A Transcriptomic Survey of Ion Channel-Based Conotoxins in the Chinese Tubular Cone Snail (Conus betulinus)
Abstract
:1. Introduction
2. Results
2.1. Summary of the Previously-Reported Transcriptome Sequencing and Achieved Data
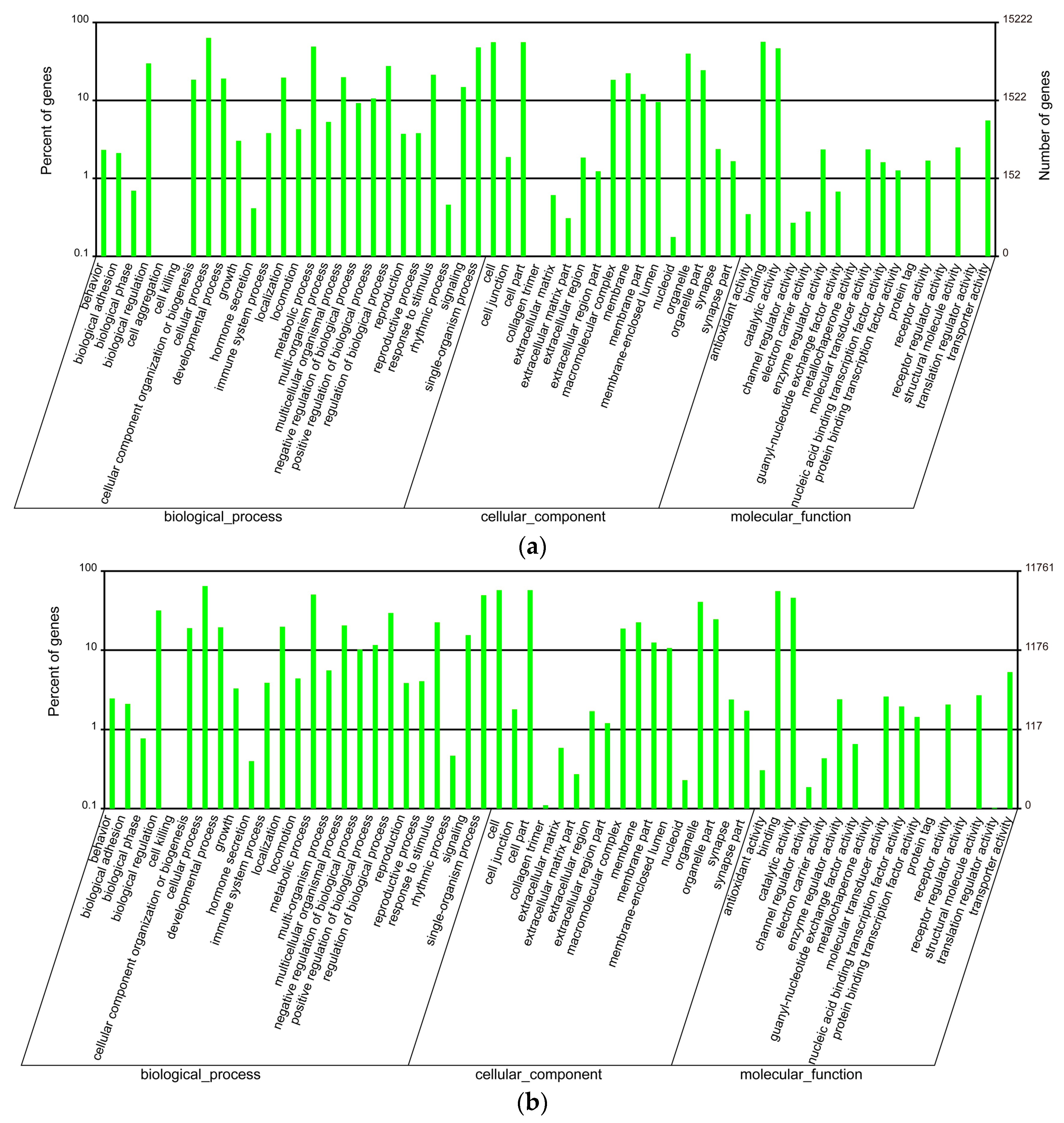
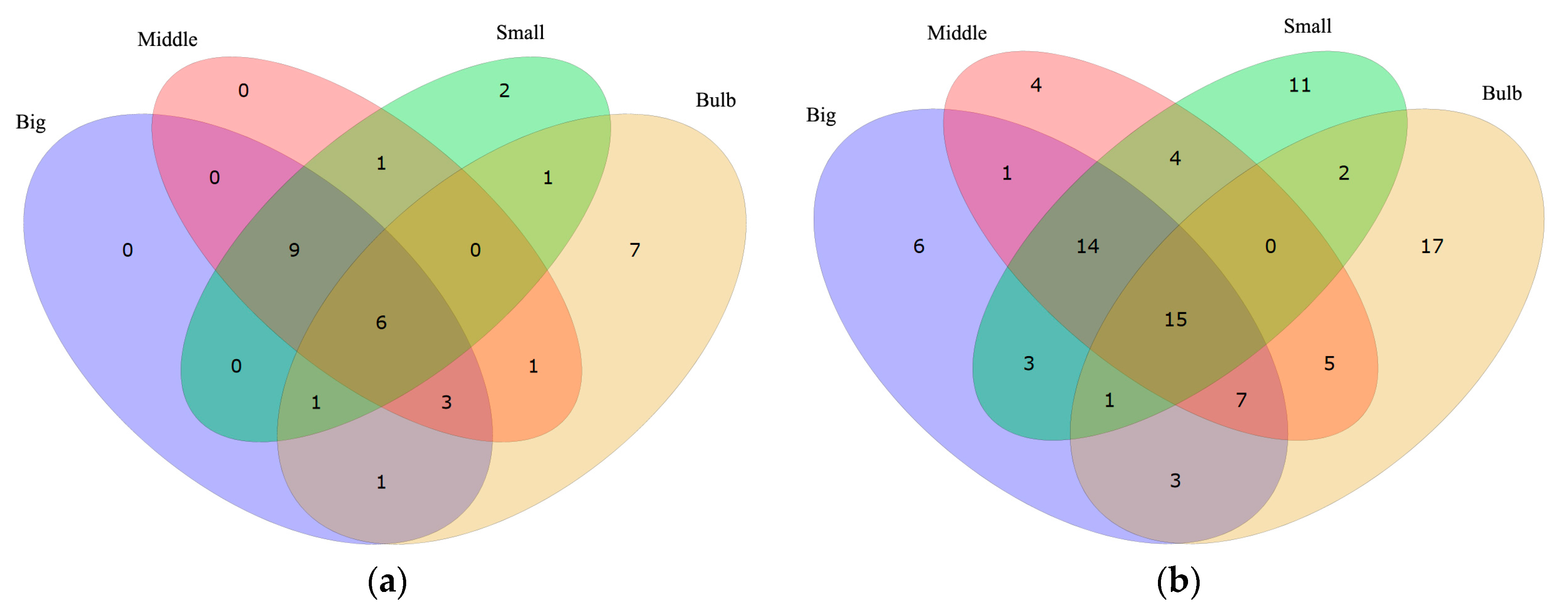
2.2. Annotation of Unigenes
2.3. Top Highly-Transcribed Channel and Ion Channel Genes in the Venom Duct and the Venom Bulb
2.4. Identified Conotoxins and Their Predicted Activities
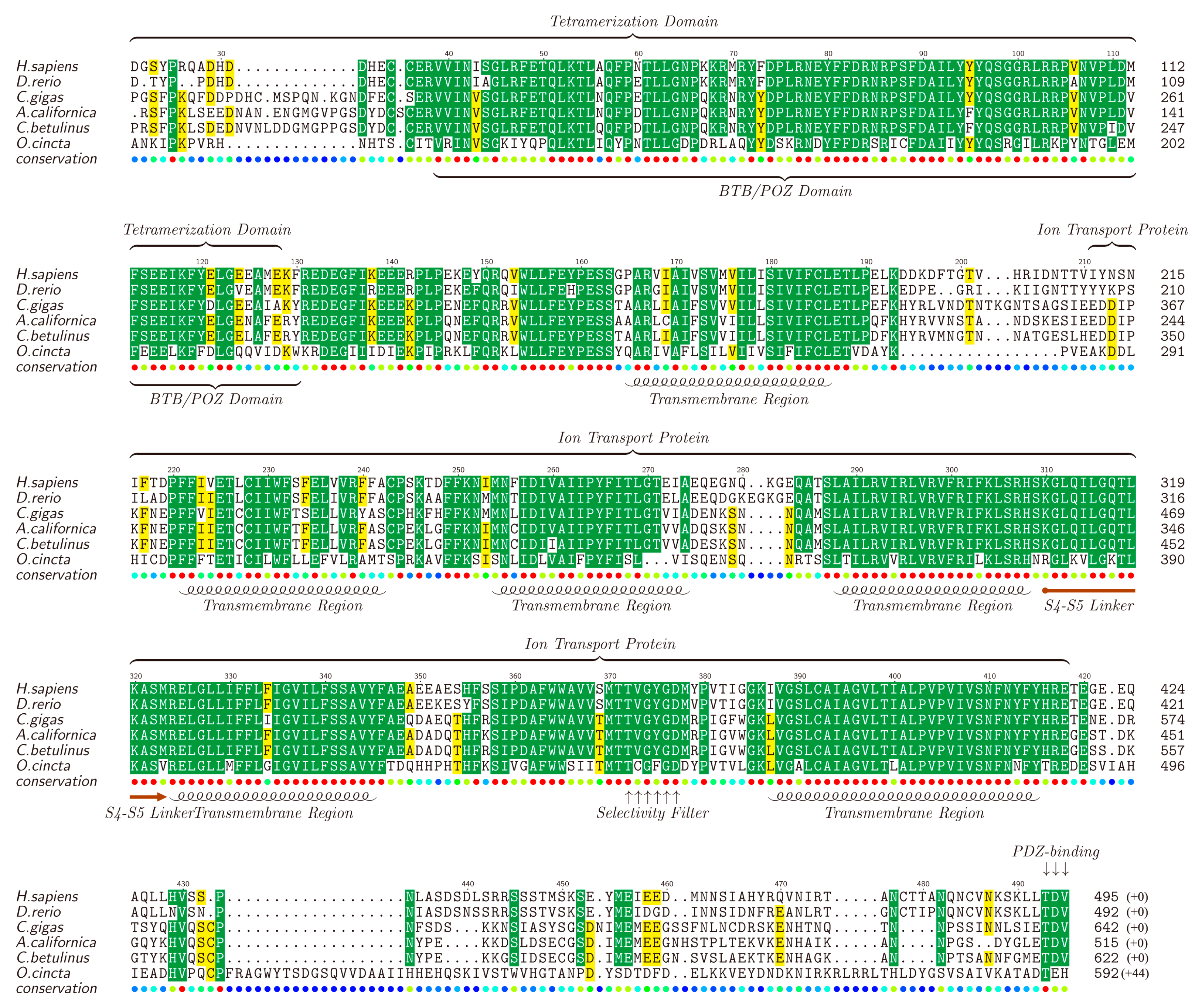
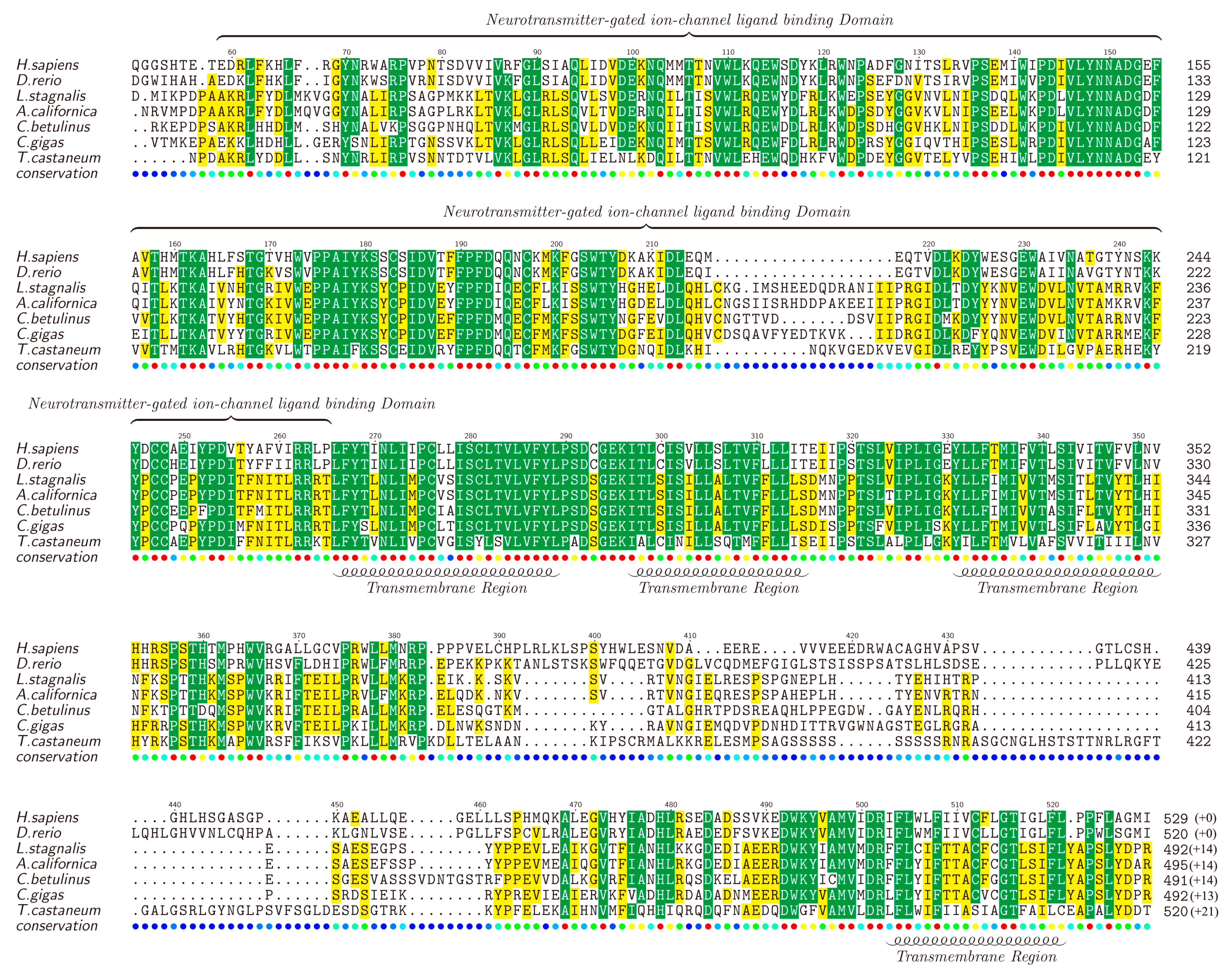
2.5. Ion Channel-Related Proteins and Homologous Analysis
3. Discussion
4. Materials and Methods
4.1. Assembly, Annotation, and RPKM Calculation
4.2. Prediction and Identification of Ion Channel-Related Proteins/Receptors
4.3. Alignment and Homology of Ion Channel Related Proteins/Receptors
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kohn, A.J. The ecology of Conus in Hawaii. Ecol. Monogr. 1959, 29, 47–90. [Google Scholar] [CrossRef]
- Puillandre, N.; Bouchet, P.; Duda, T.F.; Kauferstein, S.; Kohn, A.J.; Olivera, B.M.; Watkins, M.; Meyer, C. Molecular phylogeny and evolution of the cone snails (Gastropoda, Conoidea). Mol. Phylogenet. Evol. 2014, 78, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Duda, T.F., Jr.; Kohn, A.J.; Palumbi, S.R. Origins of diverse feeding ecologies within Conus, a genus of venomous marine gastropods. Biol. J. Linn. Soc. 2001, 73, 391–409. [Google Scholar] [CrossRef]
- Duda, T.F., Jr.; Kohn, A.J. Species-level phylogeography and evolutionary history of the hyperdiverse marine gastropod genus Conus. Mol. Phylogenet. Evol. 2005, 34, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Olivera, B.M. Conus venom peptides, receptor and ion channel targets and drug design: 50 million years of neuropharmacology. Mol. Biol. Cell 1997, 8, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, P.; Kantor, Y.I.; Sysoev, A.; Puillandre, N. A new operational classification of the conoidea (Gastropoda). J. Molluscan Stud. 2011, 77, 273–308. [Google Scholar] [CrossRef]
- Modica, M.V.; Holford, M. The Neogastropoda: Evolutionary innovations of predatory marine snails with remarkable pharmacological potential. In Evolutionary Biology—Concepts, Molecular and Morphological Evolution; Pontarotti, P., Ed.; Springer: Heidelberg, Germany, 2010; pp. 249–270. [Google Scholar]
- Prashanth, J.R.; Dutertre, S.; Jin, A.H.; Lavergne, V.; Hamilton, B.; Cardoso, F.C.; Griffin, J.; Venter, D.J.; Alewood, P.F.; Lewis, R.J. The role of defensive ecological interactions in the evolution of conotoxins. Mol. Ecol. 2016, 25, 598–615. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, S.; Jin, A.H.; Vetter, I.; Hamilton, B.; Sunagar, K.; Lavergne, V.; Dutertre, V.; Fry, B.G.; Antunes, A.; Venter, D.J.; et al. Evolution of separate predation- and defence-evoked venoms in carnivorous cone snails. Nat. Commun. 2014, 5, 3521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Violette, A.; Biass, D.; Dutertre, S.; Koua, D.; Piquemal, D.; Pierrat, F.; Stocklin, R.; Favreau, P. Large-scale discovery of conopeptides and conoproteins in the injectable venom of a fish-hunting cone snail using a combined proteomic and transcriptomic approach. J. Proteom. 2012, 75, 5215–5225. [Google Scholar] [CrossRef] [PubMed]
