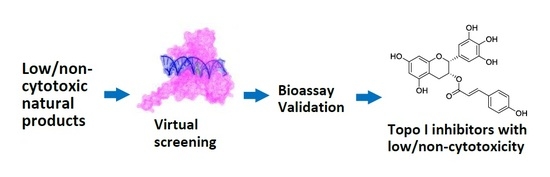
Discovery of DNA Topoisomerase I Inhibitors with Low-Cytotoxicity Based on Virtual Screening from Natural Products
Abstract
:1. Introduction
2. Results and Discussion
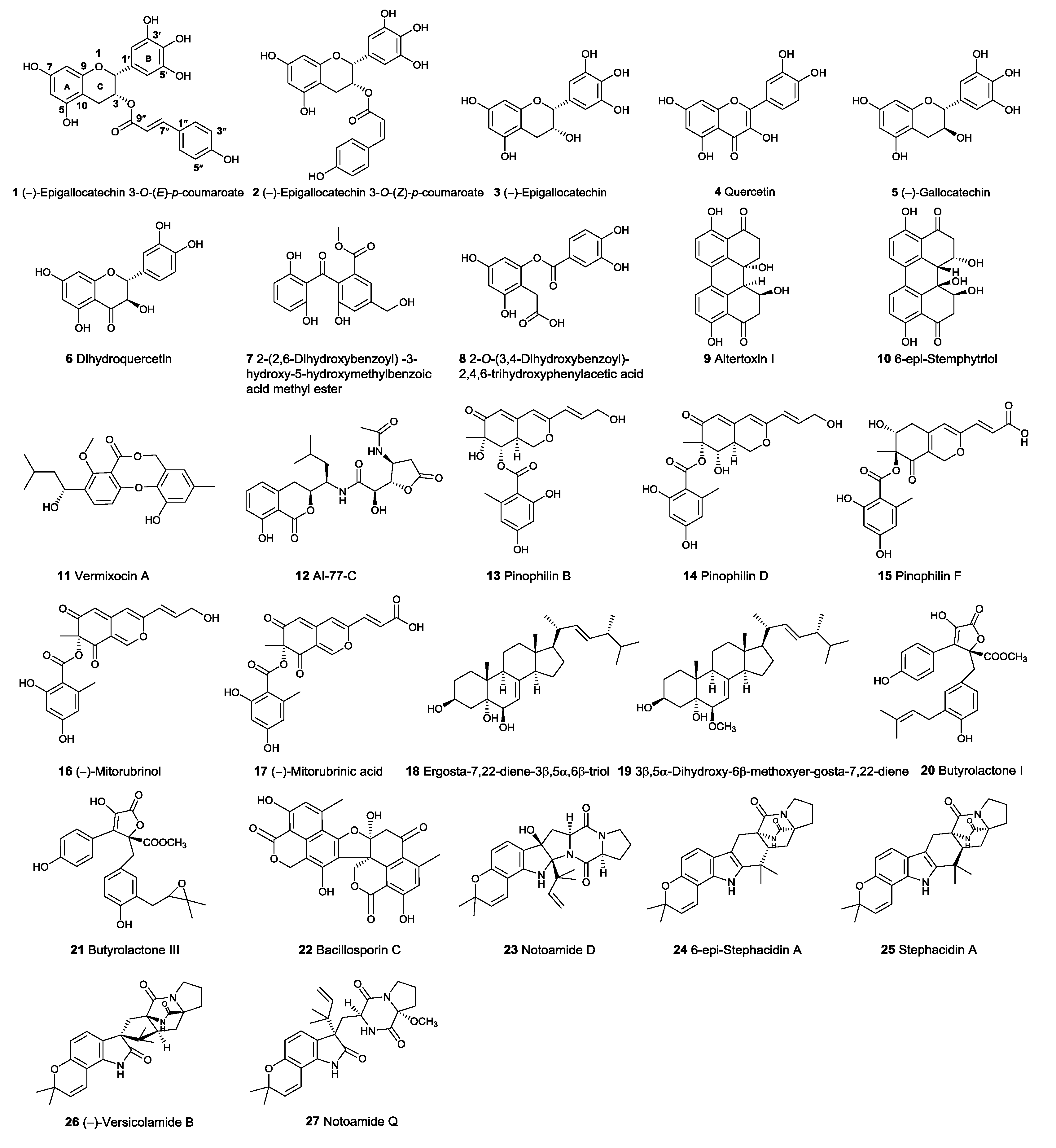
2.1. Virtual Screening
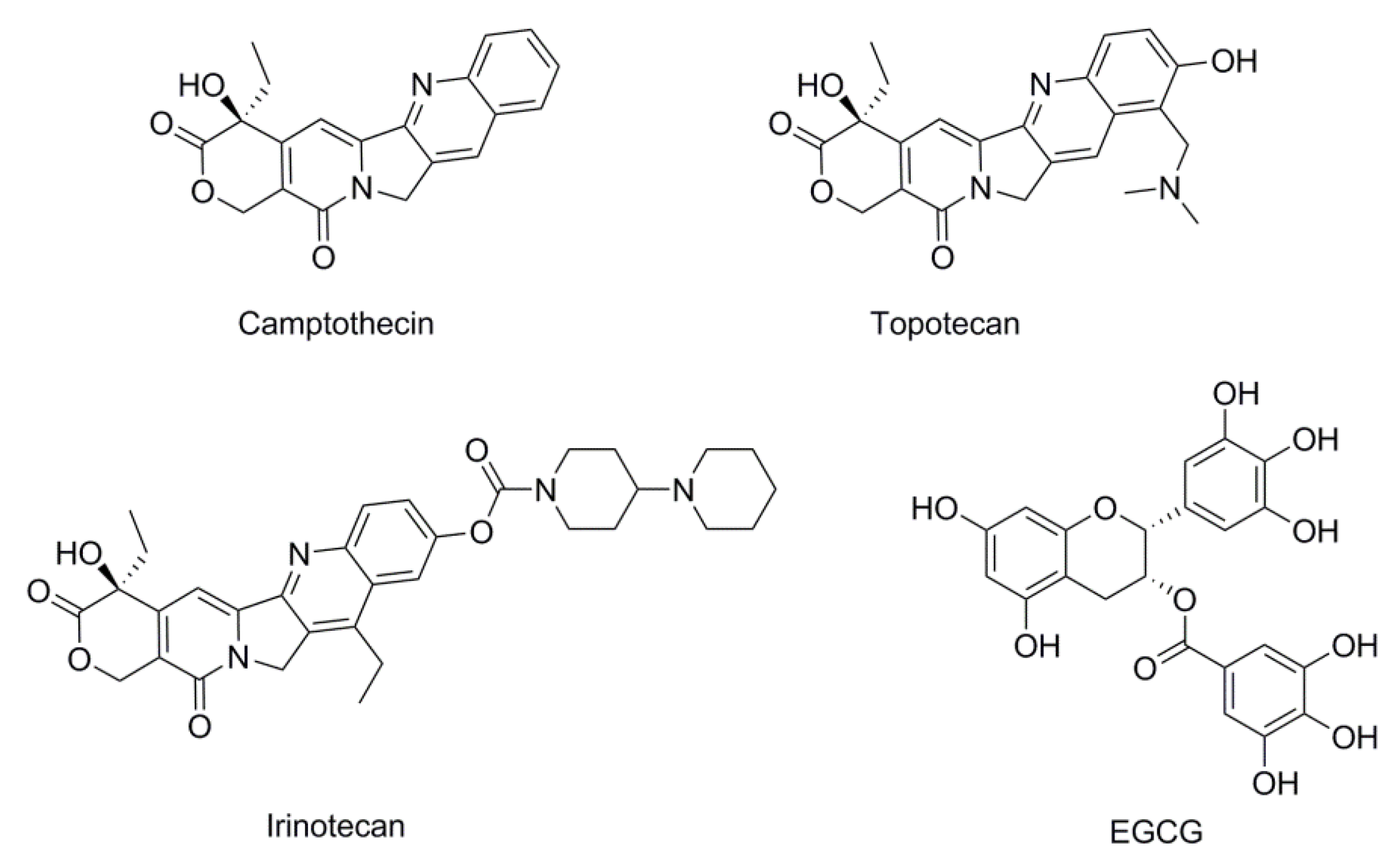
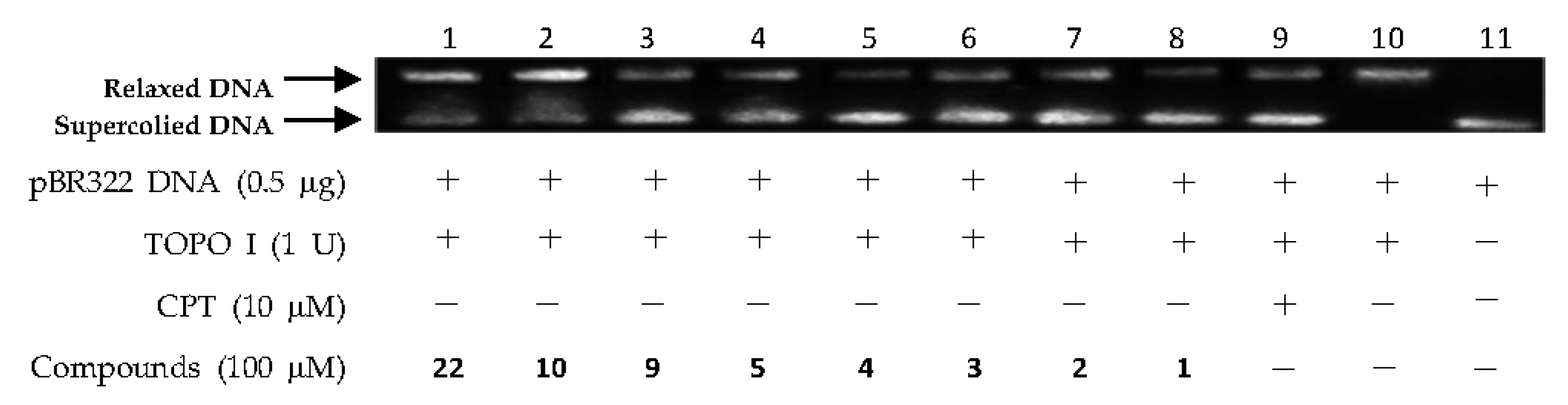
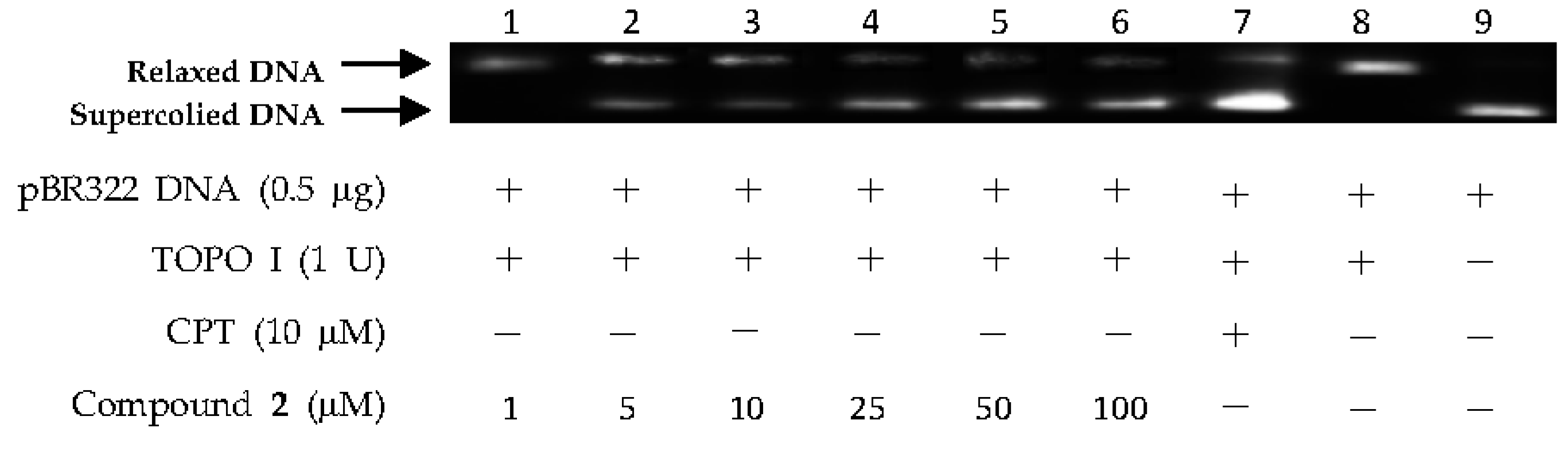
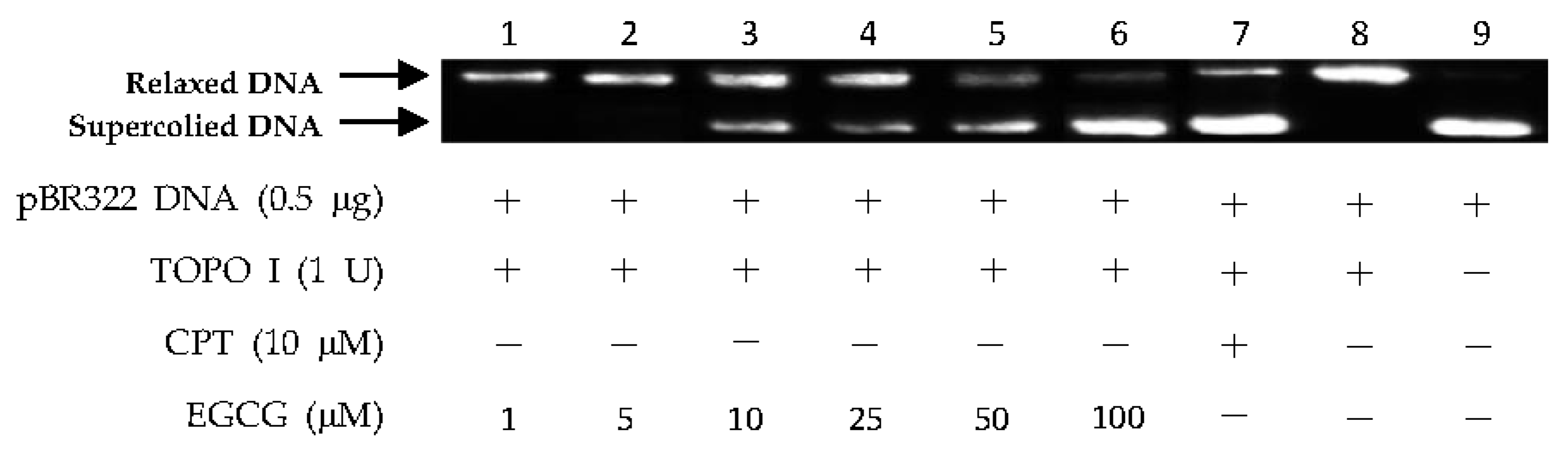
2.2. DNA Topo I Inhibitory Activity Assay
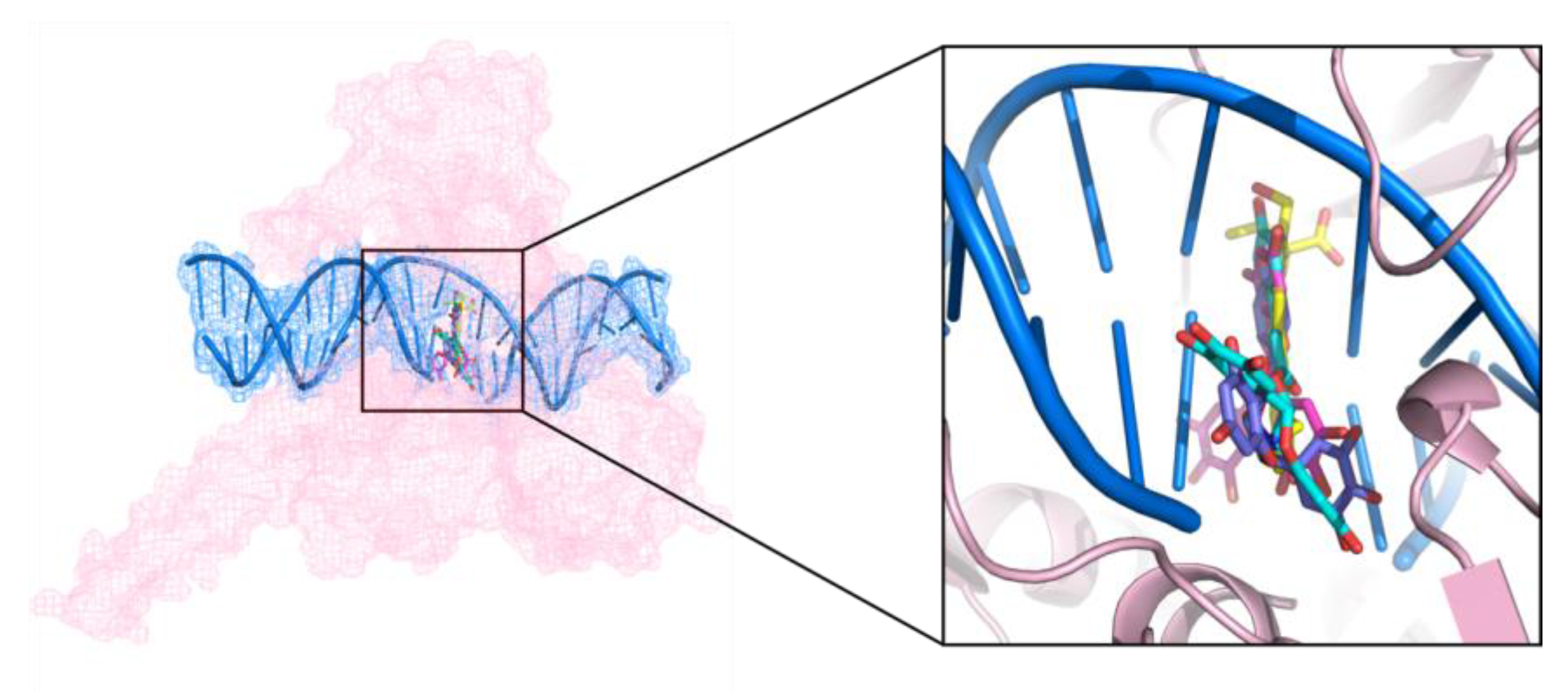
2.3. Binding Mode of the Representative New Topo I Inhibitors
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Molecular Docking
3.3. Preparation of the Tested Compounds
3.4. DNA Topoisomerase I Inhibitory Activity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Redinbo, M.R.; Stewart, L.; Kuhn, P.; Champoux, J.J.; Hol, W.G.J. Crystal structures of human topoisomerase I in covalent and noncovalent complexes with DNA. Science 1998, 279, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C. DNA topoisomerases. Annu. Rev. Biochem. 1996, 65, 635–692. