Use of Antibiotics for Maintenance of Axenic Cultures of Amphidinium carterae for the Analysis of Translation
Abstract
:1. Introduction
2. Results
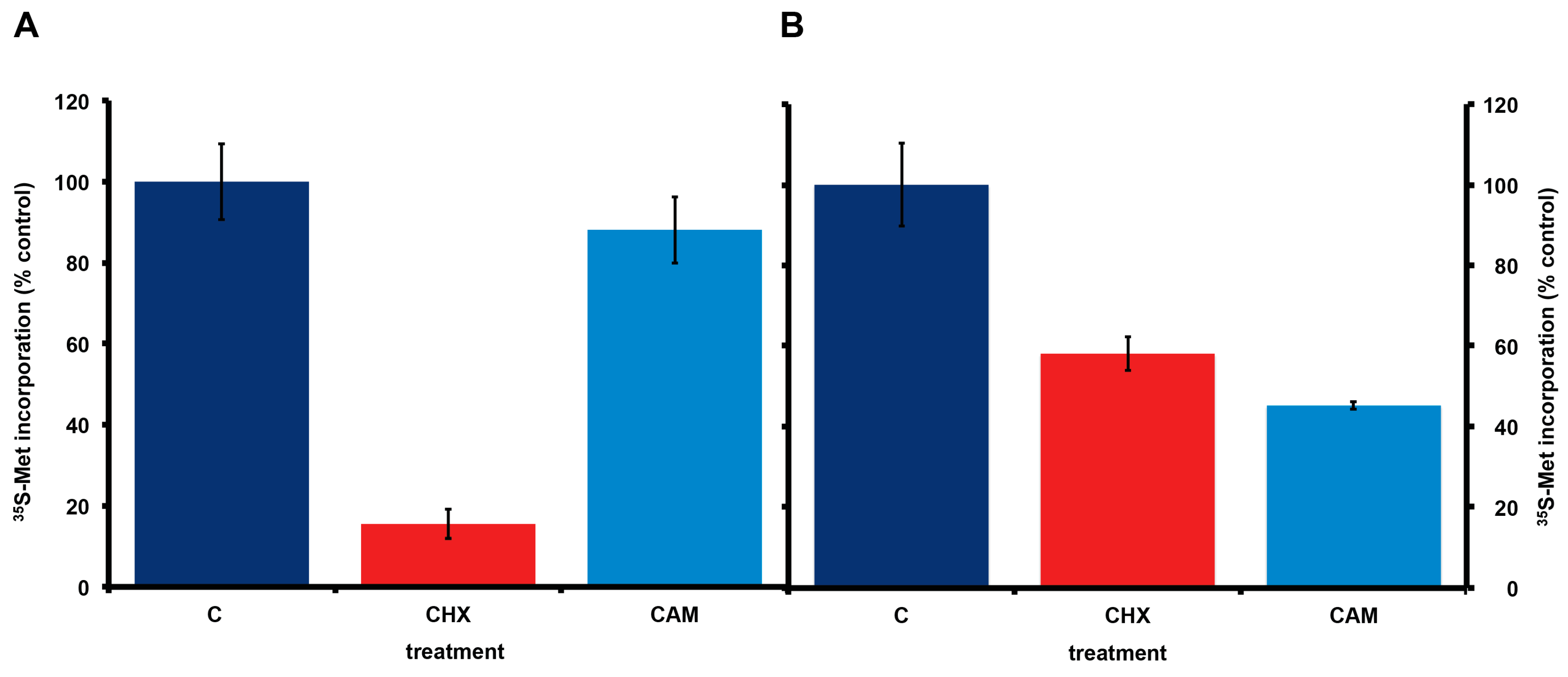
2.1. Bacterized, But Not Axenic A. carterae Show Atypical Responses to Protein Synthesis Inhibitors
2.2. Atypical Responses to Protein Synthesis Inhibitors and Bacteria Reappear with Time in Culture
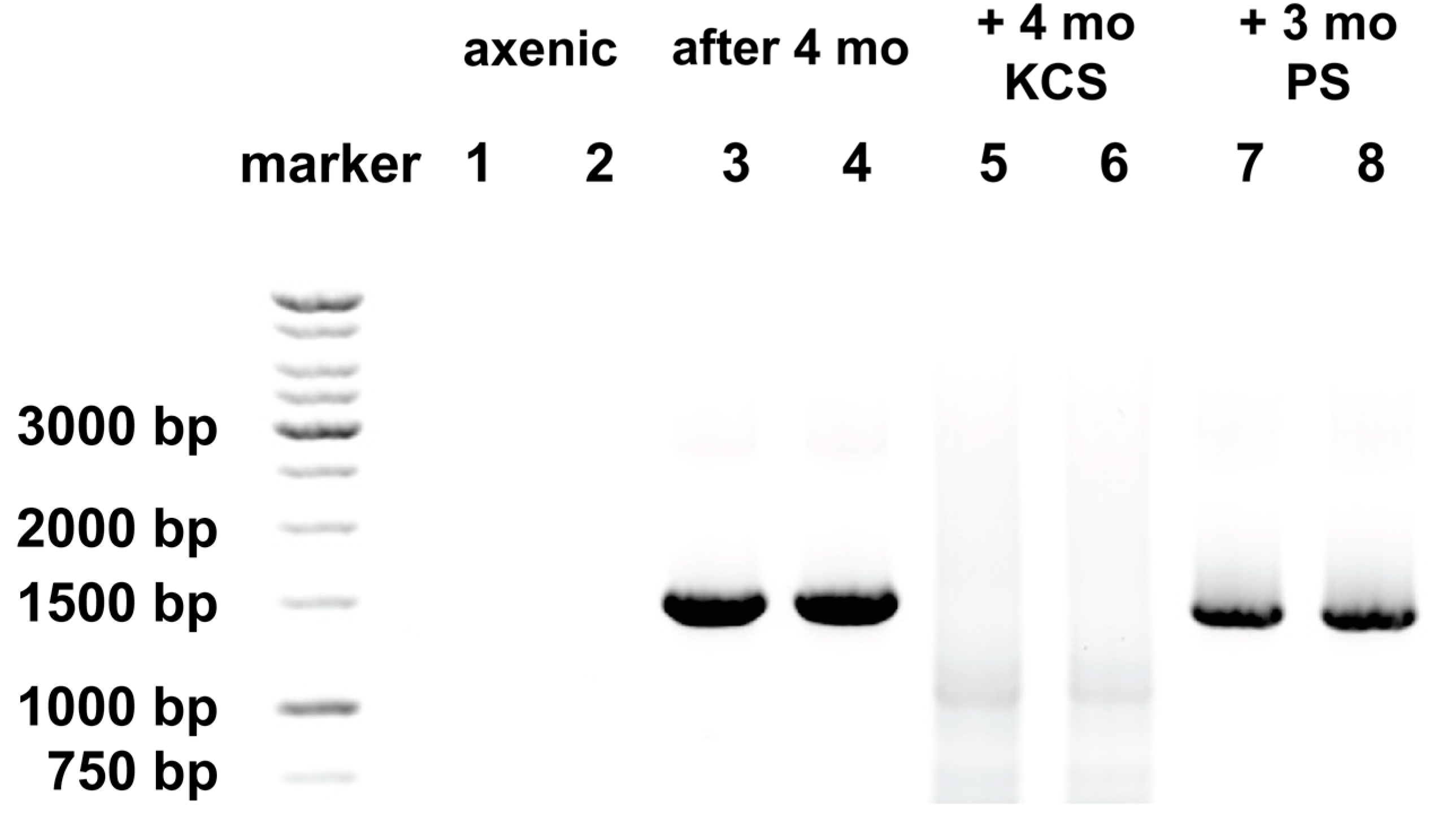
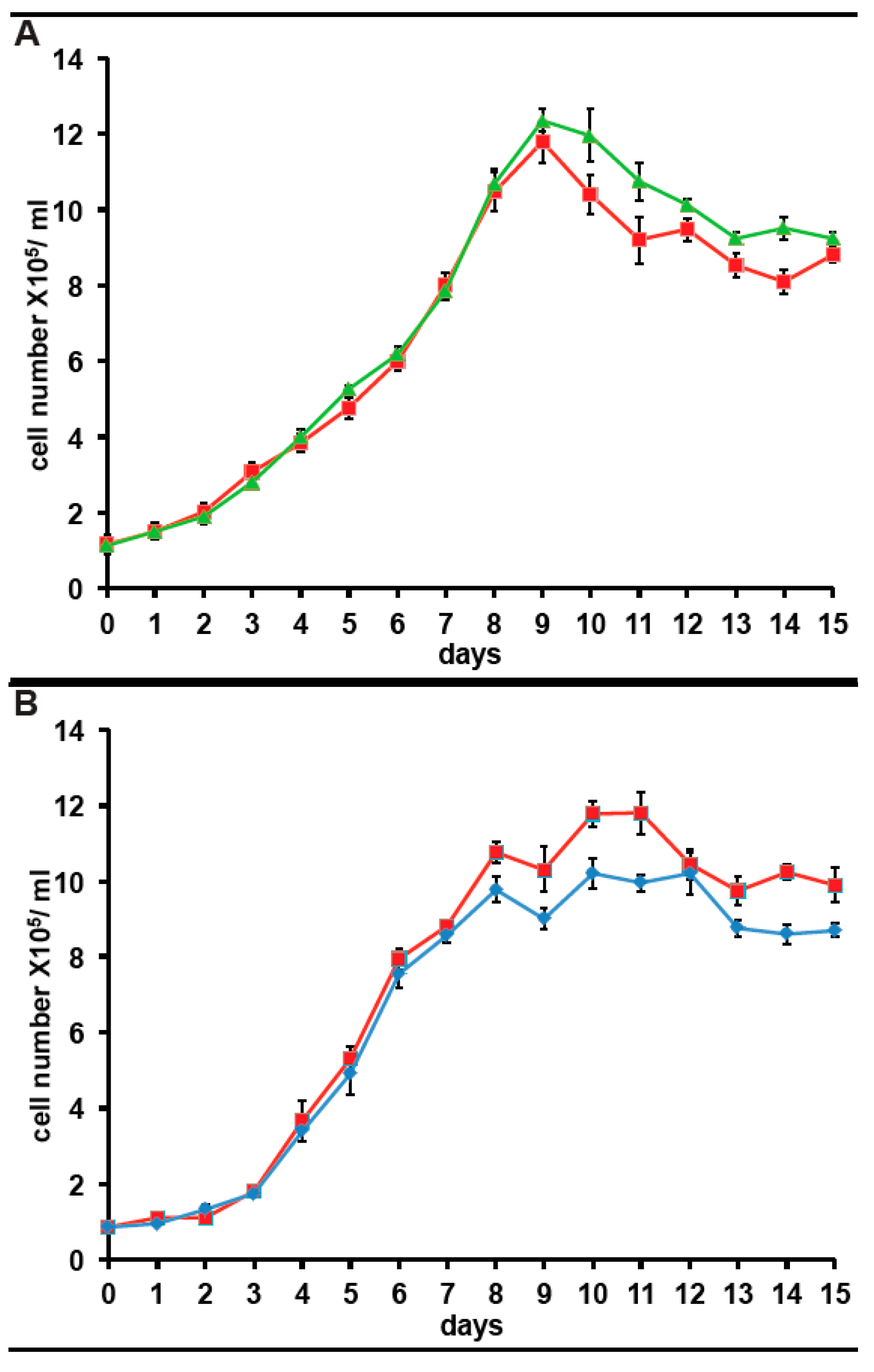
2.3. KCS, But Not PS, Prevents Re-Emergence of Bacteria
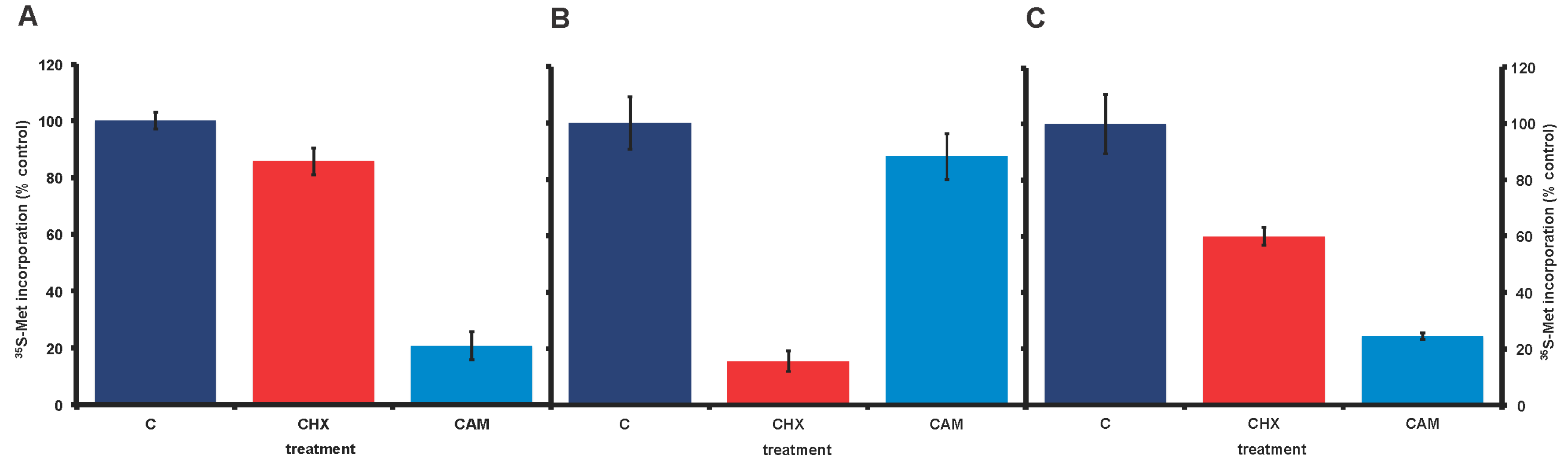
2.4. KCS, But Not PS, Prevents Atypical Responses to Protein Synthesis Inhibitors, But Does Not Impact the Growth Rate of A. carterae
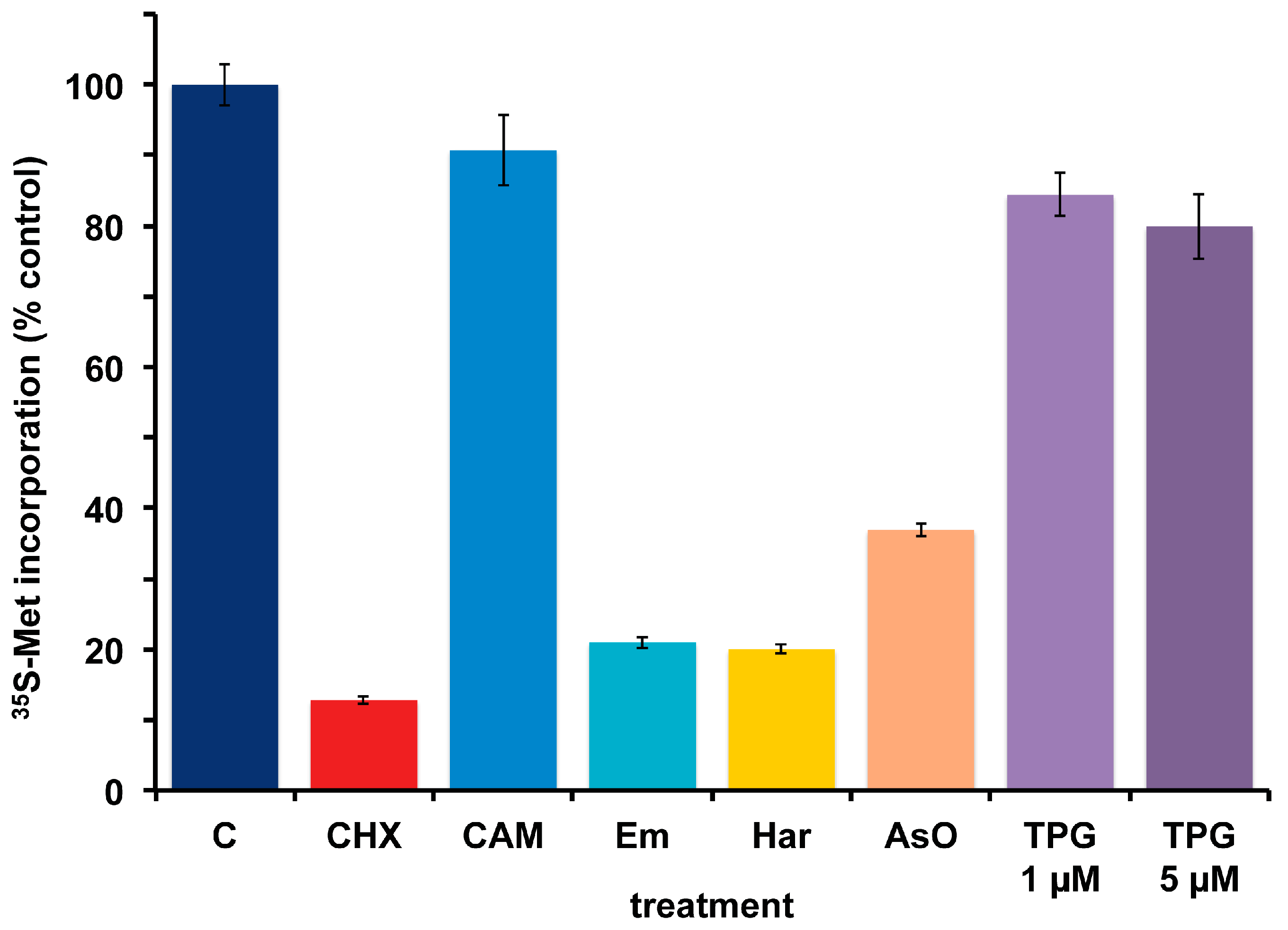
2.5. Effect of Other Protein Synthesis Inhibitors on 35S-Methionine Incorporation in Axenic A. carterae Maintained in KCS
3. Discussion
4. Materials and Methods
4.1. Antibiotics and Protein Synthesis Inhibitors
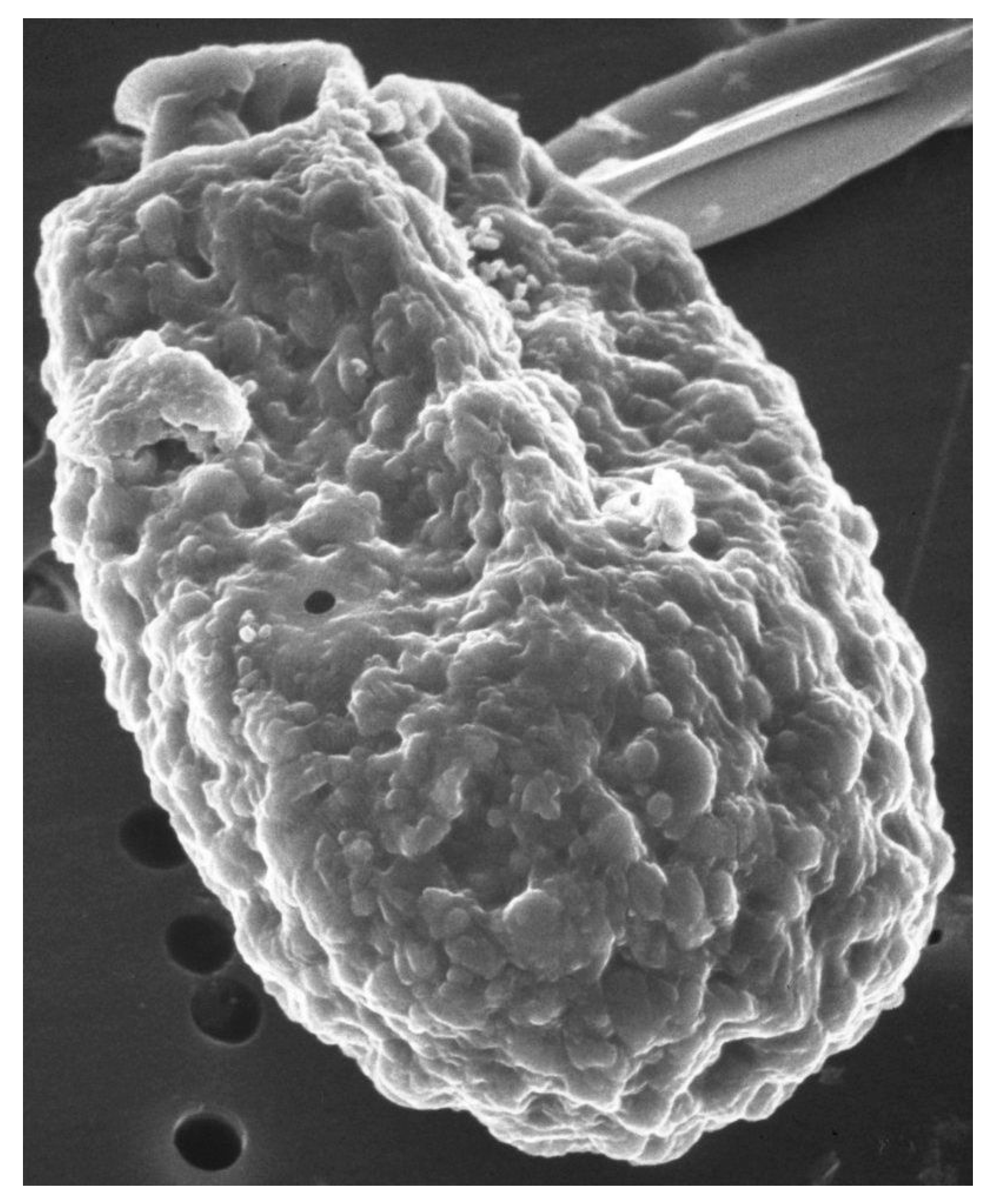
4.2. A. carterae Cell Culture
