The Epiphytic Genus Gambierdiscus (Dinophyceae) in the Kermadec Islands and Zealandia Regions of the Southwestern Pacific and the Associated Risk of Ciguatera Fish Poisoning
Abstract
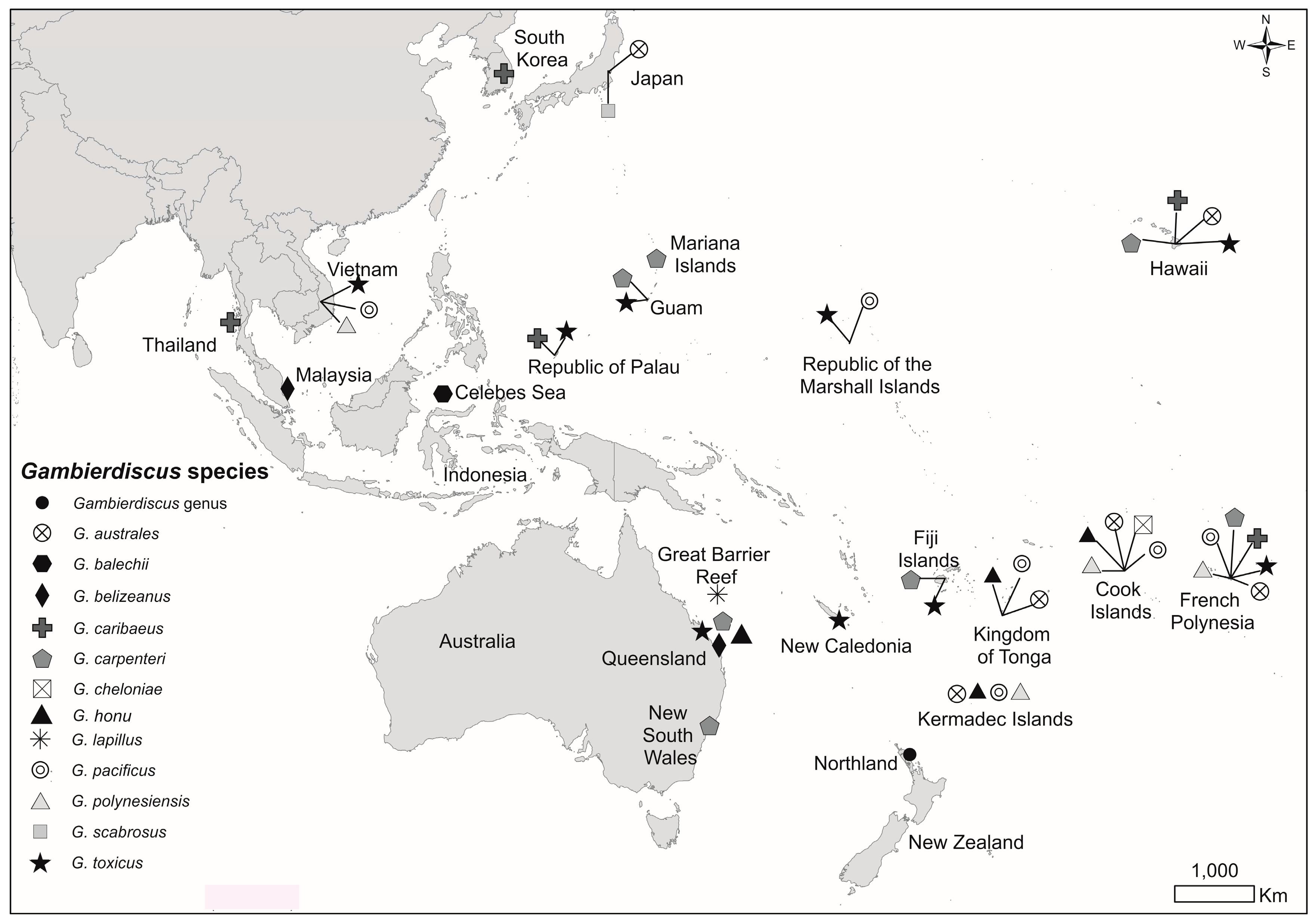
:1. Introduction
2. Results
2.1. Dinoflagellate Species Identified
2.2. Toxin Production
3. Discussion
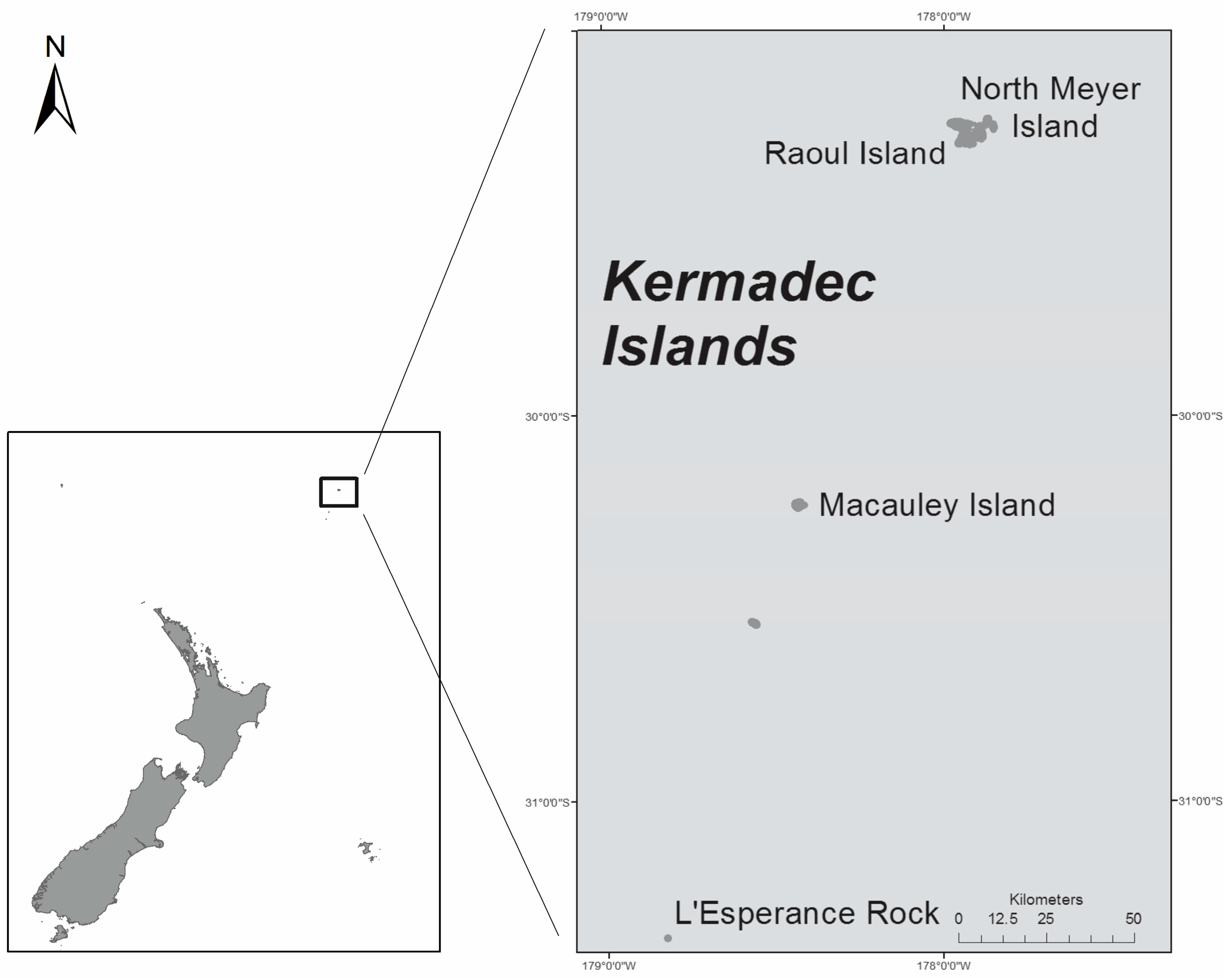
4. Materials and Methods
4.1. Sampling, Isolation and Culture
4.2. DNA Sequencing and Phylogenetic Analyses of Dinoflagellate Cultures
4.3. Toxin Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Parsons, M.L.; Settlemier, C.J.; Ballauer, J.M. An examination of the epiphytic nature of Gambierdiscus toxicus, a dinoflagellate involved in ciguatera fish poisoning. Harmful Algae 2011, 10, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.; Aligizaki, A.; Bottein, M.-Y.D.; Fraga, S.; Morton, S.; Penna, A.; Rhodes, L. Gambierdiscus and Ostreopsis: Reassessment of the state of knowledge of their taxonomy, geography, ecophysiology and toxicology. Harmful Algae 2012, 14, 107–129. [Google Scholar] [CrossRef]
- Selwood, A.; Rhodes, L.; Smith, K.; Harwood, D.T. Development of two novel UPLC-MS/MS methods for the analysis of maitotoxin from micro-algal cultures. In Marine and Fresh-Water Harmful Algae, Proceedings of the 16th International Conference on Harmful Algae, Wellington, New Zealand, 27–31 October 2014; Mackenzie, L., Ed.; Cawthron Institute and ISSHA: Nelson, New Zealand, 2015; pp. 66–69. [Google Scholar]
- Rongo, T.; van Woesik, R. Socioeconomic consequences of ciguatera poisoning in Rarotonga, southern Cook Islands. Harmful Algae 2012, 20, 92–100. [Google Scholar] [CrossRef]
- Schep, L.J.; Slaughter, R.J.; Temple, W.A.; Beasley, D.M.G. Ciguatera poisoning: An interesting occurrence in New Zealand. N. Z. Med. J. 2010, 123, 100–102. [Google Scholar] [PubMed]
- Palafox, N.A.; Jain, L.G.; Pinano, A.Z.; Gulik, T.M.; Williams, R.K.; Schatz, I.J. Successful treatment of ciguatera fish poisoning with intravenous mannitol. J. Am. Med. Assoc. 1988, 259, 2740–2742. [Google Scholar] [CrossRef]
