Urinary Excretion of Tetrodotoxin Modeled in a Porcine Renal Proximal Tubule Epithelial Cell Line, LLC-PK1
Abstract
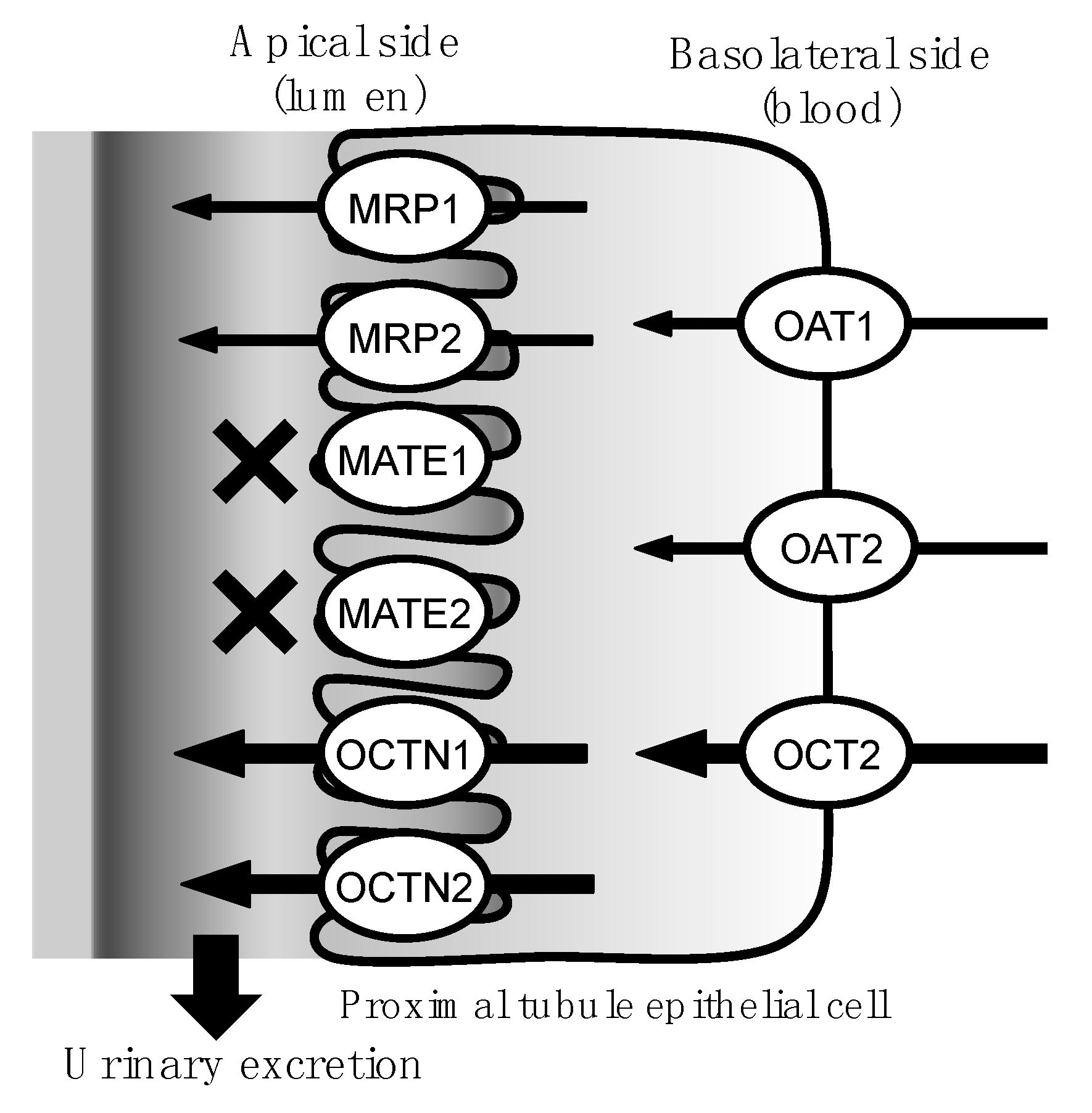
:1. Introduction
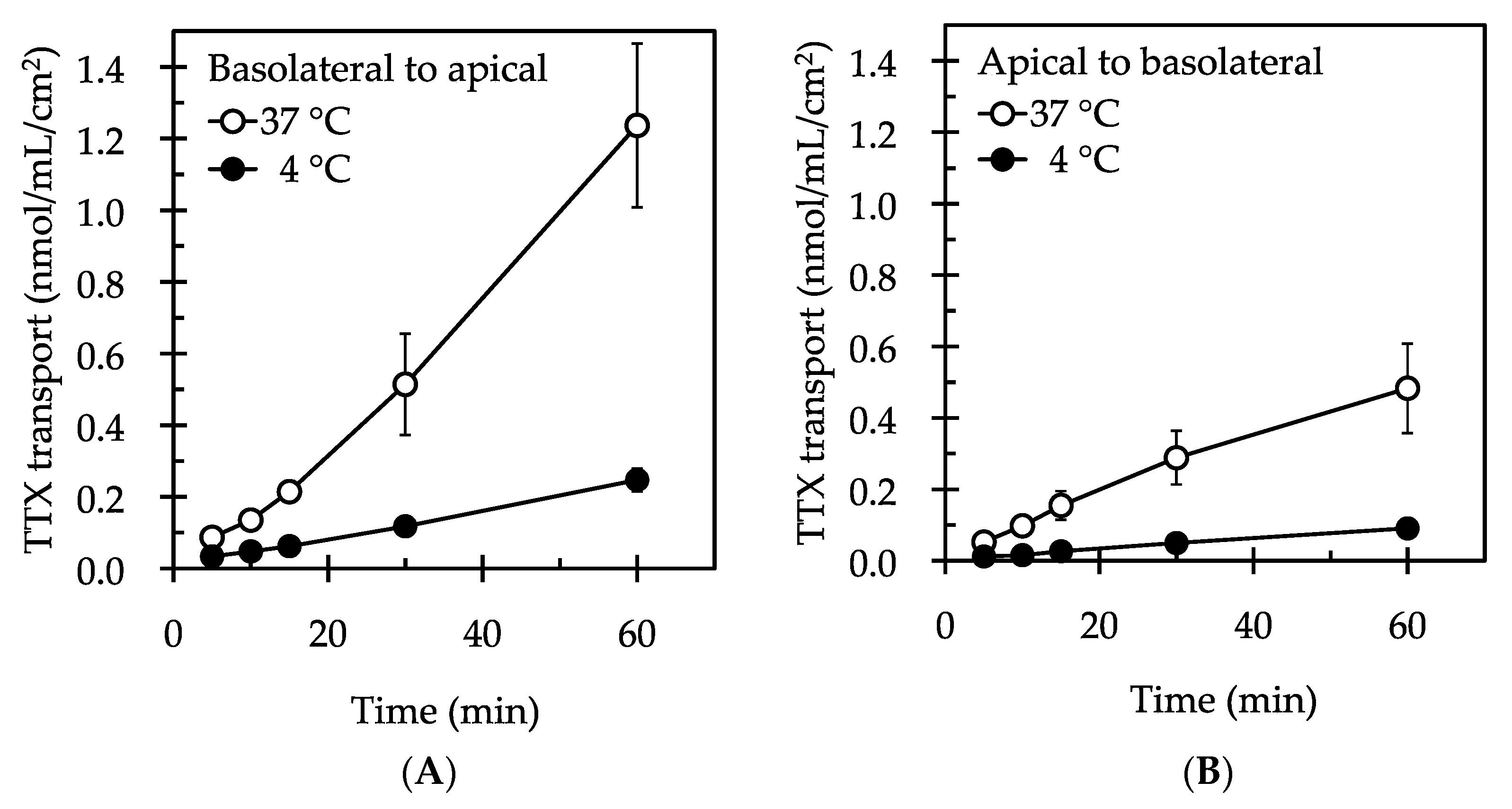
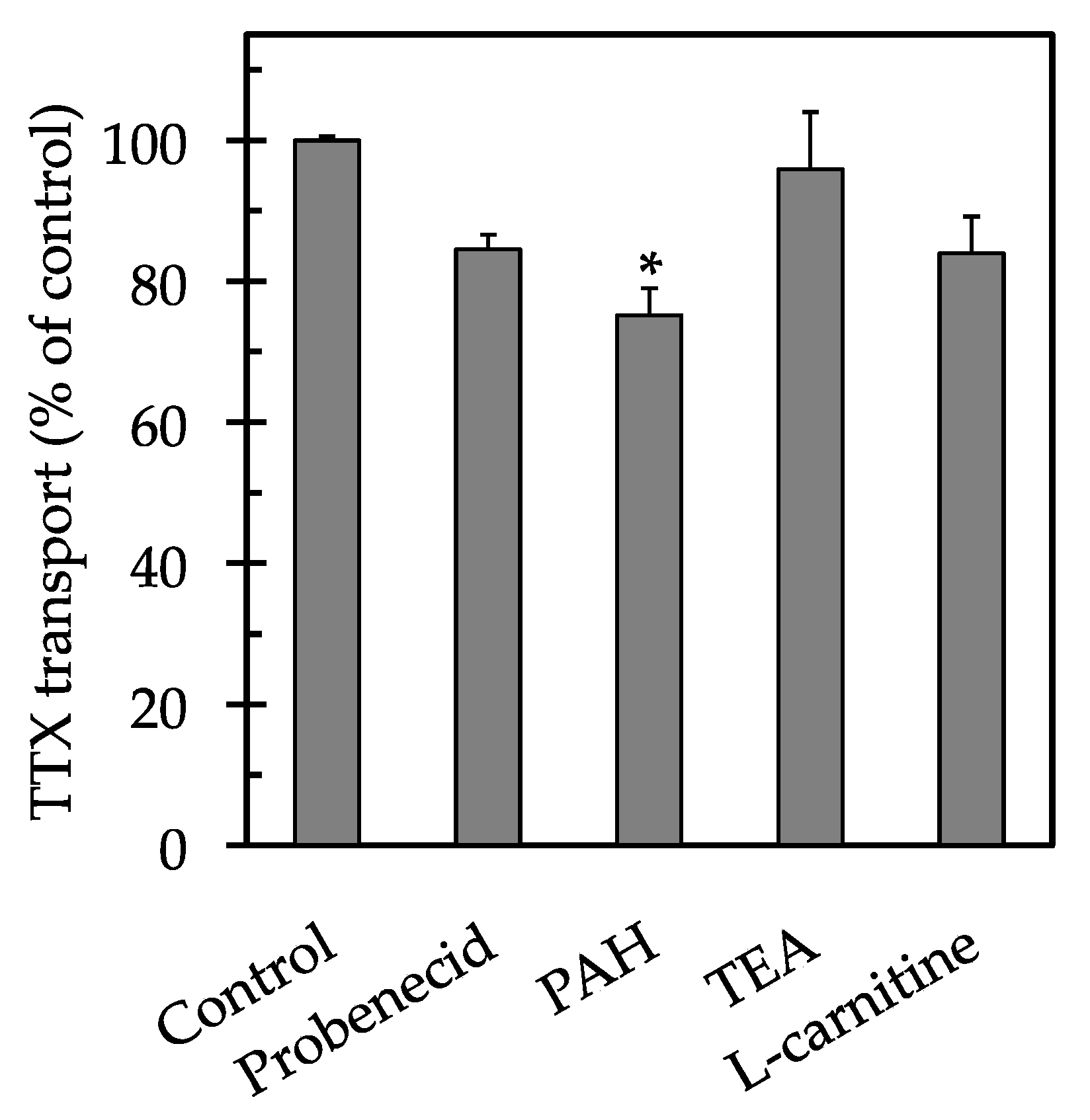
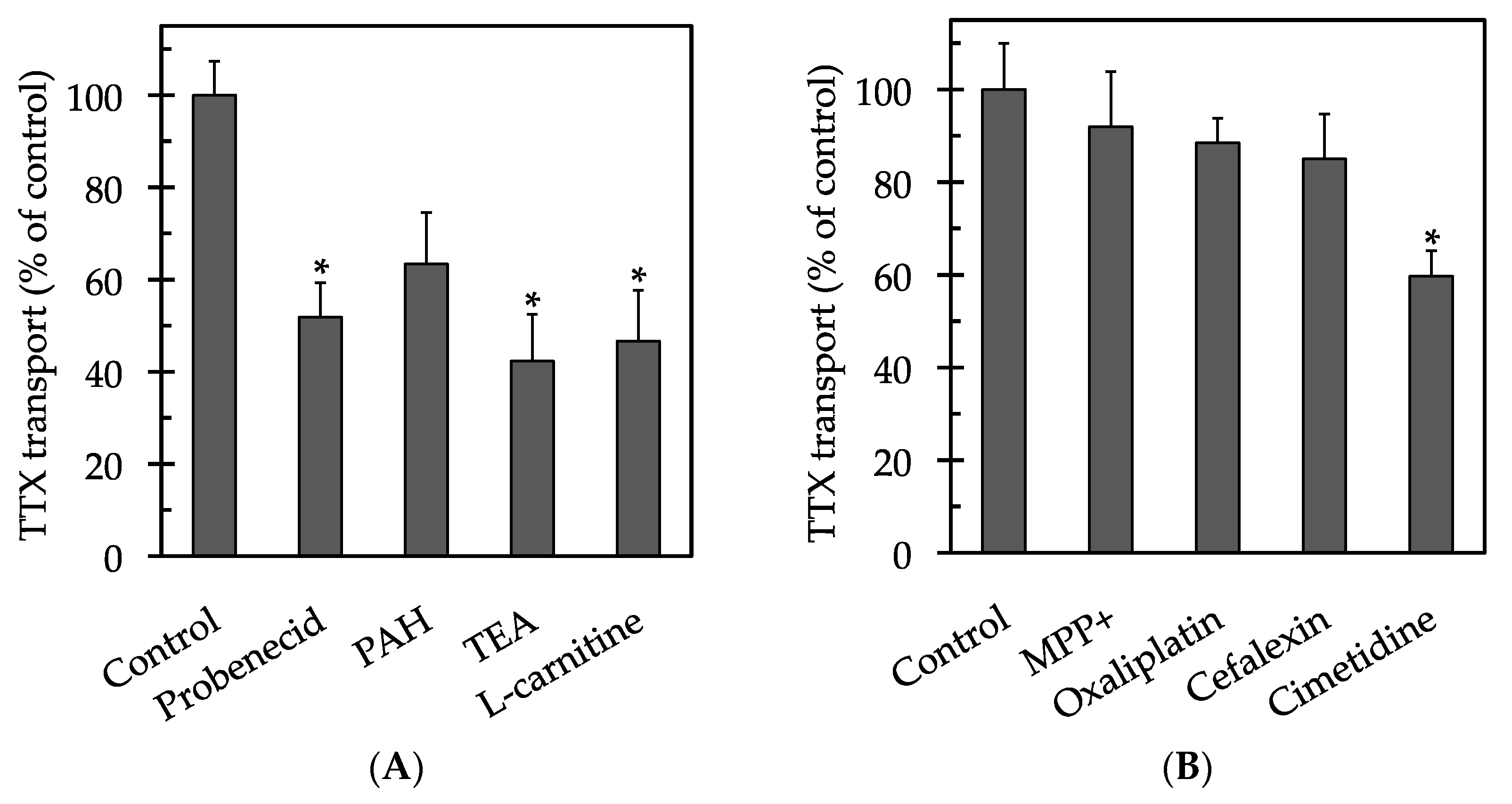
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Transepithelial Electrical Resistance (TEER) Measurements
4.4. Transport Studies
4.5. Determination of TTX Levels by LC-MS/MS
4.6. Pharmacokinetics and Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Narahashi, T.; Moore, J.W.; Scott, W.R. Tetrodotoxin blockage of sodium conductance increase in lobster giant axons. J. Gen. Physiol. 1964, 47, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Takata, M.; Moore, J.W.; Kao, C.Y.; Fuhrman, F.A. Blockage of sodium conductance increase in lobster giant axon by tarichatoxin (tetrodotoxin). J. Gen. Physiol. 1966, 49, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Noguchi, T. Distribution and origin of tetrodotoxin. J. Toxicol. Toxin Rev. 2001, 20, 11–33. [Google Scholar] [CrossRef]
- Habu, J.; Kim, M.; Katayama, M.; Komiya, H. Jomon subsistence-settlement systems at the Sannai Maruyama site. Bull. Indo-Pac. Prehist. Assoc. 2001, 21, 9–21. [Google Scholar]
- Ishida, Y.; Yamada, A.; Adachi, H.; Yagisawa, I.; Tadokoro, K.; Geiger, H.J. Salmon distribution in the northern Japan during the Jomon Period, 2000–8000 years ago, and its implications for future global warming. NPAFC Bull. 2009, 5, 287–292. [Google Scholar]
- O’Connor, S.; Ono, R.; Clarkson, C. Pelagic fishing at 42,000 years before the present and the maritime skills of modern humans. Science 2011, 334, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Isbister, G.K.; Son, J.; Wang, F.; Maclean, C.J.; Lin, C.S.; Ujma, J.; Balit, C.R.; Smith, B.; Milder, D.G.; Kiernan, M.C. Puffer fish poisoning: A potentially life-threatening condition. Med. J. Aust. 2002, 177, 650–653. [Google Scholar] [PubMed]