4.3. Determination of Cell Number
4.4. Detection of Bacteria by PCR Amplification
4.5. 35S-Methionine Incorporation
4.6. Effect of Inhibitors of Protein Synthesis on 35S-Methionine Incorporation
Acknowledgments
Author Contributions
Conflict of Interest
References
- Zhang, H.; Bhattacharya, D.; Lin, S. A three-gene dinoflagellate phylogeny suggests monophyly of prorocentrales and a basal position for Amphidinium and Heterocapsa. J. Mol. Evol. 2007, 65, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Bachvaroff, T.R.; Handy, S.M.; Place, A.R.; Delwiche, C.F. Alveolate phylogeny inferred using concatenated ribosomal proteins. J. Eukaryot. Microbiol. 2011, 58, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Bachvaroff, T.R.; Gornik, S.G.; Concepcion, G.T.; Waller, R.F.; Mendez, G.S.; Lippmeier, J.C.; Delwiche, C.F. Dinoflagellate phylogeny revisited: Using ribosomal proteins to resolve deep branching dinoflagellate clades. Mol. Phylogenet. Evol. 2014, 70, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Janouškovec, J.; Gavelis, G.S.; Burki, F.; Dinh, D.; Bachvaroff, T.R.; Gornik, S.G.; Bright, K.J.; Imanian, B.; Strom, S.L.; Delwiche, C.F.; et al. Major transitions in dinoflagellate evolution unveiled by phylotranscriptomics. Proc. Natl. Acad. Sci. USA 2017, 114, E171–E180. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.A.; Garby, T.; Hoppenrath, M.; Neilan, B.A. Genetic diversity, morphological uniformity and polyketide production in dinoflagellates (Amphidinium, Dinoflagellata). PLoS ONE 2012, 7, e38253. [Google Scholar] [CrossRef] [PubMed]
- LaJeunesse, T.C.; Lambert, G.; Andersen, R.A.; Coffroth, M.A.; Galbraith, D.W. Symbiodinium (pyrrhophyta) genome (DNA content) are smallest among dinoflagelates. J. Phycol. 2005, 41, 880–886. [Google Scholar] [CrossRef]
- Fuentes-Grünewald, C.; Bayliss, C.; Fonlut, F.; Chapuli, E. Long-term dinoflagellate culture performance in a commercial photobioreactor: Amphidinium carterae case. Bioresour. Technol. 