- Schnorf, H.; Taurarii, M.; Cundy, T. Ciguatera fish poisoning—A double-blind randomized trial of mannitol therapy. Neurology 2002, 58, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.; Smith, K.F.; Haywood, T.; Murray, S.; Biessy, L.; Argyle, P.; Munday, R. Is Gambierdiscus expanding its geographic range in the Pacific region? Harmful Algae News 2017, 56, 1–4. [Google Scholar]
- Rhodes, L.; Smith, K.; Verma, A.; Curley, B.; Harwood, T.; Murray, S.; Kohli, G.S.; Solomona, D.; Rongo, T.; Munday, R.; et al. A new species of Gambierdiscus (Dinophyceae) from the south-west Pacific: Gambierdiscus honu. Harmful Algae 2017, 65, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kohli, G.S.; Murray, S.A.; Neilan, B.A.; Rhodes, L.L.; Harwood, T.; Smith, K.; Mayer, L.; Capper, A.; Brett, S.; Hallegraeff, G. High abundance of the potentially maitotoxic dinoflagellate Gambierdiscus carpenteri in temperate waters of New South Wales, Australia. Harmful Algae 2014, 39, 134–145. [Google Scholar] [CrossRef]
- Chang, F.H. Shellfish toxin update. Seafood New Zealand, February 1996; 26. [Google Scholar]
- Rhodes, L.; Gimenez Papiol, G.; Smith, K.; Harwood, T. Gambierdiscus cf. yasumotoi (Dinophyceae) isolated from New Zealand’s sub-tropical northern coastal waters. N. Z. J. Mar. Freshw. Res. 2013, 48, 303–310. [Google Scholar] [CrossRef]
- Llewellyn, L.E. Revisiting the association between sea surface temperature and the epidemiology of fish poisoning in the South Pacific: Reassessing the link between ciguatera and climate change. Toxicon 2010, 56, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Kohli, G.S.; Farrell, H.; Murray, S.A. Gambierdiscus, the cause of ciguatera fish poisoning: An increased human health threat influenced by climate change. In Climate Change and Marine and Freshwater Toxins; Botana, L.M., Louzao, C., Vilariño, N., Eds.; De Gruyter: Berlin, Germany, 2015; pp. 273–312. [Google Scholar]
- Vergés, A.; Steinberg, P.D.; Hay, M.E.; Poore, A.G.B.; Campbell, A.H.; Ballesteros, E.; Heck, K.L., Jr.; Booth, D.J.; Coleman, M.A.; Feary, D.A.; et al. The tropicalisation of temperate marine ecosystems: Climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B 2014, 281, 20140846. [Google Scholar] [CrossRef] [PubMed]
- Bryan, S.E.; Cook, A.G.; Evans, J.P.; Hebden, K.; Hurrey, L.; Colls, P.; Jell, J.S.; Weatherley, D.; Firn, J. Rapid, long-distance dispersal by pumice rafting. PLoS ONE 2012, 7, e40583. [Google Scholar] [CrossRef] [PubMed]