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.T.; Yang, L.X. Design, synthesis and development of novel camptothecin drugs. Curr. Pharm. Des. 2008, 14, 1078–1097. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y. DNA topoisomerase I inhibitors: Chemistry, biology, and interfacial inhibition. Chem. Rev. 2009, 109, 2894–2902. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Tanizawa, A.; Kohn, K.W. Mechanisms of topoisomerase I inhibition by anticancer drugs. Adv. Pharmacol. 1994, 29, 73–92. [Google Scholar]
- Takagi, K.; Dexheimer, T.S.; Redon, C.; Sordet, O.; Agama, K.; Lavielle, G.; Pierre, A.; Bates, S.E.; Pommier, Y. Novel E-ring camptothecin keto analogues (S38809 and S39625) are stable, potent, and selective topoisomerase I inhibitors without being substrates of drug efflux transporters. Mol. Cancer Ther. 2007, 6, 3229–3238. [Google Scholar] [CrossRef] [PubMed]
- Bjornsti, M.A.; Benedetti, P.; Viglianti, G.A.; Wang, J.C. Expression of human DNA topoisomerase I in yeast cells lacking yeast DNA topoisomerase I: Restoration of sensitivity of the cells to the antitumor drug camptothecin. Cancer Res. 1989, 49, 6318–6323. [Google Scholar] [PubMed]
