The Positive Correlation of the Enhanced Immune Response to PCV2 Subunit Vaccine by Conjugation of Chitosan Oligosaccharide with the Deacetylation Degree
Abstract
:1. Introduction
2. Results
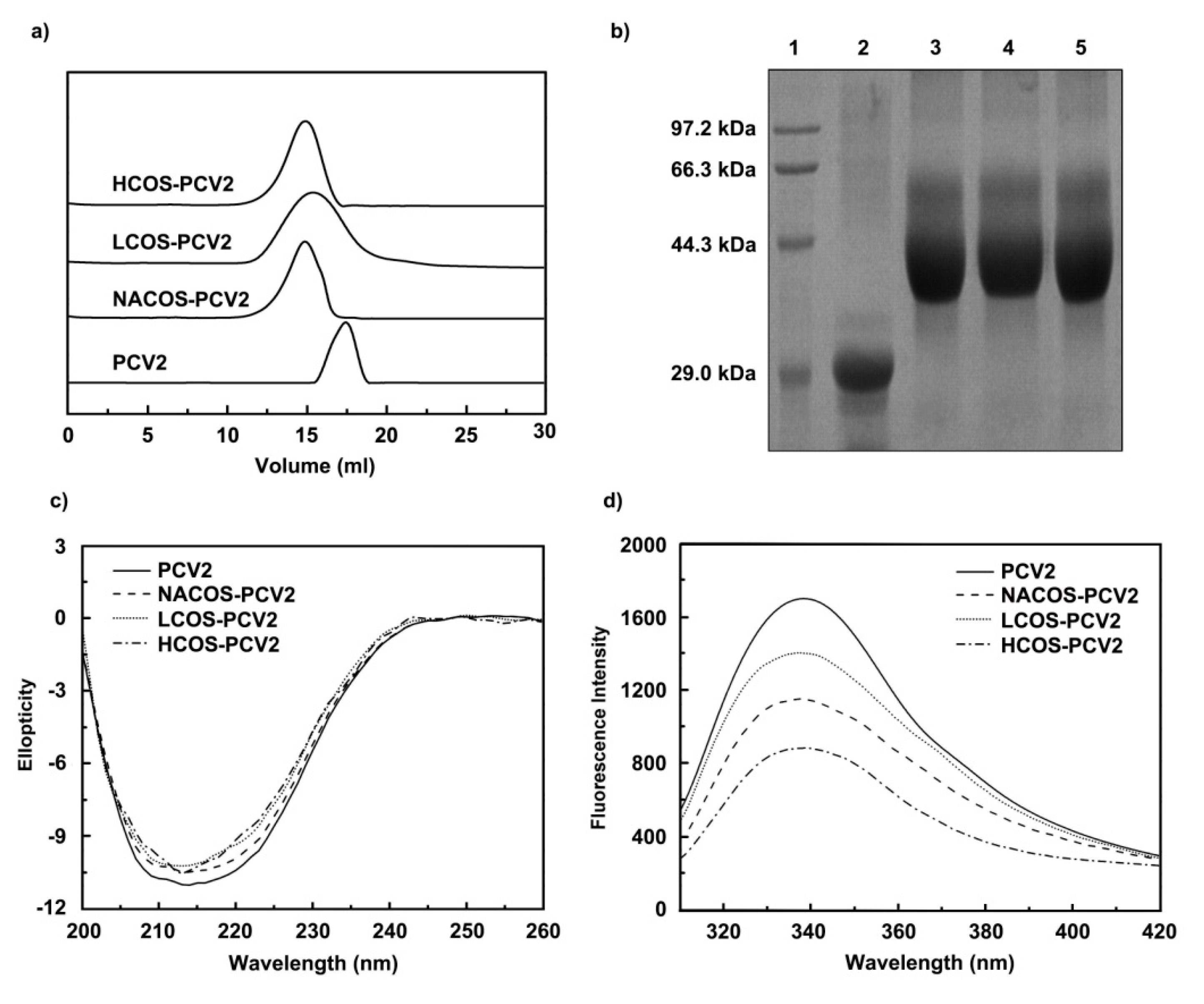
2.1. Purification and Quantitative Analysis of Conjugates
2.2. Physicochemical Characterization
2.2.1. Circular Dichroism (CD) Spectroscopy Assay
2.2.2. Fluorescence Measurement
2.2.3. Dynamic Light Scattering (DLS) Analysis
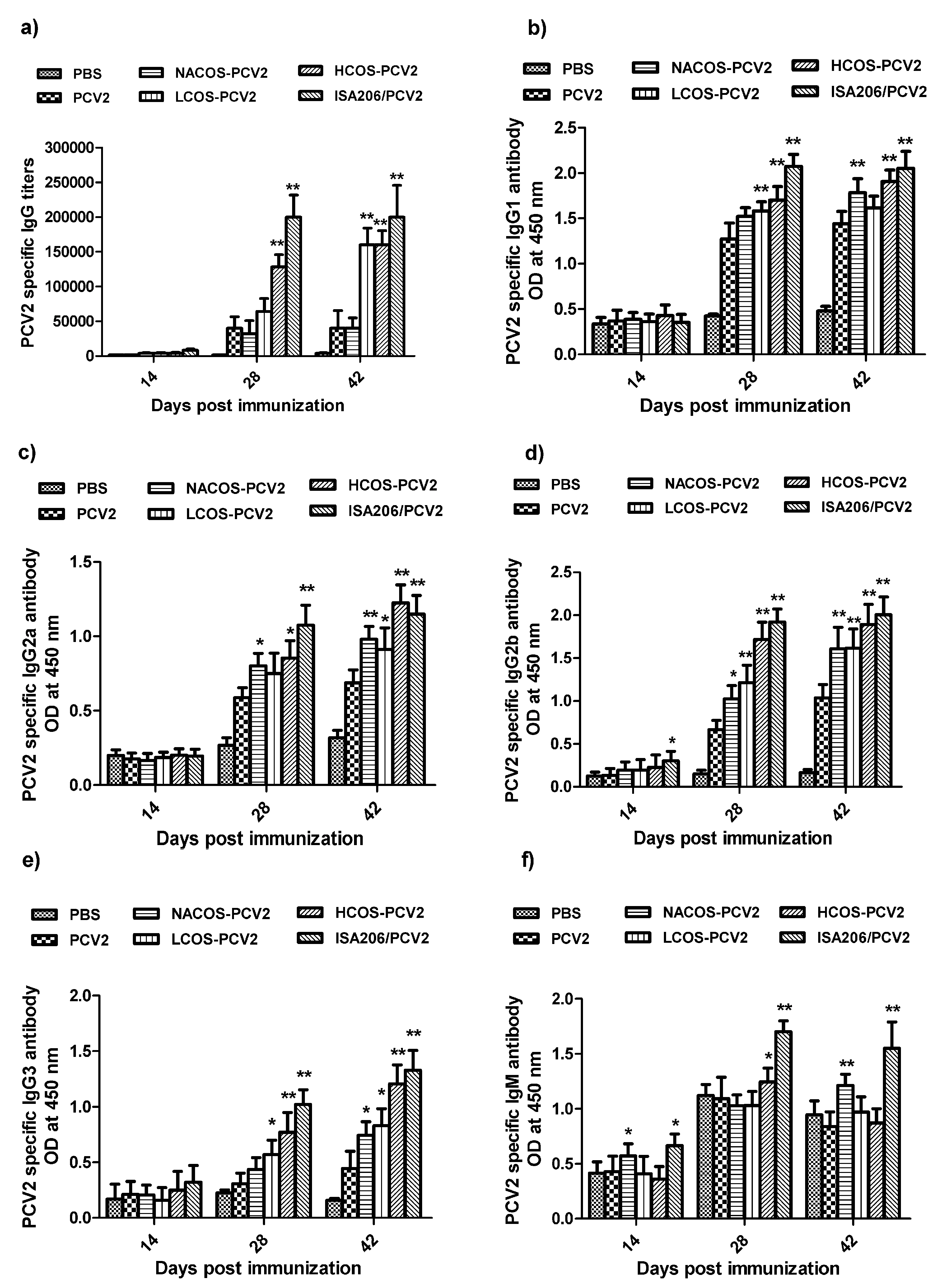
2.3. PCV2-Specific Antibody Production
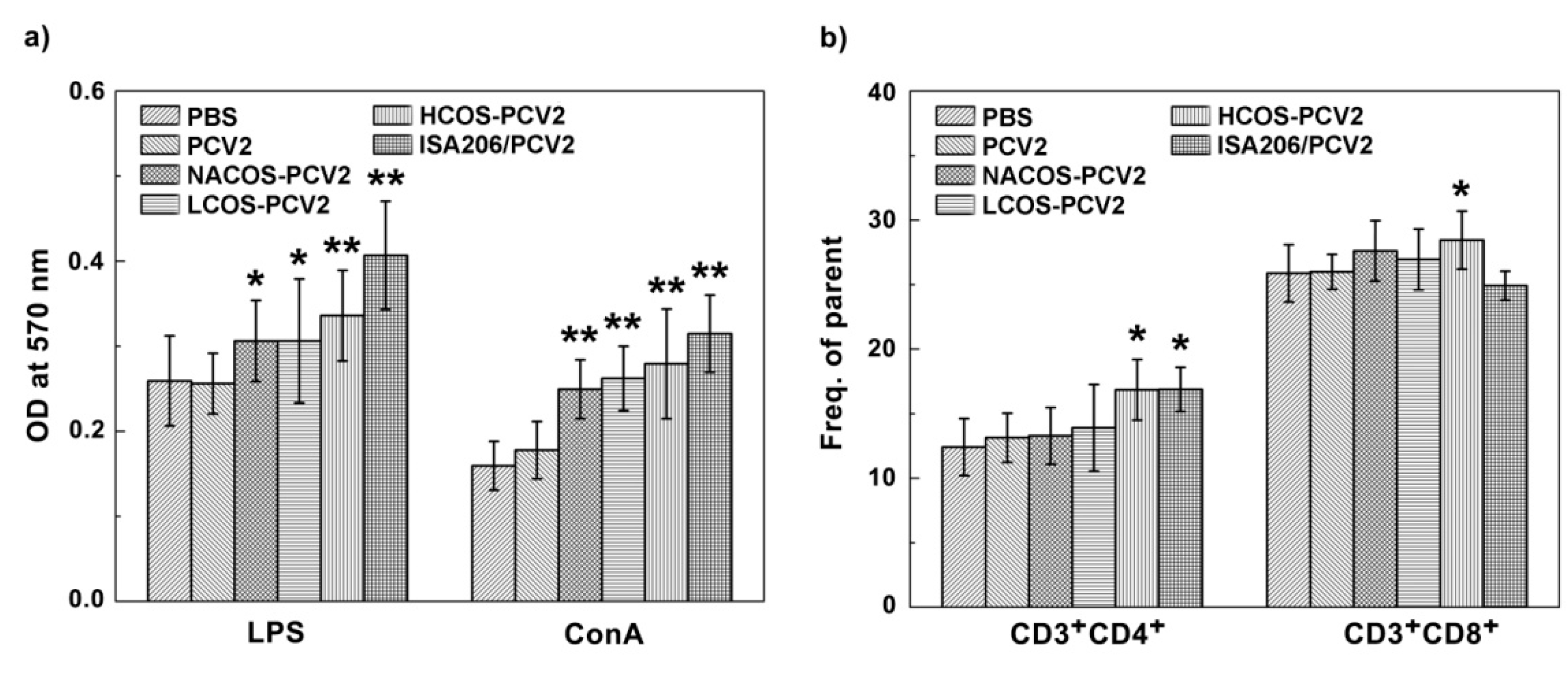
2.4. Lymphocyte Proliferation Assay
2.5. Subpopulation Analysis of CD3+ T Cells
2.6. Cytokine Assays
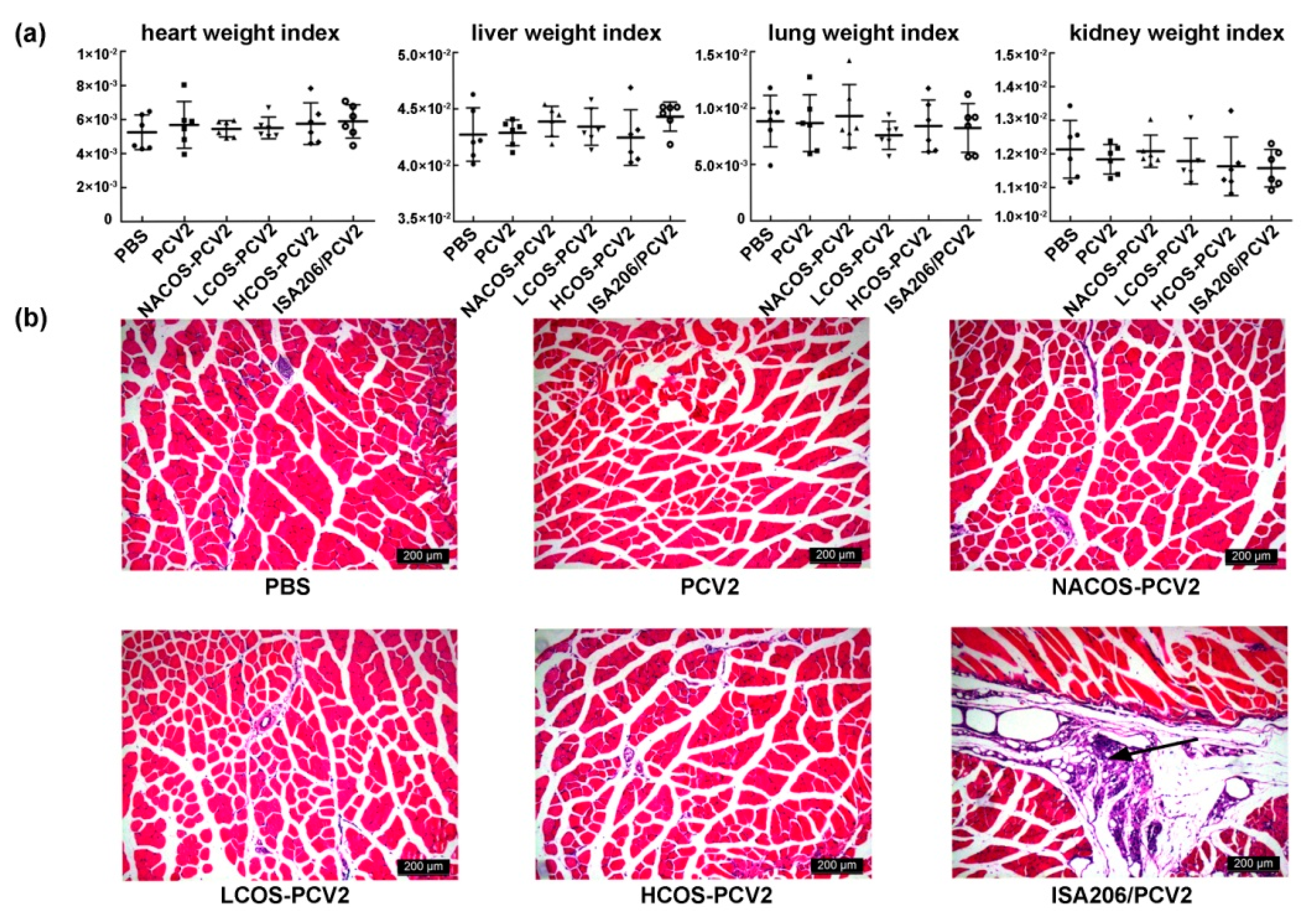
2.7. Viscera Index and Histopathological Assay
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation and Purification of COS-PCV2 Conjugates
4.3. Characterization Analysis on COS–PCV2 Conjugates
4.3.1. Quantitative Assay
4.3.2. CD Spectroscopy Assay
4.3.3. DLS Analysis
4.3.4. Fluorescence Measurement
4.4. Animal Immunization
4.5. Detection of PCV2-Specific Antibodies
4.6. Lymphocyte Proliferation Assay
4.7. Flow Cytometry Analysis on T Cell Subpopulation
4.8. Cytokine Assay
4.9. Viscera Index and Histopathological Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ngo, D.H.; Vo, T.S.; Ngo, D.N.; Kang, K.H.; Je, J.Y.; Pham, H.N.D.; Byun, H.G.; Kim, S.K. Biological effects of chitosan and its derivatives. Food Hydrocoll. 