- Global Volcanism Program. Report on Havre Seamount (New Zealand). In Bulletin of the Global Volcanism Network; Wunderman, R., Ed.; Smithsonian Institution: Washington, DC, USA, 2012; Volume 37, p. 242005. [Google Scholar]
- Mortimer, N.; Campbell, H.J.; Tulloch, A.J.; King, P.R.; Stagpoole, V.M.; Wood, R.A.; Rattenbury, M.S.; Sutherland, R.; Adams, C.J.; Collot, J.; et al. Zealandia: Earth’s Hidden Continent. GSA Today 2017, 27, 27–35. [Google Scholar] [CrossRef]
- Baumann, F.; Bourrat, M.-B.; Pauillac, S. Prevalence, symptoms and chronicity of ciguatera in New Caledonia: Results from an adult population survey conducted in Noumea during 2005. Toxicon 2010, 56, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Chinain, M.; Faust, M.A.; Pauillac, S. Morphology and molecular analyses of three species of Gambierdiscus (Dinophyceae): G. pacificus, sp. nov., G. australes, sp. nov., and G. polynesiensis, sp. nov. J. Phycol. 1999, 35, 1282–1296. [Google Scholar] [CrossRef]
- Litaker, R.W.; Vandersea, M.W.; Faust, M.A.; Kibler, S.R.; Chinain, M.; Holmes, M.J.; Holland, W.C.; Tester, P.A. Taxonomy of Gambierdiscus including four new species, Gambierdiscus caribaeus, Gambierdiscus carolinianus, Gambierdiscus carpenteri and Gambierdiscus ruetzleri (Gonyaulacales, Dinophyceae). Phycologia 2009, 48, 344–390. [Google Scholar] [CrossRef]
- Laurent, D.; Kerbrat, A.-S.; Darius, H.T.; Girard, E.; Golubic, S.; Benoit, E.; Sauviat, M.-P.; Chinain, M.; Molgo, J.; Pauillac, S. Are cyanobacteria involved in Ciguatera Fish Poisoning-like outbreaks in New Caledonia? Harmful Algae 2008, 7, 827–838. [Google Scholar] [CrossRef]
- Roué, M.; Darius, H.T.; Picot, S.; Ung, A.; Viallon, J.; Gaertner-Mazouni, N.; Sibat, M.; Amzil, Z.; Chinain, M. Evidence of the bioaccumulation of ciguatoxins in giant clams (Tridacna maxima) exposed to Gambierdiscus spp. cells. Harmful Algae 2016, 57, 78–87. [Google Scholar] [CrossRef]
- Marsden, I.D.; Contreras, A.M.; Mackenzie, L.; Munro, M.H.G. A comparison of the physiological responses, behaviour and biotransformation of paralytic shellfish poisoning toxins in a surf-clam (Paphies donacina) and the green-lipped mussel (Perna canaliculus). Mar. Freshw. Res. 2015, 67, 1163–1174. [Google Scholar] [CrossRef]
