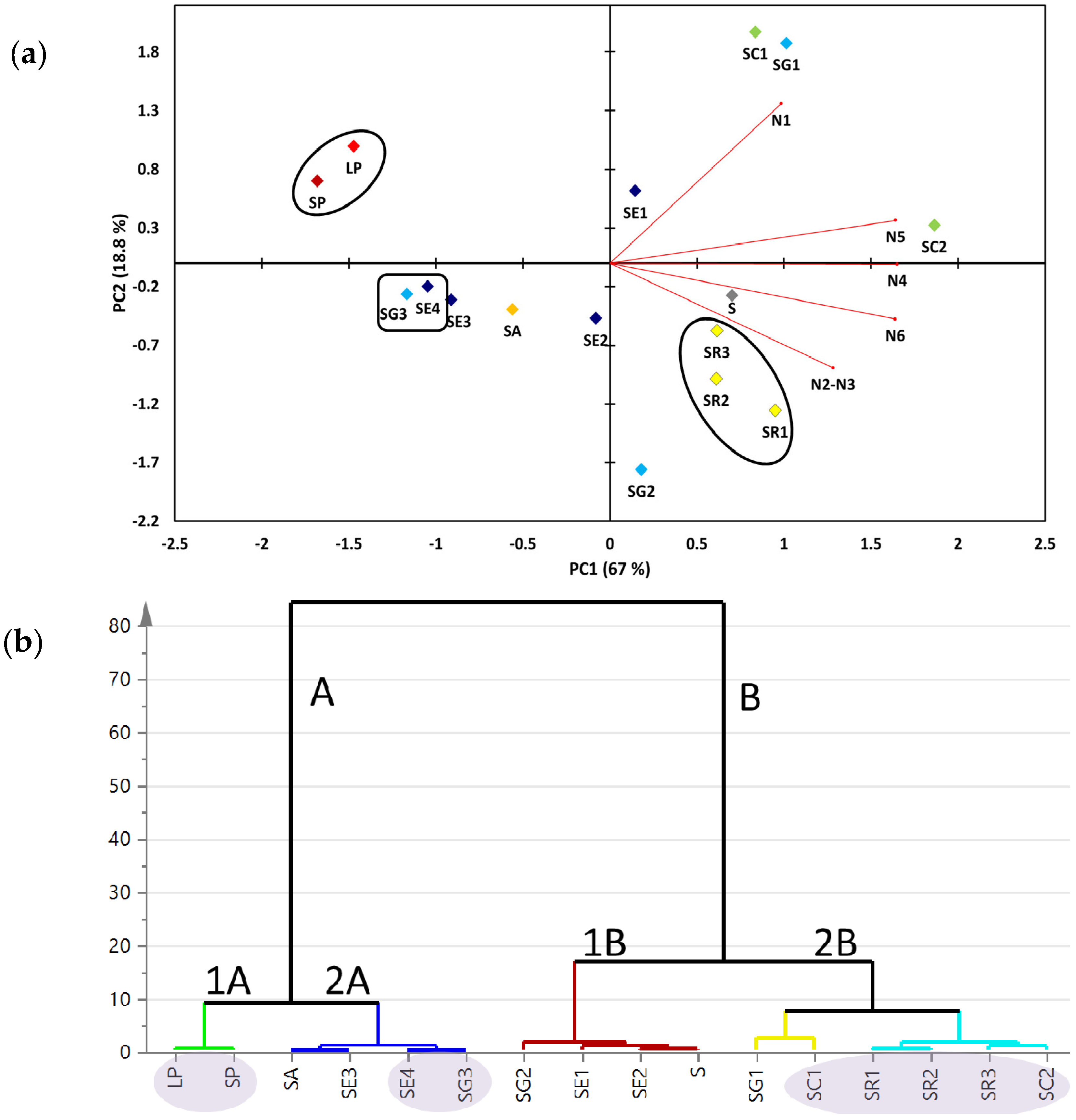
2.2. Multivariate Data Analysis of the Targeted Metabolite Profiling by qNMR of Soft Corals
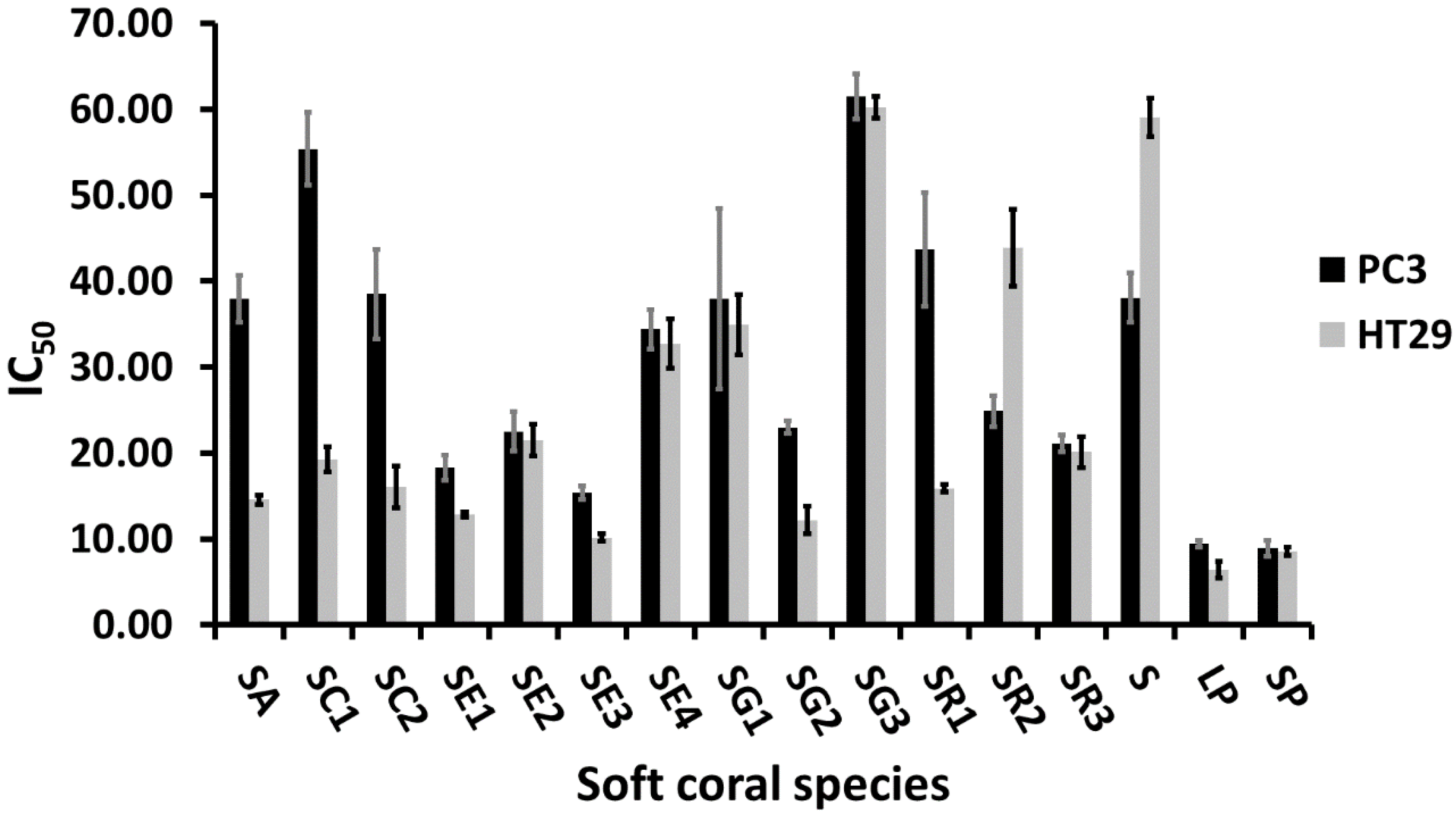
2.3. Cytotoxic Activity of Soft Corals
2.4. Metabolite/Metabolite and Metabolite/Cytotoxicity Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Soft Coral Material Collection and Identification
4.2. Chemicals and Reagents
4.3. Soft Coral Extraction, Sample Preparation, and NMR Analyses
4.4. NMR Quantification
- mT: mass of the target compound [µg] in the solution used for 1H NMR measurement
- MT: molecular weight of the target compound [g/mol]
- IT: relative integral value of the 1H NMR signal of the target compound
- ISt: relative integral value of the 1H NMR signal of the standard compound
- xSt: number of protons belonging to the 1H NMR signal of the standard compound
- xT: number of protons belonging to the 1H NMR signal of the target compound
- cSt: concentration of internal standard (HMDS) in the solution used for 1H NMR measurement [mmol/L]
- vSt: volume of solution used for 1H NMR measurement [mL]
4.5. Cytotoxicity Assay
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Ethical Approval
References
- Danovaro, R.; Company, J.B.; Corinaldesi, C.; D’Onghia, G.; Galil, B.; Gambi, C.; Gooday, A.J.; Lampadariou, N.; Luna, G.M.; Morigi, C.; et al. Deep-sea biodiversity in the Mediterranean Sea: The known, the unknown, and the unknowable. PLoS ONE 2010, 5, e11832. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Ozogul, F.; Ozogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Abraham, I.; El Sayed, K.; Chen, Z.-S.; Guo, H. Current status on marine products with reversal effect on cancer multidrug resistance. Mar. Drugs 2012, 10, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- Adla, S.K.; Sasse, F.; Kelter, G.; Fiebig, H.-H.; Lindel, T. Doubly prenylated tryptamines: Cytotoxicity, antimicrobial activity and cyclisation to the marine natural product flustramine A. Org. Biomol. Chem. 2013, 11, 6119–6130. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.; Mohamed, T.A.; Alhammady, M.A.; Shaheen, A.M.; Reda, E.H.; Elshamy, A.I.; Aziz, M.; Pare, P.W. Molecular architecture and biomedical leads of terpenes from red sea marine invertebrates. Mar. Drugs 2015, 13, 3154–3181. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef] [PubMed]