- Safavi-Hemami, H.; Siero, W.A.; Gorasia, D.G.; Young, N.D.; Macmillan, D.; Williamson, N.A.; Purcell, A.W. Specialisation of the venom gland proteome in predatory cone snails reveals functional diversification of the conotoxin biosynthetic pathway. J. Proteome Res. 2011, 10, 3904–3919. [Google Scholar] [CrossRef] [PubMed]
- Endean, R.; Duchemin, C. The venom apparatus of Conus magus. Toxicon 1967, 4, 275–284. [Google Scholar] [CrossRef]
- Peng, C.; Yao, G.; Gao, B.; Fan, C.; Bian, C.; Wang, J.; Cao, Y.; Wen, B.; Zhu, Y.; Ruan, Z.; et al. High-throughput identification of novel conotoxins from the chinese tubular cone snail (Conus betulinus) by multitranscriptome sequencing. Gigascience 2016, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, J.M.; Jones, R.M. Cone venom—From accidental stings to deliberate injection. Toxicon 2001, 39, 1447–1451. [Google Scholar] [CrossRef]
- Dutertre, S.; Jin, A.H.; Kaas, Q.; Jones, A.; Alewood, P.F.; Lewis, R.J. Deep venomics reveals the mechanism for expanded peptide diversity in cone snail venom. Mol. Cell Proteom. 2013, 12, 312–329. [Google Scholar] [CrossRef] [PubMed]
- Barghi, N.; Concepcion, G.P.; Olivera, B.M.; Lluisma, A.O. Comparison of the Venom Peptides and Their Expression in Closely Related Conus Species: Insights into Adaptive Post-speciation Evolution of Conus Exogenomes. Genome Biol. Evol. 2015, 7, 1797–1814. [Google Scholar] [CrossRef] [PubMed]
- Himaya, S.W.A.; Jin, A.H.; Dutertre, S.; Giacomotto, J.; Mohialdeen, H.; Vetter, I.; Alewood, P.F.; Lewis, R.J. Comparative Venomics Reveals the Complex Prey Capture Strategy of the Piscivorous Cone Snail Conus catus. J. Proteome Res. 2015, 14, 4372–4381. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, V.; Harliwong, I.; Jones, A.; Miller, D.; Taft, R.J.; Alewood, P.F. Optimized deep-targeted proteotranscriptomic profiling reveals unexplored Conus toxin diversity and novel cysteine frameworks. Proc. Natl. Acad. Sci. USA 2015, 112, E3782–E3791. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zheng, Y.; Tang, H. Identifying the types of ion channel-targeted conotoxins by incorporating new properties of residues into pseudo amino acid composition. BioMed Res. Int. 2016, 2016, 3981478. [Google Scholar] [CrossRef] [PubMed]
- Woodward, S.R.; Cruz, L.J.; Olivera, B.M.; Hillyard, D.R. Constant and hypervariable regions in conotoxin propeptides. EMBO J. 1990, 9, 1015–1020. [Google Scholar] [PubMed]
- Olivera, B.M.; Walker, C.; Cartier, G.E.; Hooper, D.; Santos, A.D.; Schoenfeld, R.; Shetty, R.; Watkins, M.; Bandyopadhyay, P.; Hillyard, D.R. Speciation of cone snails and interspecific hyperdivergence of their venom peptides. Potential evolutionary significance of introns. Ann. N. Y. Acad. Sci. 1999, 870, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Lebbe, E.K.; Tytgat, J. In the picture: Disulfide-poor conopeptides, a class of pharmacologically interesting compounds. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Koua, D.; Favreau, P.; Olivera, B.M.; Stocklin, R. Molecular phylogeny, classification and evolution of conopeptides. J. Mol. Biol. 2012, 74, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Vetter, I.; Lewis, R.J. Therapeutic potential of cone snail venom peptides (conopeptides). Curr. Top. Med. Chem. 2012, 12, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Akondi, K.B.; Muttenthaler, M.; Dutertre, S.; Kaas, Q.; Craik, D.J.; Lewis, R.J.; Alewood, P.F. Discovery, synthesis, and structure-activity relationships of conotoxins. Chem. Rev. 2014, 114, 5815–5847. [Google Scholar] [CrossRef] [PubMed]