2015, 51, 200–216. [Google Scholar] [CrossRef]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sørlie, M.; Vårum, K.M.; Eijsink, V.G. Production of chitooligosaccharides and their potential applications in medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.Y.; Wu, M.F.; Shang, H.S.; Chang, J.B.; Shih, Y.L.; Chen, Y.L.; Hung, H.F.; Lu, H.F.; Yeh, C.; Wood, W.G.; et al. Effects of chitosan on xenograft models of melanoma in C57BL/6 mice and hepatoma formation in SCID mice. Anticancer Res. 2013, 33, 4867–4873. [Google Scholar] [PubMed]
- Jeon, Y.J.; Kim, S.K. Production of chitooligosaccharides using an ultrafiltration membrane reactor and their antibacterial activity. Carbohydr. Polym. 2000, 41, 133–141. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhang, H.; Gao, Y.; Zhang, Y.; Wu, H.Z.; Zhang, Y.X. Efficacy of chitosan oligosaccharide as aquatic adjuvant administrated with a formalin-inactivated Vibrio anguillarum vaccine. Fish Shellfish Immun. 2015, 47, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Le Van Hiep, M.T.T.; Van, D.T.H.; Khanh, V.T.P.; Dzung, N.A. Chitosan oligomer as a hopeful adjuvant for H5N1 influenza vaccin. Chitosan J. Chitin 2008, 13, 6–8. [Google Scholar]
- Zhang, G.; Jia, P.; Gong, C.; Jiao, S.; Ren, L.; Ji, S.; Tao, H.; Liu, H.; Du, Y. Enhanced immune response to inactivated porcine circovirus type 2 (PCV2) vaccine by conjugation of chitosan oligosaccharides. Carbohydr. Polym. 2017, 166, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Khor, E.; Lim, L.Y. Uptake and cytotoxicity of chitosan molecules and nanoparticles: Effects of molecular weight and degree of deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Aam, B.B.; Wang, W.; Norberg, A.L.; Sørlie, M.; Eijsink, V.G.; Du, Y. Inhibition of angiogenesis by chitooligosaccharides with specific degrees of acetylation and polymerization. Carbohydr. Polym. 