- Januar, H.I.; Zamani, N.P.; Soedharma, D.; Chasanah, E. New Cytotoxic Cembranoid from Indonesian Soft Coral Sarcophyton sp. Pharmacogn. Res. 2017, 9, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Wessjohann, L.A.; Ruijter, E.; Garcia-Rivera, D.; Brandt, W. What can a chemist learn from nature’s macrocycles?—A brief, conceptual view. Mol. Divers. 2005, 9, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.-E.F.; Eldeen, A.M.G.; Shahat, A.A.; Abdel-Latif, F.F.; Mohamed, T.A.; Whittlesey, B.R.; Pare, P.W. Bioactive Hydroperoxyl Cembranoids from the Red Sea Soft Coral Sarcophyton glaucum. Mar. Drugs 2012, 10, 209–222. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, K.A.; Hamann, M.T.; Waddling, C.A.; Jensen, C.; Lee, S.K.; Dunstan, C.A.; Pezzuto, J.M. Structurally novel bioconversion products of the marine natural product sarcophine effectively inhibit JB6 cell transformation. J. Org. Chem. 1998, 63, 7449–7455. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Ezzat, S.M.; Salama, M.M.; Tadros, M.G. Anti-acetylcholinesterase potential and metabolome classification of 4 Ocimum species as determined via UPLC/qTOF/MS and chemometric tools. J. Pharm. Biomed. Anal. 2016, 125, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Otify, A.; Porzel, A.; Michel, C.G.; Elsayed, A.; Wessjohann, L.A. Comparative metabolite profiling and fingerprinting of genus Passiflora leaves using a multiplex approach of UPLC-MS and NMR analyzed by chemometric tools. Anal. Bioanal. Chem. 2016, 408, 3125–3143. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Porzel, A.; Mahrous, E.A.; El-Massry, M.M.; Wessjohann, L.A. Integrated comparative metabolite profiling via MS and NMR techniques for Senna drug quality control analysis. Anal. Bioanal. Chem. 2015, 407, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Porzel, A.; Wessjohann, L.A. Unraveling the active hypoglycemic agent trigonelline in Balanites aegyptiaca date fruit using metabolite fingerprinting by NMR. J. Pharm. Biomed. Anal. 2015, 115, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Pieri, V.; Stuppner, H. Quantification of cynaropicrin in artichoke leaf extracts by 1H NMR spectroscopy. Planta Med. 2011, 77, 1756–1758. [Google Scholar] [CrossRef] [PubMed]