- Rhodes, L.; Smith, K.; Harwood, T.; Selwood, A.; Argyle, P.; Bedford, C.; Munday, R. Gambierdiscus and Ostreopsis from New Zealand, the Kermadec Islands and the Cook Islands and the risk of ciguatera fish poisoning in New Zealand. In Marine and Fresh-Water Harmful Algae, Proceedings of the 16th International Conference on Harmful Algae, Wellington, New Zealand, 27–31 October 2014; Mackenzie, L., Ed.; Cawthron Institute and ISSHA: Nelson, New Zealand, 2015; pp. 180–183. [Google Scholar]
- Gentry, S. Raoul and the Kermadecs. New Zealand’s Northernmost Islands; Steele Roberts: Wellington, New Zealand, 2013; 346p. [Google Scholar]
- Trnski, T.; de Lange, P.J. Introduction to the Kermadec Biodiscovery Expedition 2011. Bull. Auckl. Mus. 2015, 20, 1–18. [Google Scholar]
- Duffy, C.A.J.; Ahyong, S.T. Annotated checklist of the marine flora and fauna of the Kermadec Islands Marine Reserve and northern Kermadec Ridge, New Zealand. Bull. Auckl. Mus. 2015, 20, 19–124. [Google Scholar]
- Rhodes, L.; Smith, K.; Harwood, T.; Bedford, C. Novel and toxin-producing epiphytic dinoflagellates isolated from sub-tropical Raoul Island, Kermadec Islands group. N. Z. J. Mar. Freshw. Res. 2014, 48, 594–599. [Google Scholar] [CrossRef]
- Rhodes, L.L.; Smith, K.F.; Verma, A.; Murray, S.; Harwood, D.T.; Trnski, T. The dinoflagellate genera Gambierdiscus and Ostreopsis from sub-tropical Raoul Island and North Meyer Island, Kermadec Islands. N. Z. J. Mar. Freshw. Res. 2017, 1–15. [Google Scholar] [CrossRef]
- Sato, S.; Nishimura, T.; Uehara, K.; Sakanari, H.; Tawong, W.; Hariganeya, N.; Smith, K.; Rhodes, L.; Yasumoto, T.; Taira, Y.; et al. Phylogeography of Ostreopsis along west Pacific coast, with special reference to a novel clade from Japan. PLoS ONE 2011, 6, e27983. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Noor, N.; Moestrup, O.; Lundholm, N.; Fraga, S.; Adam, A.; Holmes, M.J.; Saleh, E. Autecology and phylogeny of Coolia tropicalis and Coolia malayensis (Dinophyceae), with emphasis on taxonomy of C. tropicalis based on light microscopy, scanning electron microscopy and LSU rDNA. J. Phycol. 