2012, 89, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, L.; Yang, S.; Niu, R.; Chu, E.; Lin, X. N-acetylchitooligosaccharide is a potent angiogenic inhibitor both in vivo and in vitro. Biochem. Biophys. Res. Commun. 2007, 357, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Chae, C. A review of porcine circovirus 2-associated syndromes and diseases. Vet. J. 2005, 169, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, P.; Rushton, J.; Wieland, B. Cost of post-weaning multi-systemic wasting syndrome and porcine circovirus type-2 subclinical infection in England—An economic disease model. Prev. Vet. Med. 2013, 110, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Chae, C. Commercial porcine circovirus type 2 vaccines: Efficacy and clinical application. Vet. J. 2012, 194, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.C.; Rodriguez, E.G. Vaccine adjuvants revisited. Vaccine 2007, 25, 3752–3762. [Google Scholar] [CrossRef] [PubMed]
- Vera-Lastra, O.; Medina, G.; Cruz-Dominguez Mdel, P.; Jara, L.J.; Shoenfeld, Y. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld’s syndrome): Clinical and immunological spectrum. Expert Rev. Clin. Immun. 2013, 9, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Annable, T.; Tomassian, T.; Jain, S.; Leibbrandt, M.; Cooke, M.P.; Deane, J.A. Using Poly I:C as an adjuvant does not induce or exacerbate models of systemic lupus erythematosus. Autoimmunity 2015, 48, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Slutter, B.; Soema, P.C.; Ding, Z.; Verheul, R.; Hennink, W.; Jiskoot, W. Conjugation of ovalbumin to trimethyl chitosan improves immunogenicity of the antigen. J. Control. Release 2010, 143, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Du, Y.; Yu, X.; Mitsutomi, M.; Aiba, S. Preparation of chitooligosaccharides from chitosan by a complex enzyme. Carbohydr. Res. 1999, 320, 257–260. [Google Scholar] [CrossRef]
- Zou, P.; Li, K.; Liu, S.; Xing, R.; Qin, Y.; Yu, H.; Zhou, M.; Li, P. Effect of chitooligosaccharides with different degrees of acetylation on wheat seedlings under salt stress. Carbohydr. Polym. 2015, 126, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Lavertu, M.; Xia, Z.; Serreqi, A.N.; Berrada, M.; Rodrigues, A.; Wang, D.; Buschmann, M.D.; Gupta, A. A validated 1H NMR method for the determination of the degree of deacetylation of chitosan. J. Pharm. Biomed. Anal. 2003, 32, 1149–1158. [Google Scholar] [CrossRef]
- Micoli, F.; Romano, M.R.; Tontini, M.; Cappelletti, E.; Gavini, M.; Proietti, D.; Rondini, S.; Swennen, E.; Santini, L.; Filippini, S.; et al. Development of a glycoconjugate vaccine to prevent meningitis in Africa caused by meningococcal serogroup X. Proc. Natl. Acad. Sci. USA 2013, 110, 19077–19082. [Google Scholar] [CrossRef] [PubMed]
- Mittrucker, H.W.; Kaufmann, S.H. Immune response to infection with Salmonella typhimurium in mice. J. Leukoc. Biol. 2000, 67, 457–463. [Google Scholar] [PubMed]