- Kanchanapongkul, J. Tetrodotoxin poisoning following ingestion of the toxic eggs of the horseshoe crab Carcinoscorpius rotundicauda, a case series from 1994 through 2006. Southeast Asian J. Trop. Med. Public Health 2008, 39, 303–306. [Google Scholar] [PubMed]
- Fernández-Ortega, J.F.; Morales-de los Santos, J.M.; Herrera-Gutiérrez, M.E.; Fernández-Sánchez, V.; Loureo, P.R.; Rancaño, A.A.; Téllez-Andrade, A. Seafood intoxication by tetrodotoxin: First case in Europe. J. Emerg. Med. 2010, 39, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Islam, Q.T.; Razzak, M.A.; Islam, M.A.; Bari, M.I.; Basher, A.; Chowdhury, F.R.; Sayeduzzaman, A.B.; Ahasan, H.A.; Faiz, M.A.; Arakawa, O.; et al. Puffer fish poisoning in Bangladesh: Clinical and toxicological results from large outbreaks in 2008. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.S.; Quek, L.S.; Lim, E.K.; Ngo, A. A case report of puffer fish poisoning in Singapore. Case Rep. Med. 2013, 2013, 206971. [Google Scholar] [CrossRef] [PubMed]
- Simões, E.M.D.S.; Mendes, T.M.; Adão, A.; Junior, V.H. Poisoning after ingestion of pufferfish in Brazil: Report of 11 cases. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Lin, C.L.; Chen, C.H.; Hsieh, C.H.; Jen, H.C.; Jian, S.J.; Hwang, D.F. Toxin and species identification of toxic octopus implicated into food poisoning in Taiwan. Toxicon 2014, 91, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.B.; Heegaard, W.G.; Deeds, J.R.; McGrath, S.C.; Handy, S.M. Tetrodotoxin poisoning outbreak from imported dried puffer fish-Minneapolis, Minnesota, 2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 63, 1222–1225. [Google Scholar] [PubMed]
- You, J.; Yue, Y.; Xing, F.; Xia, W.; Lai, S.; Zhang, F. Tetrodotoxin poisoning caused by Goby fish consumption in southeast China: A retrospective case series analysis. Clinics 2015, 70, 24–29. [Google Scholar] [CrossRef]