- Espiritu, D.J.; Watkins, M.; Dia-Monje, V.; Cartier, G.E.; Cruz, L.J.; Olivera, B.M. Venomous cone snails: Molecular phylogeny and the generation of toxin diversity. Toxicon 2001, 39, 1899–1916. [Google Scholar] [CrossRef]
- Lu, A.; Yang, L.; Xu, S.; Wang, C. Various conotoxin diversifications revealed by a venomic study of Conus flavidus. Mol. Cell Proteom. 2014, 13, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Kaas, Q.; Westermann, J.C.; Craik, D.J. Conopeptide characterization and classifications: An analysis using ConoServer. Toxicon 2010, 55, 1491–1509. [Google Scholar] [CrossRef] [PubMed]
- Kaas, Q.; Yu, R.; Jin, A.H.; Dutertre, S.; Craik, D.J. ConoServer: Updated content, knowledge, and discovery tools in the conopeptide database. Nucleic Acids Res. 2012, 40, D325–D330. [Google Scholar] [CrossRef] [PubMed]
- Biggs, J.S.; Watkins, M.; Puillandre, N.; Ownby, J.P.; Lopez-Vera, E.; Christensen, S.; Moreno, K.J.; Bernaldez, J.; Licea-Navarro, A.; Corneli, P.S.; et al. Evolution of Conus peptide toxins: Analysis of Conus californicus Reeve, 1844. Mol. Phylogenet. Evol. 2010, 56, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kancherla, A.K.; Meesala, S.; Jorwal, P.; Palanisamy, R.; Sikdar, S.K.; Sarma, S.P. A Disulfide Stabilized β-Sandwich Defines the Structure of a New Cysteine Framework M-Superfamily Conotoxin. ACS Chem. Biol. 2015, 10, 1847–1860. [Google Scholar] [CrossRef] [PubMed]
- Bernáldez, J.; Román-González, S.A.; Martínez, O.; Jiménez, S.; Vivas, O.; Arenas, I.; Corzo, G.; Arreguín, R.; García, D.E.; Possani, L.D.; et al. A Conus regularis conotoxin with a novel eight-cysteine framework inhibits CaV2.2 channels and displays an anti-nociceptive activity. Mar. Drugs 2013, 11, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- Olivera, B.M.; Cruz, L.J. Conotoxins, in retrospect. Toxicon 2001, 39, 7–14. [Google Scholar] [CrossRef]
- Dutton, J.L.; Craik, D.J. alpha-Conotoxins: Nicotinic acetylcholine receptor antagonists as pharmacological tools and potential drug leads. Curr. Med. Chem. 2001, 8, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J. Conotoxins: Molecular and therapeutic targets. Prog. Mol. Subcell. Biol. 2009, 46, 45–65. [Google Scholar] [PubMed]
- Vincler, M.; McIntosh, J.M. Targeting the alpha9alpha10 nicotinic acetylcholine receptor to treat severe pain. Expert Opin. Ther. Targets 2007, 11, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Twede, V.D.; Miljanich, G.; Olivera, B.M.; Bulaj, G. Neuroprotective and cardioprotective conopeptides: An emerging class of drug leads. Curr. Opin. Drug Discov. Dev. 2009, 12, 231–239. [Google Scholar]
- Clark, R.J.; Jensen, J.; Nevin, S.T.; Callaghan, B.P.; Adams, D.J.; Craik, D.J. The engineering of an orally active conotoxin for the treatment of neuropathic pain. Angew. Chem. Int. Ed. 2010, 49, 6545–6548. [Google Scholar] [CrossRef] [PubMed]
- Miljanich, G.P. Ziconotide: Neuronal calcium channel blocker for treating severe chronic pain. Curr. Med. Chem. 2004, 11, 3029–3040. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Dutertre, S.; Vetter, I.; Christie, M.J. Conus venom peptide pharmacology. Pharmacol. Rev. 2012, 64, 259–298. [Google Scholar] [CrossRef] [PubMed]