- Gomez-Laguna, J.; Salguero, F.J.; Pallares, F.J.; de Fernandez Marco, M.; Barranco, I.; Ceron, J.J.; Martinez-Subiela, S.; Van Reeth, K.; Carrasco, L. Acute phase response in porcine reproductive and respiratory syndrome virus infection. Comp. Immun. Microbiol. Infect. Dis. 2010, 33, e51–e58. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N. Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Saf. 2015, 38, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; O’Hagan, D. Advances in vaccine adjuvants. Nat. Biotechnol. 1999, 17, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Segales, J. Best practice and future challenges for vaccination against porcine circovirus type 2. Expert Rev. Vaccines 2015, 14, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Carasova, P.; Celer, V.; Takacova, K.; Trundova, M.; Molinkova, D.; Lobova, D.; Smola, J. The levels of PCV2 specific antibodies and viremia in pigs. Res. Vet. Sci. 2007, 83, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Seder, R.A.; Hill, A.V. Vaccines against intracellular infections requiring cellular immunity. Nature 2000, 406, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yu, W.; Hu, T. Potent Antigen-Adjuvant Delivery System by Conjugation of Mycobacterium tuberculosis Ag85B-HspX Fusion Protein with Arabinogalactan-Poly(I:C) Conjugate. Bioconj. Chem. 2016, 27, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Yin, H.; Ye, Z.; Zhao, X.; Yuan, J.; Du, Y. A comparison study on the interactions of two oligosaccharides with tobacco cells by time-resolved fluorometric method. Carbohydr. Polym. 2012, 90, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.G.; Wei, W.; Lv, P.P.; Yue, H.; Wang, L.Y.; Su, Z.G.; Ma, G.H. Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhao, L.; Yu, Z.; Feng, J.; Yu, Q. Role of mannose receptor in oligochitosan-mediated stimulation of macrophage function. Int. Immunopharmacol. 2005, 5, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.J.; Tsai, G.J. Chitooligosaccharides in combination with interferon-gamma increase nitric oxide production via nuclear factor-kappaB activation in murine RAW264.7 macrophages. Food Chem. Toxicol. 2007, 45, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Blander, J.M.; Medzhitov, R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 2006, 440, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Bijker, M.S.; Weterings, J.J.; Tanke, H.J.; Adema, G.J.; Van, H.T.; Drijfhout, J.W.; Melief, C.J.; Overkleeft, H.S.; Ga, V.D.M. Distinct uptake mechanisms but similar intracellular processing of two different toll-like receptor ligand-peptide conjugates in dendritic cells. J. Biol. Chem. 2007, 282, 21145–21159. [Google Scholar] [CrossRef] [PubMed]
- Stefanetti, G.; Rondini, S.; Lanzilao, L.; Saul, A.; MacLennan, C.A.; Micoli, F. Impact of conjugation chemistry on the immunogenicity of S. Typhimurium conjugate vaccines. Vaccine 2014, 32, 6122–6129. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Ji, S.; Zhao, Y.; Hu, T. Conjugation of beta-glucan markedly increase the immunogencity of meningococcal group Y polysaccharide conjugate vaccine. Vaccine 2015, 33, 2066–2072. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, T.; Liu, Y.; Zhang, G.; Ma, G.; Su, Z. Kinetic and stoichiometric analysis of the modification process for N-terminal PEGylation of staphylokinase. Anal. Biochem. 