- Noguchi, T.; Ebesu, J.S.M. Puffer poisoning: Epidemiology and treatment. J. Toxicol. Toxin Rev. 2001, 20, 1–10. [Google Scholar] [CrossRef]
- Isbister, G.K.; Kiernan, M.C. Neurotoxic marine poisoning. Lancet Neurol. 2005, 4, 219–228. [Google Scholar] [CrossRef]
- Hwang, D.F.; Noguchi, T. Tetrodotoxin poisoning. Adv. Food Nutr. Res. 2007, 52, 141–236. [Google Scholar] [PubMed]
- Oda, K.; Araki, K.; Totoki, T.; Shibasaki, H. Nerve conduction study of human tetrodotoxication. Neurology 1989, 39, 743–745. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.A.; Schneider, J.J.; Isbister, G.K. Use of high performance liquid chromatography to measure tetrodotoxin in serum and urine of poisoned patients. Toxicon 2004, 44, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Tsai, Y.H.; Deng, J.F.; Cheng, C.A.; Ho, P.H.; Hwang, D.F. Identification of tetrodotoxin in a marine gastropod (Nassarius glans) responsible for human morbidity and mortality in Taiwan. J. Food Prot. 2005, 68, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Yu, C.F.; Tam, S.; Yu, P.H. Rapid screening of tetrodotoxin in urine and plasma of patients with puffer fish poisoning by HPLC with creatinine correction. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sasaya, M.; Oda, M.; Endo, T.; Saitoh, H.; Takada, M. The transport of ciprofloxacin in cultured kidney epithelial cells LLC-PK1. Biol. Pharm. Bull. 1997, 20, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Soares-Da-Silva, P.; Serrão, M.P. Molecular modulation of inward and outward apical transporters of l-dopa in LLC-PK1 cells. Am. J. Physiol. Ren. Physiol. 2000, 279, F736–F746. [Google Scholar]
- Fouda, A.K.; Fauth, C.; Roch-Ramel, F. Transport of organic cations by kidney epithelial cell line LLC-PK1. J. Pharmacol. Exp. Ther. 1990, 252, 286–292. [Google Scholar] [PubMed]