- Pommier, Y.; Pourquier, P.; Urasaki, Y.; Wu, J.; Laco, G.S. Topoisomerase I inhibitors: Selectivity and cellular resistance. Drug Resist. Updates 1999, 2, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y. Diversity of DNA topoisomerases I and inhibitors. Biochimie 1998, 80, 255–270. [Google Scholar] [CrossRef]
- Rasheed, Z.A.; Rubin, E.H. Mechanisms of resistance to topoisomerase I-targeting drugs. Oncogene 2003, 22, 7296–7304. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Zu, Y.G.; Shi, R.Z.; Yao, L.P. Review camptothecin: Current perspectives. Curr. Med. Chem. 2006, 13, 2021–2039. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Liu, L.F. DNA topoisomerases: Essential enzymes and lethal targets. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.D.; Berger, N.A. Mutagenicity and carcinogenicity of topoisomerase-interactive agents. Mutat. Res. Fund. Mol. Mech. 1994, 309, 109–142. [Google Scholar] [CrossRef]
- Landis-Piwowar, K.R.; Kuhn, D.J.; Wan, S.B.; Chen, D.; Chan, T.H.; Dou, Q.P. Evaluation of proteasome-inhibitory and apoptosis-inducing potencies of novel (−)-EGCG analogs and their prodrugs. Int. J. Mol. Med. 2005, 15, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, H.; Koga, K.; Fujimura, Y.; Yamada, K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004, 11, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.J.; Gupta, S.; Belfi, C.A.; Gosky, D.M.; Mukhtar, H. Green tea constituent (−)-epigallocatechin-3-gallate inhibits topoisomerase I activity in human colon carcinoma cells. Biochem. Bioph. Res. Commun. 2001, 288, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Feyes, D.K.; Agarwal, R.; Mukhtar, H.; Nieminen, A.L. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J. Natl. Cancer Inst. 1997, 89, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Sheng, C.; Wang, S.; Miao, Z.; Yao, J.; Zhang, W. Selection of evodiamine as a novel topoisomerase I inhibitor by structure-based virtual screening and hit optimization of evodiamine derivatives as antitumor agents. J. Med. Chem. 2010, 53, 7521–7531. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, S.T.; Li, C.Y.; Hu, M.H.; Chen, S.B.; Yao, P.F.; Huang, S.L.; Ou, T.M.; Tan, J.H.; An, L.K.; Li, D.; et al. Synthesis and biological evaluation of benzo [a] phenazine derivatives as a dual inhibitor of topoisomerase I and II. Org. Biomol. Chem. 2013, 11, 3989–4005. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Shao, Z.; Dexheimer, T.S.; Scher, E.S.; Pommier, Y.; Cushman, M. Structure-Based Design, Synthesis and Biological Studies of New Anticancer Norindenoisoquinoline Topoisomerase I Inhibitors. J. Med. Chem. 2010, 53, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Shao, C.L.; Guo, Z.Y.; Chen, J.F.; Deng, D.S.; Yang, K.L.; Chen, Y.Y.; Fu, X.M.; She, Z.G.; Lin, Y.C.; et al. Bioactive hydroanthraquinones and anthraquinone dimers from a soft coral-derived Alternaria sp. fungus. J. Nat. Prod. 2012, 75, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Wu, Z.H.; Shao, C.L.; Pang, S.; Liang, X.Y.; Voogd, N.J.D.; Wang, C.Y. Cytotoxic scalarane sesterterpenoids from the South China Sea sponge Carteriospongia Foliascens. Org. Biomol. Chem. 2015, 13, 4016–4024. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, X.D.; Zhao, Q.; Wang, C.Y. Topsensterols A–C, Cytotoxic Polyhydroxylated Sterol Derivatives from a Marine Sponge Topsentia sp. Mar. Drugs 2016, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.L.; Shao, C.L.; Gan, L.S.; Wang, M.; Wang, C.Y. Chromone derivatives from a sponge-derived strain of the fungus Corynespora cassiicola. J. Nat. Prod. 2015, 78, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Staker, B.L.; Feese, M.D.; Cushman, M.; Pommier, Y.; Zembower, D.; Stewart, L.; Burgin, A.B. Structures of three classes of anticancer agents bound to the human topoisomerase I-DNA covalent complex. J. Med. Chem. 2005, 48, 2336–2345. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.S.; Zheng, N.; Li, D.M.; Zheng, H.L.; Zhang, L.L.; Ge, H.; Liu, W.D. cyp51A-based mechanism of azole resistance in Aspergillus fumigatus: Illustration by a new 3D Structural Model of Aspergillus fumigatus CYP51A protein. Med. Mycol. 2016, 54, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Mamidala, R.; Majumdar, P.; Jha, K.K.; Bathula, C.; Agarwal, R.; Chary, M.T.; Mazumdar, H.K.; Munshi, P.; Sen, S. Identification of Leishmania donovani Topoisomerase 1 