- Han, T.S.; Teichert, R.W.; Olivera, B.M.; Bulaj, G. Conus venoms—A rich source of peptide-based therapeutics. Curr. Pharm. Des. 2008, 14, 2462–2479. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.R.; Luque, A.; Olivera, B.M.; Barrett, J.; Cruz, L.J. Peptide toxins from Conus geographus venom. J. Biol. Chem. 1981, 256, 4734–4740. [Google Scholar] [PubMed]
- Fainzilber, M.; Nakamura, T.; Lodder, J.C.; Zlotkin, E.; Kits, K.S.; Burlingame, A.L. gamma-Conotoxin-PnVIIA, a gamma-carboxyglutamate-containing peptide agonist of neuronal pacemaker cation currents. Biochemistry 1998, 37, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Fainzilber, M.; Gordon, D.; Hasson, A.; Spira, M.E.; Zlotkin, E. Mollusc-specific toxins from the venom of Conus textile neovicarius. Eur. J. Biochem. 1991, 202, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Rigby, A.C.; Lucas-Meunier, E.; Kalume, D.E.; Czerwiec, E.; Hambe, B.; Dahlqvist, I.; Fossier, P.; Baux, G.; Roepstorff, P.; Baleja, J.D.; et al. A conotoxin from Conus textile with unusual posttranslational modifications reduces presynaptic Ca2+ influx. Proc. Natl. Acad. Sci. USA 1999, 96, 5758–5763. [Google Scholar] [CrossRef] [PubMed]
- Buczek, O.; Wei, D.; Babon, J.J.; Yang, X.; Fiedler, B.; Chen, P.; Yoshikami, D.; Olivera, B.M.; Bulaj, G.; Norton, R.S. Structure and sodium channel activity of an excitatory I1-superfamily conotoxin. Biochemistry 2007, 46, 9929–9940. [Google Scholar] [CrossRef] [PubMed]
- Terlau, H.; Shon, K.J.; Grilley, M.; Stocker, M.; Stühmer, W.; Olivera, B.M. Strategy for rapid immobilization of prey by a fish-hunting marine snail. Nature 1996, 381, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.J.; Gray, W.R.; Olivera, B.M.; Zeikus, R.D.; Kerr, L.; Yoshikami, D.; Moczydlowski, E. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. J. Biol. Chem. 1985, 260, 9280–9288. [Google Scholar] [PubMed]
- Sharpe, I.A.; Gehrmann, J.; Loughnan, M.L.; Thomas, L.; Adams, D.A.; Atkins, A.; Palant, E.; Craik, D.J.; Adams, D.J.; Alewood, P.F.; et al. Two new classes of conopeptides inhibit the alpha1-adrenoceptor and noradrenaline transporter. Nat. Neurosci. 2001, 4, 902–907. [Google Scholar] [CrossRef] [PubMed]
- England, L.J.; Imperial, J.; Jacobsen, R.; Craig, A.G.; Gulyas, J.; Akhtar, M.; Rivier, J.; Julius, D.; Olivera, B.M. Inactivation of a serotonin-gated ion channel by a polypeptide toxin from marine snails. Science 1998, 281, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Petrel, C.; Hocking, H.G.; Reynaud, M.; Upert, G.; Favreau, P.; Biass, D.; Paolini-Bertrand, M.; Peigneur, S.; Tytgat, J.; Gilles, N.; et al. Identification, structural and pharmacological characterization of τ-CnVA, a conopeptide that selectively interacts with somatostatin sst3 receptor. Biochem. Pharmacol. 2013, 85, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Kerr, L.M.; Yoshikami, D. A venom peptide with a novel presynaptic blocking action. Nature 1984, 308, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Bairoch, A.; Apweiler, R. The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucleic Acids Res. 2000, 28, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef] [PubMed]
- GO-EBI. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 2004, 32, D258–D261. [Google Scholar]