- Saito, H.; Yamamoto, M.; Inui, K.; Hori, R. Transcellular transport of organic cation across monolayers of kidney epithelial cell line LLC-PK1. Am. J. Physiol. 1992, 262, C59–C66. [Google Scholar] [PubMed]
- Saladik, D.T.; Soler, A.P.; Lewis, S.A.; Mullin, J.M. Cell division does not increase transepithelial permeability of LLC-PK1 cell sheets. Exp. Cell Res. 1995, 220, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A. Impact of transporter-mediated drug absorption, distribution, elimination and drug interactions in antimicrobial chemotherapy. J. Infect. Chemother. 2006, 12, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, G.; Wolff, N.A. Structure of renal organic anion and cation transporters. Am. J. Physiol. Ren. Physiol. 2000, 278, F853–F866. [Google Scholar]
- Motohashi, H.; Nakao, Y.; Masuda, S.; Katsura, T.; Kamba, T.; Ogawa, O.; Inui, K. Precise comparison of protein localization among OCT, OAT, and MATE in human kidney. J. Pharm. Sci. 2013, 102, 3302–3308. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, J. Renal drug transporters and their significance in drug-drug interactions. Acta Pharm. Sin. B 2016, 6, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Inui, K.; Masuda, S.; Saito, H. Cellular and molecular aspects of drug transport in the kidney. Kidney Int. 2000, 58, 944–958. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Otsuki, Y.; Hashimoto, Y.; Inui, K. Kinetic analysis of tetraethylammonium transport in the kidney epithelial cell line, LLC-PK1. Pharm. Res. 1997, 14, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Gründemann, D.; Babin-Ebell, J.; Martel, F.; Ording, N.; Schmidt, A.; Schömig, E. Primary structure and functional expression of the apical organic cation transporter from kidney epithelial LLC-PK1 cells. J. Biol. Chem. 1997, 272, 10408–10413. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kishi, Y.; Takahashi, S.; Hirata, Y. Tetrodotoxin. Tetrahedron 1965, 21, 2059–2088. [Google Scholar] [CrossRef]