inhibitors via intuitive scaffold hopping and bioisosteric modification of known Top 1 inhibitors. Sci. Rep. 2016, 6, 26003. [Google Scholar]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, L.-T.; Liu, L.; Shao, C.-L.; Yu, R.-L.; Chen, F.-L.; Yue, S.-J.; Wang, M.; Guo, Z.-L.; Fan, Y.-C.; Guan, H.-S.; et al. Discovery of DNA Topoisomerase I Inhibitors with Low-Cytotoxicity Based on Virtual Screening from Natural Products. Mar. Drugs 2017, 15, 217. https://doi.org/10.3390/md15070217
Xin L-T, Liu L, Shao C-L, Yu R-L, Chen F-L, Yue S-J, Wang M, Guo Z-L, Fan Y-C, Guan H-S, et al. Discovery of DNA Topoisomerase I Inhibitors with Low-Cytotoxicity Based on Virtual Screening from Natural Products. Marine Drugs. 2017; 15(7):217. https://doi.org/10.3390/md15070217
Chicago/Turabian StyleXin, Lan-Ting, Lu Liu, Chang-Lun Shao, Ri-Lei Yu, Fang-Ling Chen, Shi-Jun Yue, Mei Wang, Zhong-Long Guo, Ya-Chu Fan, Hua-Shi Guan, and et al. 2017. "Discovery of DNA Topoisomerase I Inhibitors with Low-Cytotoxicity Based on Virtual Screening from Natural Products" Marine Drugs 15, no. 7: 217. https://doi.org/10.3390/md15070217