- Gika, H.G.; Wilson, I.D.; Theodoridis, G.A. LC-MS-based holistic metabolic profiling. Problems, limitations, advantages, and future perspectives. J. Chromatogr. B 2014, 966, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Moing, A.; Maucourt, M.; Renaud, C.; Gaudillère, M.; Brouquisse, R.; Lebouteiller, B.; Gousset-Dupont, A.; Vidal, J.; Granot, D.; Denoyes-Rothan, B. Quantitative metabolic profiling by 1-dimensional 1H-NMR analyses: Application to plant genetics and functional genomics. Funct. Plant Biol. 2004, 31, 889–902. [Google Scholar] [CrossRef]
- Farag, M.A.; Porzel, A.; Al-Hammady, M.A.; Hegazy, M.-E.F.; Meyer, A.; Mohamed, T.A.; Westphal, H.; Wessjohann, L.A. Soft Corals Biodiversity in the Egyptian Red Sea: A Comparative MS and NMR Metabolomics Approach of Wild and Aquarium Grown Species. J. Proteome Res. 2016, 15, 1274–1287. [Google Scholar] [CrossRef] [PubMed]
- Hielscher-Michael, S.; Griehl, C.; Buchholz, M.; Demuth, H.U.; Arnold, N.; Wessjohann, L.A. Natural Products from Microalgae with Potential against Alzheimer’s Disease: Sulfolipids Are Potent Glutaminyl Cyclase Inhibitors. Mar. Drugs 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, K.A.; Hamann, M.T. A new norcembranoid dimer from the Red Sea soft coral Sinularia gardineri. J. Nat. Prod. 1996, 59, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Weigend, M.; Luebert, F.; Brokamp, G.; Wessjohann, L.A. Phytochemical, phylogenetic, and anti-inflammatory evaluation of 43 Urtica accessions (stinging nettle) based on UPLC–Q–TOF–MS metabolomic profiles. Phytochemistry 2013, 96, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Bilasy, S.E.-S.; Khalifa, S.I.; Saleh, S.M.; El-Ela, S.H.A. HPLC method for the quantitative determination of sarcophine, a source of cembranoids with cancer chemopreventive activity. J. Pharm. Biomed. Anal. 2008, 46, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Cuong, N.X.; Tuan, T.A.; Kiem, P.V.; Minh, C.V.; Choi, E.M.; Kim, Y.H. New cembranoid diterpenes from the Vietnamese soft coral Sarcophyton mililatensis stimulate osteoblastic differentiation in MC3T3-E1 cells. Chem. Pharm. Bull. 2008, 56, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Coll, J.C. The chemistry and chemical ecology of octocorals (Coelenterata, Anthozoa, Octocorallia). Chem. Rev. 1992, 92, 613–631. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Macdonald, C.A. Drug discovery from uncultivable microorganisms. Drug Discov. Today 2010, 15, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Calado, R.; Sheridan, C.; Alimonti, A.; Osinga, R. Coral aquaculture to support drug discovery. Trends Biotechnol. 