- Moran, O. Molecular simulation of the interaction of kappa-conotoxin-PVIIA with the Shaker potassium channel pore. Eur. Biophys. J. 2001, 30, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Terlau, H.; Olivera, B.M. Conus venoms: A rich source of novel ion channel-targeted peptides. Physiol. Rev. 2004, 84, 41–68. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Alewood, P.F.; Craik, D.J. Three-dimensional solution structure of mu-conotoxin GIIIB, a specific blocker of skeletal muscle sodium channels. Biochemistry 1996, 35, 8824–8835. [Google Scholar] [CrossRef] [PubMed]
- Halai, R.; Craik, D.J. Conotoxins: Natural product drug leads. Nat. Prod. Rep. 2009, 26, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Green, B.R.; Bulaj, G.; Norton, R.S. Structure and function of μ-conotoxins, peptide-based sodium channel blockers with analgesic activity. Future Med. Chem. 2014, 6, 1677–1698. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J. Conotoxins as selective inhibitors of neuronal ion channels, receptors and transporters. IUBMB Life 2004, 56, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Favreau, P.; Le Gall, F.; Benoit, E.; Molgó, J. A review on conotoxins targeting ion channels and acetylcholine receptors of the vertebrate neuromuscular junction. Acta Physiol. Pharmacol. Ther. Latinoam. 1999, 49, 257–267. [Google Scholar] [PubMed]
- Deuis, J.R.; Dekan, Z.; Inserra, M.C.; Lee, T.H.; Aguilar, M.I.; Craik, D.J.; Lewis, R.J.; Alewood, P.F.; Mobli, M.; Schroeder, C.I.; et al. Development of a μO-Conotoxin Analogue with Improved Lipid Membrane Interactions and Potency for the Analgesic Sodium Channel NaV1.8. J. Biol. Chem. 2016, 291, 11829–11842. [Google Scholar] [CrossRef] [PubMed]
- Green, B.R.; Gajewiak, J.; Chhabra, S.; Skalicky, J.J.; Zhang, M.M.; Rivier, J.E.; Bulaj, G.; Olivera, B.M.; Yoshikami, D.; Norton, R.S. Structural Basis for the Inhibition of Voltage-gated Sodium Channels by Conotoxin μO§-GVIIJ. J. Biol. Chem. 2016, 291, 7205–7220. [Google Scholar] [CrossRef] [PubMed]
- Bellacchio, E. Mechanism of neurotoxicity of prion and Alzheimer’s disease-related proteins: Molecular insights from bioinformatically identified ω-conotoxin-like pharmacophores. Crit. Rev. Eukaryot. Gene Expr. 2013, 23, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Reimers, C.; Lee, C.H.; Kalbacher, H.; Tian, Y.; Hung, C.H.; Schmidt, A.; Prokop, L.; Kauferstein, S.; Mebs, D.; Chen, C.C.; et al. Identification of a cono-RFamide from the venom of Conus textile that targets ASIC3 and enhances muscle pain. Proc. Natl. Acad. Sci. USA 2017, 114, E3507–E3515. [Google Scholar] [CrossRef] [PubMed]
- Jayamanne, A.; Jeong, H.J.; Schroeder, C.I.; Lewis, R.J.; Christie, M.J.; Vaughan, C.W. Spinal actions of ω-conotoxins, CVID, MVIIA and related peptides in a rat neuropathic pain model. Br. J. Pharmocol. 2013, 170, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Gutman, G.A.; Chandy, K.G.; Grissmer, S.; Lazdunski, M.; McKinnon, D.; Pardo, L.A.; Robertson, G.A.; Rudy, B.; Sanguinetti, M.C.; Stühmer, W.; et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol. Rev. 2005, 57, 473–508. [Google Scholar] [CrossRef] [PubMed]
- Grizel, A.V.; Glukhov, G.S.; Sokolova, O.S. Mechanisms of Activation of Voltage-Gated Potassium Channels. Acta Nat. 2014, 6, 10–26. [Google Scholar]