2013, 49, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Pisapia, F.; Holland, W.C.; Hardison, D.R.; Litaker, R.W.; Fraga, S.; Nishimura, T.; Adachi, M.; Nguyen-Ngoc, L.; Séchet, V.; Amzil, Z.; et al. Toxicity screening of 13 Gambierdiscus strains using neuro-2a and erythrocyte lysis bioassays. Harmful Algae 2017, 63, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Munday, R.; Murray, S.; Rhodes, L.L.; Larsson, M.; Harwood, D.T. Ciguatoxins and maitotoxins in extracts of 17 Gambierdiscus isolates from the South Pacific and their toxicity to mice by intraperitoneal and oral administration. Mar. Drugs 2017, 15, 208. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.F.; Biessy, L.; Argyle, P.; Trnski, T.; Halafihi, T.; Rhodes, L. Molecular identification of Gambierdiscus and Fukuyoa from environmental samples. Mar. Drugs 2017, unpublished. [Google Scholar]
- Rhodes, L.L.; Smith, K.F.; Gimenez Papiol, G.; Adamson, J.E.; Harwood, T.; Munday, R. Epiphytic dinoflagellates in sub-tropical New Zealand, in particular the genus Coolia Meunier. Harmful Algae 2014, 34, 36–41. [Google Scholar] [CrossRef]
- Guillard, R.R. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.H., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 29–60. [Google Scholar]
- Smith, K.F.; Rhodes, L.; Verma, A.; Curley, B.G.; Harwood, D.T.; Kohli, G.S.; Solomona, D.; Rongo, T.; Munday, R.; Munday, S.A. A new Gambierdiscus species (Dinophyceae) from Rarotonga, Cook Islands: Gambierdiscus cheloniae sp. nov. Harmful Algae 2016, 60, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Litaker, R.W.; Vandersea, M.W.; Faust, M.A.; Kibler, S.R.; Nau, A.W.; Holland, W.C.; Chinain, M.; Holmes, M.J.; Tester, P.A. Global distribution of ciguatera causing dinoflagellates in the genus Gambierdiscus. Toxicon 2010, 56, 711–730. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. Mrbayes: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J.A.A. MrModeltest v2 Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Holmes, M.J.; Lewis, R.J. Purification and characterisation of large and small maitotoxins from cultured Gambierdiscus toxicus. Nat. Toxins 1994, 2, 64–72. [Google Scholar] [CrossRef] [PubMed]