2013, 31, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Osinga, R.; Schutter, M.; Griffioen, B.; Wijffels, R.H.; Verreth, J.A.; Shafir, S.; Henard, S.; Taruffi, M.; Gili, C.; Lavorano, S. The biology and economics of coral growth. Mar. Biotechnol. 2011, 13, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, M.K.; Beeftink, H.; Wijffels, R. Light-dependency of growth and secondary metabolite production in the captive zooxanthellate soft coral Sinularia flexibilis. Mar. Biotechnol. 2009, 11, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Porzel, A.; Wessjohann, L.A. Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC-MS, LC-MS and 1D NMR techniques. Phytochemistry 2012, 76, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Putra, M.Y.; Bavestrello, G.; Cerrano, C.; Renga, B.; D’Amore, C.; Fiorucci, S.; Fattorusso, E.; Taglialatela-Scafati, O. Polyhydroxylated sterols from the Indonesian soft coral Sinularia sp. and their effect on farnesoid X-activated receptor. Steroids 2012, 77, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Kinamoni, Z.; Groweiss, A.; Carmely, S.; Kashman, Y.; Loya, Y. Several new cembranoid diterpenes from three soft corals of the Red Sea. Tetrahedron 1983, 39, 1643–1648. [Google Scholar] [CrossRef]
- Feller, M.; Rudi, A.; Berer, N.; Goldberg, I.; Stein, Z.; Benayahu, Y.; Schleyer, M.; Kashman, Y. Isoprenoids of the Soft Coral Sarcophyton g laucum: Nyalolide, a New Biscembranoid, and Other Terpenoids. J. Nat. Prod. 2004, 67, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Ishizaka, T.; Mitsuhashi, H. Marine sterols X. Minor constituents of the sterols of the soft coral Sarcophyton glaucum. Steroids 1982, 40, 209–221. [Google Scholar] [CrossRef]
- Carvalho, J.F.; Silva, M.M. C.; Moreira, J.N.; Simões, S.R.; Sá e Melo, M.L. Sterols as anticancer agents: synthesis of ring-B oxygenated steroids, cytotoxic profile, and comprehensive SAR analysis. J. Med. Chem. 2010, 53, 7632–7638. [Google Scholar] [CrossRef] [PubMed]
- Bennani, H.; Drissi, A.; Giton, F.; Kheuang, L.; Fiet, J.; Adlouni, A. Antiproliferative effect of polyphenols and sterols of virgin argan oil on human prostate cancer cell lines. Cancer Detect. Prev. 2007, 31, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Bobach, C.; Schurwanz, J.; Franke, K.; Denkert, A.; Van Sung, T.; Kuster, R.; Mutiso, P.C.; Seliger, B.; Wessjohann, L.A. Multiple readout assay for hormonal (androgenic and antiandrogenic) and cytotoxic activity of plant and fungal extracts based on differential prostate cancer cell line behavior. J. Ethnopharmacol. 