- Li, G.R.; Deng, X.L. Functional ion channels in stem cells. World J. Stem Cells 2011, 3, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardo, L.A. Voltage-Gated Potassium Channels in Cell Proliferation. Physiology 2004, 19, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Archer, S.L.; Allalunis-Turner, J.; Haromy, A.; Beaulieu, C.; Thompson, R.; Lee, C.T.; Lopaschuk, G.D.; Puttagunta, L.; Bonnet, S.; et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 2007, 11, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, T.; Zagotta, W.N.; Aldrich, R.W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 1990, 250, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Q.; Purhonen, P.; Hebert, H. Structure of potassium channels. Cell Mol. Life Sci. 2015, 72, 3677–3693. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.; Nordberg, A. Neuronal nicotinic receptors in the human brain. Prog. Neurobiol. 2000, 61, 75–111. [Google Scholar] [CrossRef]
- Dani, J.A.; Bertrand, D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 699–729. [Google Scholar] [CrossRef] [PubMed]
- Changeux, J.P. The nicotinic acetylcholine receptor: The founding father of the pentameric ligand-gated ion channel superfamily. J. Biol. Chem. 2012, 287, 40207–40215. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, G.K.; Williams, M. Neuronal nicotinic acetylcholine receptors as novel drug targets. J. Pharmacol. Exp. Ther. 2000, 292, 461–467. [Google Scholar] [PubMed]
- Kawasaki, Y.; Freire, E. Finding a better path to drug selectivity. Drug Discov. Today 2011, 16, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Alkondon, M.; Albuquerque, E.X. The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Prog. Brain Res. 2004, 145, 109–120. [Google Scholar] [PubMed]
- Albuquerque, E.X.; Pereira, E.F.R.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef] [PubMed]
- Barghi, N.; Concepcion, G.P.; Olivera, B.M.; Lluisma, A.O. High conopeptide diversity in Conus tribblei revealed through analysis of venom duct transcriptome using two high-throughput sequencing platforms. Mar. Biotechnol. 2015, 17, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, V.; Dutertre, S.; Jin, A.H.; Lewis, R.J.; Taft, R.J.; Alewood, P.F. Systematic interrogation of the Conus marmoreus venom duct transcriptome with ConoSorter reveals 158 novel conotoxins and 13 new gene superfamilies. BMC Genom. 2013, 14, 708. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Bandyopadhyay, P.K.; Olivera, B.M.; Yandell, M. Elucidation of the molecular envenomation strategy of the cone snail Conus geographus through transcriptome sequencing of its venom duct. BMC Genom. 2012, 13, 284. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.; Kelley, W.P.; Rubakhin, S.S.; Bingham, J.P.; Sweedler, J.V.; Gilly, W.F. Anatomical correlates of venom production in Conus californicus. Biol. Bull. 2002, 203, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Safavi-Hemami, H.; Young, N.D.; Williamson, N.A.; Purcell, A.W. Proteomic interrogation of venom delivery in marine cone snails: novel insights into the role of the venom bulb. J. Proteome Res. 2010, 9, 5610–5619. [Google Scholar] [CrossRef] [PubMed]
- Bardwell, V.J.; Treisman, R. The POZ domain: A conserved protein-protein interaction motif. Genes Dev. 1994, 8, 1664–1677. [Google Scholar] [CrossRef] [PubMed]