- Kungsuwan, A.; Nagashima, Y.; Noguchi, T.; Shida, Y.; Suvapeepan, S.; Suwansakornkul, P.; Hashimoto, K. Tetrodotoxin in the horseshoe crab Carcinoscorpius rotundicauda inhabiting Thailand. Nippon Suisan Gakkaishi 1987, 53, 261–266. [Google Scholar] [CrossRef]
- Feller, N.; Broxterman, H.J.; Währer, D.C.; Pinedo, H.M. ATP-dependent efflux of calcein by the multidrug resistance protein (MRP): No inhibition by intracellular glutathione depletion. FEBS Lett. 1995, 368, 385–388. [Google Scholar] [CrossRef]
- Evers, R.; Zaman, G.J.; van Deemter, L.; Jansen, H.; Calafat, J.; Oomen, L.C.; Oude-Elferink, R.P.; Borst, P.; Schinkel, A.H. Basolateral localization and export activity of the human multidrug resistance-associated protein in polarized pig kidney cells. J. Clin. Investig. 1996, 97, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Goh, L.B.; Spears, K.J.; Yao, D.; Ayrton, A.; Morgan, P.; Roland, W.C.; Friedberg, T. Endogenous drug transporters in vitro and in vivo models for the prediction of drug disposition in man. Biochem. Pharmacol. 2002, 64, 1569–1578. [Google Scholar] [CrossRef]
- Tamai, I.; Ohashi, R.; Nezu, J.; Yabuuchi, H.; Oku, A.; Shimane, M.; Sai, Y.; Tsuji, A. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J. Biol. Chem. 1998, 273, 20378–20382. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, H.; Tamai, I.; Nezu, J.; Sakamoto, K.; Oku, A.; Shimane, M.; Sai, Y.; Tsuji, A. Novel membrane transporter OCTN1 mediates multispecific, bidirectional, and pH-dependent transport of organic cations. J. Pharmacol. Exp. Ther. 1999, 289, 768–773. [Google Scholar] [PubMed]