2014, 155, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Janes, M.P. Laboratory Methods for the Identification of Soft Corals (Octocorallia: Alcyonacea). In Proceedings of the 1st International Symposium of Coral Husbandry in Public Aquaria, Arnhem, The Netherlands, 2008. [Google Scholar]
- Porzel, A.; Farag, M.A.; Mülbradt, J.; Wessjohann, L.A. Metabolite profiling and fingerprinting of Hypericum species: a comparison of MS and NMR metabolomics. Metabolomics 2014, 10, 574–588. [Google Scholar] [CrossRef]
- Jia, R.; Guo, Y.-W.; Chen, P.; Yang, Y.-M.; Mollo, E.; Gavagnin, M.; Cimino, G. Biscembranoids and Their Probable Biogenetic Precursor from the Hainan Soft Coral Sarcophyton tortuosum. J. Nat. Prod. 2007, 70, 1158–1166. [Google Scholar] [CrossRef] [PubMed]


| Variables | N1 | N2-3 | N4 | N5 | N6 | PC3 | HT29 |
|---|---|---|---|---|---|---|---|
| N1 | 0.064 | 0.246 | 0.393 | 0.236 | 0.061 | 0.031 | |
| N2-3 | 0.167 | 0.599 | 0.512 | 0.735 | 0.297 | 0.235 | |
| N4 | 0.411 | 0.532 | 0.878 | 0.884 | 0.501 | 0.434 | |
| N5 | 0.572 | 0.481 | 0.864 | 0.826 | 0.435 | 0.224 | |
| N6 | 0.294 | 0.721 | 0.839 | 0.764 | 0.485 | 0.359 | |
| PC3 | 0.268 | 0.128 | 0.340 | 0.316 | 0.404 | 0.638 | |
| HT29 | −0.079 | −0.079 | 0.279 | 0.026 | 0.225 | 0.565 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farag, M.A.; Fekry, M.I.; Al-Hammady, M.A.; Khalil, M.N.; El-Seedi, H.R.; Meyer, A.; Porzel, A.; Westphal, H.; Wessjohann, L.A. Cytotoxic Effects of Sarcophyton sp. Soft Corals—Is There a Correlation to Their NMR Fingerprints? Mar. Drugs 2017, 15, 211. https://doi.org/10.3390/md15070211
Farag MA, Fekry MI, Al-Hammady MA, Khalil MN, El-Seedi HR, Meyer A, Porzel A, Westphal H, Wessjohann LA. Cytotoxic Effects of Sarcophyton sp. Soft Corals—Is There a Correlation to Their NMR Fingerprints? Marine Drugs. 2017; 15(7):211. https://doi.org/10.3390/md15070211
Chicago/Turabian StyleFarag, Mohamed A., Mostafa I. Fekry, Montasser A. Al-Hammady, Mohamed N. Khalil, Hesham R. El-Seedi, Achim Meyer, Andrea Porzel, Hildegard Westphal, and Ludger A. Wessjohann. 2017. "Cytotoxic Effects of Sarcophyton sp. Soft Corals—Is There a Correlation to Their NMR Fingerprints?" Marine Drugs 15, no. 7: 211. https://doi.org/10.3390/md15070211