- Brejc, K.; van Dijk, W.J.; Klaassen, R.V.; Schuurmans, M.; van Der Oost, J.; Smit, A.B.; Sixma, T.K. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 2001, 411, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Kukhtina, V.; Kottwitz, D.; Strauss, H.; Heise, B.; Chebotareva, N.; Tsetlin, V.; Hucho, F. Intracellular domain of nicotinic acetylcholine receptor: the importance of being unfolded. J. Neurochem. 2006, 97, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Kracun, S.; Harkness, P.C.; Gibb, A.J.; Millar, N.S. Influence of the M3-M4 intracellular domain upon nicotinic acetylcholine receptor assembly, targeting and function. Br. J. Pharmacol. 2008, 153, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatcis 2008, 24, 713–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Trinity: Reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Pertea, G.; Huang, X.; Liang, F.; Antonescu, V.; Sultana, R.; Karamycheva, S.; Lee, Y.; White, J.; Cheung, F.; Parvizi, B.; et al. TIGR Gene Indices clustering tools (TGICL): A software system for fast clustering of large EST datasets. Bioinformatics 2003, 19, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Fang, L.; Zheng, H.; Zhang, Y.; Chen, J.; Zhang, Z.; Wang, J.; Li, S.; Li, R.; Bolund, L.; et al. WEGO: A web tool for plotting GO annotations. Nucleic Acids Res. 2006, 34, W293–W297. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and abundance estimation from RNA-Seq reveals thousands of new transcripts and switching among isoforms. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Beitz, E. TEXshade: Shading and labeling multiple sequence alignments using LATEX2 epsilon. Bioinformatics 2000, 16, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, S. Protein data bank. J. Pharmacol. Pharmocother. 2012, 3, 351–352. [Google Scholar] [CrossRef] [PubMed]
| Family | Definition | Reference |
|---|---|---|
| α (alpha) | Nicotinic acetylcholine receptors (nAChR) | [42] |
| γ (gamma) | Neuronal pacemaker cation currents (inward cation current) | [43] |
| δ (delta) | Voltage-gated Na channels (agonist, delay inactivation) | [44] |
| ε (epsilon) | Presynaptic Ca channels or G protein-coupled presynaptic receptors | [45] |
| ι (iota) | Voltage-gated Na channels (agonist, no delayed inactivation) | [46] |
| κ (kappa) | Voltage-gated K channels (blocker) | [47] |
| μ (mu) | Voltage-gated Na channels (antagonist, blocker) | [48] |
| ρ (rho) | Alpha1-adrenoceptors (GPCR) | [49] |
| σ (sigma) | Serotonin-gated ion channels (5-HT3R) | [50] |
| τ (tau) | Somatostatin receptor | [51] |
| χ (chi) | Neuronal noradrenaline transporter | [49] |
| ω (omega) | Voltage-gated Ca channels (blocker) | [52] |
| Sample | Venom Duct | Bulb | All | |||
|---|---|---|---|---|---|---|
| Unigene | Big | Middle | Small | |||
| Total Number | 94,026 | 52,387 | 114,057 | 124,004 | 300,069 | |
| Total Length | 37,880,261 | 23,128,493 | 44,918,779 | 67,451,577 | 128,471,163 | |
| Mean Length | 403 | 441 | 394 | 544 | 428 | |
| N50 | 413 | 464 | 398 | 681 | 554 | |
| Big | Middle | Small | Bulb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conotoxin | RPKM | Predicted Activity 1 | Conotoxin | RPKM | Predicted Activity | Conotoxin | RPKM | Predicted Activity | Conotoxin | RPKM | Predicted Activity |
| Bt035 | 84,466.31 | NMDARi 2 | Bt055 | 77,776.43 | κ | Bt035 | 58,816.45 | NMDARi | Bt070 | 179.21 | α, ι, κ, μ |
| Bt057 | 72,563.89 | κ | Bt018 | 71,975.85 | unknown | Bt018 | 32,503.63 | unknown | Bt035 | 143.27 | NMDARi |
| Bt018 | 57,233.21 | unknown | Bt082 | 57,532.96 | unknown | Bt075 | 25,533.62 | α, ι, κ, μ | Bt055 | 106.38 | κ |
| Bt005 | 21,270.26 | α, ρ | Bt035 | 53,262.22 | NMDARi | Bt055 | 20,861.06 | κ | Bt141 | 91.35 | δ, γ, κ, μ, ω |