2016, 218, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Rodríguez, J.; Sánchez-Mirón, A.; García-Camacho, F.; López-Rosales, L.; Chisti, Y.; Molina-Grima, E. Bioactives from microalgal dinoflagellates. Biotechnol. Adv. 2012, 30, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, G.; Cutignano, A.; Sardo, A.; Fontana, A. Antifungal amphidinol 18 and its 7-sulfate derivative from the marine dinoflagellate Amphidinium carterae. J. Nat. Prod. 2014, 77, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.; Evans, A.N.; Kulis, D.M.; Hackett, J.D.; Erdner, D.L.; Anderson, D.M.; Bhattacharya, D. Transcriptome profiling of a toxic dinoflagellate reveals a gene-rich protist and a potential impact on gene expression due to bacterial presence. PLoS ONE 2010, 5, e9688. [Google Scholar] [CrossRef] [PubMed]
- Morey, J.S.; Van Dolah, F.M. Global Analysis of mRNA Half-Lives and de novo transcription in a dinoflagellate, Karenia brevis. PLoS ONE 2013, 8, e66347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.; Morse, D. Transcription and maturation of mRNA in dinoflagellates. Microorganisms 2013, 1, 71–99. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Beauchemin, M.; Dagenais-Bellefeuille, S.; Letourneau, L.; Cappadocia, M.; Morse, D. The Lingulodinium circadian system lacks rhythmic changes in transcript abundance. BMC Biol. 2014, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Milos, P.; Morse, D.; Hastings, J.W. Circadian control over synthesis of many Gonyaulax proteins is at a translational level. Naturwissenschaften 1990, 77, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Miller-Morey, J.S.; Van Dolah, F.M. Differential responses of stress proteins, antioxidant enzymes, and photosynthetic efficiency to physiological stresses in the Florida red tide dinoflagellate, Karenia brevis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 138, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, H.; Wu, C.; Kinumi, T.; Ohmiya, Y. Biological rhythmicity in expressed proteins of the marine dinoflagellate Lingulodinium polyedrum demonstrated by chronological proteomics. Biochem. Biophys. Res. Commun. 