2011, 412, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Li, D.; Wang, J.; Wang, Q.; Liang, Y.; Su, Y.; Ma, G.; Su, Z.; Wang, S. Propylbenzmethylation at Val-1(alpha) markedly increases the tetramer stability of the PEGylated hemoglobin: A comparison with propylation at Val-1(alpha). Biochim. Biophys. Acta 2012, 1820, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Suo, X.; Lu, X.; Hu, T.; Ma, G.; Su, Z. A solid-phase adsorption method for PEGylation of human serum albumin and staphylokinase: Preparation, purification and biochemical characterization. Biotechnol. Lett. 2009, 31, 1191–1196. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Cheng, G.; Jia, P.; Jiao, S.; Feng, C.; Hu, T.; Liu, H.; Du, Y. The Positive Correlation of the Enhanced Immune Response to PCV2 Subunit Vaccine by Conjugation of Chitosan Oligosaccharide with the Deacetylation Degree. Mar. Drugs 2017, 15, 236. https://doi.org/10.3390/md15080236
Zhang G, Cheng G, Jia P, Jiao S, Feng C, Hu T, Liu H, Du Y. The Positive Correlation of the Enhanced Immune Response to PCV2 Subunit Vaccine by Conjugation of Chitosan Oligosaccharide with the Deacetylation Degree. Marine Drugs. 2017; 15(8):236. https://doi.org/10.3390/md15080236
Chicago/Turabian StyleZhang, Guiqiang, Gong Cheng, Peiyuan Jia, Siming Jiao, Cui Feng, Tao Hu, Hongtao Liu, and Yuguang Du. 2017. "The Positive Correlation of the Enhanced Immune Response to PCV2 Subunit Vaccine by Conjugation of Chitosan Oligosaccharide with the Deacetylation Degree" Marine Drugs 15, no. 8: 236. https://doi.org/10.3390/md15080236