| Bt082 | 20,710.97 | unknown | Bt213 | 43,598.05 | ε, μ, τ | Bt005 | 19,553.90 | α, ρ | Bt145 | 77.8 | δ, γ, κ, μ, ω |
| Bt055 | 19,799.15 | κ | Bt013 | 38,601.60 | NMDARi | Bt070 | 11,309.30 | α, ι, κ, μ | Bt043 | 56.26 | δ, γ, κ, μ, ω |
| Bt087 | 11,394.34 | α, ι, κ, μ | Bt076 | 27,361.60 | α, ι, κ, μ | Bt043 | 11,144.72 | δ, γ, κ, μ, ω | Bt017 | 51.57 | NMDARi |
| Bt043 | 10,995.23 | δ, γ, κ, μ, ω | Bt072 | 25,964.06 | α, ι, κ, μ | Bt213 | 10,602.62 | ε, μ, τ | Bt041 | 42.9 | unknown |
| Bt200 | 7552.24 | unknown | Bt071 | 22,016.06 | α, ι, κ, μ | Bt111 | 9823.73 | unknown | Bt076 | 34.75 | α, ι, κ, μ |
| Bt044 | 6067.80 | δ, γ, κ, μ, ω | Bt077 | 21,344.69 | α, ι, κ, μ | Bt057 | 9268.87 | κ | Bt005 | 34.05 | α |
| Bt186 | 5760.52 | δ, γ, κ, μ, ω | Bt125 | 18,909.36 | unknown | Bt013 | 8930.31 | NMDARi | Bt018 | 28.91 | unknown |
| Bt213 | 5745.23 | ε, μ, τ | Bt145 | 18,505.50 | δ, γ, κ, μ, ω | Bt210 | 6581.46 | ε, μ, τ | Bt186 | 27.08 | δ, γ, κ, μ, ω |
| Bt141 | 4616.86 | δ, γ, κ, μ, ω | Bt185 | 17,584.41 | δ, γ, κ, μ, ω | Bt081 | 5527.25 | α, ι, κ, μ | Bt054 | 16.35 | κ |
| Bt075 | 4512.67 | α, ι, κ, μ | Bt043 | 14,968.06 | δ, γ, κ, μ, ω | Bt082 | 3706.28 | unknown | Bt044 | 15.82 | δ, γ, κ, μ, ω |
| Bt081 | 4368.21 | α, ι, κ, μ | Bt192 | 12,120.02 | unknown | Bt086 | 3465.02 | α, ι, κ, μ | Bt075 | 15.36 | α, ι, κ, μ |
| Bt100 | 4154.73 | KCb 3 | Bt075 | 11,667.17 | α, ι, κ, μ | Bt058 | 3431.21 | κ | Bt077 | 13.29 | α, ι, κ, μ |
| Bt042 | 4079.54 | unknown | Bt141 | 11,364.97 | δ, γ, κ, μ, ω | Bt087 | 3429.93 | α, ι, κ, μ | Bt150 | 11.54 | δ, γ, κ, μ, ω |
| Bt138 | 3860.72 | δ, γ, κ, μ, ω | Bt136 | 10,248.83 | δ, γ, κ, μ, ω | Bt186 | 3353.26 | δ, γ, κ, μ, ω | Bt100 | 10.06 | KCb |
| Bt041 | 3662.06 | unknown | Bt005 | 9612.27 | α,ρ | Bt072 | 3110.04 | α, ι, κ, μ | Bt048 | 9.73 | ι |
| Bt172 | 3633.23 | γ | Bt040 | 8273.34 | unknown | Bt044 | 2827.06 | δ, γ, κ, μ, ω | Bt020 | 8.01 | NMDARi |
| Gene Name | Genbank Accession No. | Species |
|---|---|---|
| Kv1.1 | NP_000208.2 | human (Homo sapiens) |
| XP_005163101.1 | zebrafish (Danio rerio) | |
| NP_001191634.1 | sea hare (Aplysia californica) | |
| MF179123 | cone snail (Conus betulinus) | |
| XP_011413619.1 | oyster (Crassostrea gigas) | |
| ODM96669.1 | springtail insect (Orchesella cincta) | |
| α2-nAchR | EAW63552.1 | human (Homo sapiens) |
| NP_001035417.1 | zebrafish (Danio rerio) | |
| NP_001267757.1 | sea hare (Aplysia californica) | |
| MF179124 | cone snail (Conus betulinus) | |
| XP_011450331.1 | oyster (Crassostrea gigas) | |
| ABA60382.1 | great pond snail (Lymnaea stagnalis) | |
| NP_001103423.1 | darkling beetle (Tribolium castaneum) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Peng, C.; Yi, Y.; Gao, B.; Shi, Q. A Transcriptomic Survey of Ion Channel-Based Conotoxins in the Chinese Tubular Cone Snail (Conus betulinus). Mar. Drugs 2017, 15, 228. https://doi.org/10.3390/md15070228
Huang Y, Peng C, Yi Y, Gao B, Shi Q. A Transcriptomic Survey of Ion Channel-Based Conotoxins in the Chinese Tubular Cone Snail (Conus betulinus). Marine Drugs. 2017; 15(7):228. https://doi.org/10.3390/md15070228
Chicago/Turabian StyleHuang, Yu, Chao Peng, Yunhai Yi, Bingmiao Gao, and Qiong Shi. 2017. "A Transcriptomic Survey of Ion Channel-Based Conotoxins in the Chinese Tubular Cone Snail (Conus betulinus)" Marine Drugs 15, no. 7: 228. https://doi.org/10.3390/md15070228