- Tanihara, Y.; Masuda, S.; Sato, T.; Katsura, T.; Ogawa, O.; Inui, K. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H+-organic cation antiporters. Biochem. Pharmacol. 2007, 74, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Matsumoto, T.; Morimoto, R.; Arioka, S.; Omote, H.; Moriyama, Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc. Natl. Acad. Sci. USA 2005, 103, 17923–17928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahara, H.; Kusuhara, H.; Endou, H.; Koepsell, H.; Imaoka, T.; Fuse, E.; Sugiyama, Y. A species difference in the transport activities of H2 receptor antagonists by rat and human renal organic anion and cation transporters. J. Pharmacol. Exp. Ther. 2005, 315, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Tanuma, D.; Tsutsumi, K.; Jeon, J.K.; Ishizaki, S.; Nagashima, Y. Plasma protein binding of tetrodotoxin in the marine puffer fish Takifugu rubripes. Toxicon 2010, 55, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.Y.; Lai, S.L.; Chen, S.S.; Hwang, D.F. Tetrodotoxin intoxication in a uraemic patient. J. Neurol. Neurosurg. Psychiatry 1999, 67, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Sunwoo, M.K.; Sunwoo, I.N. Serial electrophysiological changes in uraemic patients with tetrodotoxin intoxication. Clin. Neurophysiol. 2011, 122, 2310–2311. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, R.; Nakata, Y.; Kameoka, M.; Hayashi, N.; Watanabe, K.; Yagi, K. A case of tetrodotoxin intoxication in a uremic patient. Chudoku Kenkyu 2007, 20, 141–145. (In Japanese) [Google Scholar] [PubMed]
- Fong, B.M.; Tam, S.; Tsui, S.H.; Leung, K.S. Development and validation of a high-throughput double solid phase extraction-liquid chromatography-tandem mass spectrometry method for the determination of tetrodotoxin in human urine and plasma. Talanta 2011, 83, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Inui, K.; Saito, H.; Hori, R. H+-gradient-dependent active transport of tetraethylammonium cation in apical-membrane vesicles isolated from kidney epithelial cell line LLC-PK1. Biochem. J. 