| Species | Isolate Code | CICCM Code | GenBank Accession Number | Toxins Produced (pg/cell) 1 | |
|---|---|---|---|---|---|
| Ciguatoxins 2 | Maitotoxin-1 | ||||
| G. polynesiensis | Mac3-o | CAWD254 | MF109032 | ND | ND |
| G. australes | Mac1-b | CAWD255 | MF109033 | ND | 36 |
| G. australes | Mac2-a | n | ND | 25 | |
| G. australes | Mac2-b | n | ND | 12 | |
| G. australes | Mac2-c | n | ND | 22 | |
| G. australes | Mac3-a | n | ND | 14 | |
| G. australes | Mac3-b | n | ND | 20 | |
| G. australes | Mac3-c | CAWD256 | MF109034 | ND | 31 |
| G. australes | Mac3-e | n | ND | 19 | |
| G. australes | Mac3-i | n | ND | 3 | |
| G. australes | Mac3-m | n | ND | 19 | |
| G. australes | Mac3-n | n | ND | 5 | |
| G. australes | Mac4-e | n | ND | 10 | |
| G. australes | Mac4-f | n | ND | 9 | |
| G. australes | Mac4-fuk | n | ND | 21 | |
| G. australes | Mac4-g | n | ND | 27 | |
| G. australes | Mac5-a | n | ND | 16 | |
| G. australes | Mac5-b | n | ND | 15 | |
| G. australes | Mac5-c | n | ND | 8 | |
| G. australes | Mac5-e | n | ND | 20 | |
| G. australes | Mac5-f | n | ND | 8 | |
| G. australes | Mac5-g | n | ND | 7 | |
| G. australes | Mac5-j | n | ND | 32 | |
| G. australes | Mac5-l | n | ND | 6 | |
| G. australes | Mac6-a | n | ND | 18 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhodes, L.L.; Smith, K.F.; Murray, S.; Harwood, D.T.; Trnski, T.; Munday, R. The Epiphytic Genus Gambierdiscus (Dinophyceae) in the Kermadec Islands and Zealandia Regions of the Southwestern Pacific and the Associated Risk of Ciguatera Fish Poisoning. Mar. Drugs 2017, 15, 219. https://doi.org/10.3390/md15070219
Rhodes LL, Smith KF, Murray S, Harwood DT, Trnski T, Munday R. The Epiphytic Genus Gambierdiscus (Dinophyceae) in the Kermadec Islands and Zealandia Regions of the Southwestern Pacific and the Associated Risk of Ciguatera Fish Poisoning. Marine Drugs. 2017; 15(7):219. https://doi.org/10.3390/md15070219
Chicago/Turabian StyleRhodes, Lesley L., Kirsty F. Smith, Sam Murray, D. Tim Harwood, Tom Trnski, and Rex Munday. 2017. "The Epiphytic Genus Gambierdiscus (Dinophyceae) in the Kermadec Islands and Zealandia Regions of the Southwestern Pacific and the Associated Risk of Ciguatera Fish Poisoning" Marine Drugs 15, no. 7: 219. https://doi.org/10.3390/md15070219