1985, 227, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Kimura, O.; Sasaya, M.; Takada, M.; Sakata, M. Na+- and energy-dependent transport of cadmium into LLC-PK1 cells. Biol. Pharm. Bull. 1995, 18, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Masago, M.; Takaai, M.; Sakata, J.; Horie, A.; Ito, T.; Ishida, K.; Taguchi, M.; Hashimoto, Y. Membrane transport mechanisms of quinidine and procainamide in renal LLC-PK1 and intestinal LS180 cells. Biol. Pharm. Bull. 2010, 33, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Takaai, M.; Suzuki, H.; Ishida, K.; Tahara, K.; Hashimoto, Y. Pharmacokinetic analysis of transcellular transport of levofloxacin across LLC-PK1 and Caco-2 cell monolayers. Biol. Pharm. Bull. 2007, 30, 2167–2172. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kiriake, A.; Ishizaki, S.; Watabe, S.; Nagashima, Y. Biliary excretion of tetrodotoxin in the cultured pufferfish Takifugu rubripes juvenile after intramuscular administration. Toxicon 2015, 93, 98–102. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, T.; Ishizaki, Y.; Mochizuki, K.; Aoyagi, M.; Mitoma, Y.; Ishizaki, S.; Nagashima, Y. Urinary Excretion of Tetrodotoxin Modeled in a Porcine Renal Proximal Tubule Epithelial Cell Line, LLC-PK1. Mar. Drugs 2017, 15, 225. https://doi.org/10.3390/md15070225
Matsumoto T, Ishizaki Y, Mochizuki K, Aoyagi M, Mitoma Y, Ishizaki S, Nagashima Y. Urinary Excretion of Tetrodotoxin Modeled in a Porcine Renal Proximal Tubule Epithelial Cell Line, LLC-PK1. Marine Drugs. 2017; 15(7):225. https://doi.org/10.3390/md15070225
Chicago/Turabian StyleMatsumoto, Takuya, Yui Ishizaki, Keika Mochizuki, Mitsuru Aoyagi, Yoshiharu Mitoma, Shoichiro Ishizaki, and Yuji Nagashima. 2017. "Urinary Excretion of Tetrodotoxin Modeled in a Porcine Renal Proximal Tubule Epithelial Cell Line, LLC-PK1" Marine Drugs 15, no. 7: 225. https://doi.org/10.3390/md15070225