2004, 315, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, H.; Kinumi, T.; Ohmiya, Y. Circadian rhythm of a TCA cycle enzyme is apparently regulated at the translational level in the dinoflagellate Lingulodinium polyedrum. J. Biol. Rhythms 2005, 20, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.W.; Morse, D.; Lo, S.C. Identification of two plastid proteins in the dinoflagellate Alexandrium affine that are substantially down-regulated by nitrogen-depletion. J. Proteome Res. 2009, 8, 5080–5092. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lo, S.C.; Matton, D.P.; Lang, B.F.; Morse, D. Daily changes in the phosphoproteome of the dinoflagellate Lingulodinium. Protist 2012, 163, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Lidie, K.; Jagus, R.; Place, A.R. Identification in dinoflagellates of two eukaryotic translation initiation factor 4E homologues. In Proceedings of the 13th International Conference on Harmful Algae, Hong Kong, China, 3–7 November 2008; pp. 103–107. [Google Scholar]
- Jones, G.D. Discovery, Phylogenetic Analysis and Characterization of eIF4E Family Members from Amphidinium carterae. Ph.D. Thesis, University of Baltimore Maryland, Baltimore, MD, USA, 2016. [Google Scholar]
- Jones, G.D.; Williams, E.P.; Place, A.R.; Jagus, R.; Bachvaroff, T.R. The alveolate translation initiation factor 4E family reveals a custom toolkit for translational control in core dinoflagellates. BMC Evol. Biol. 2015, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.D.; Williams, E.P.; Place, A.R.; Bachvaroff, T.R.; Jagus, R. New tools in the Karlodinium veneficum translation initiation toolbox. In Proceedings of the 16th International Conference on Harmful Algae, Wellington, New Zealand, 27–31 October 2014; MacKenzie, A.L., Ed.; Cawthron Institute: Nelson, New Zealand, 2014; pp. 237–240. [Google Scholar]
- Lidie, K.B.; van Dolah, F.M. Spliced leader RNA-mediated trans-splicing in a dinoflagellate, Karenia brevis. J. Eukaryot. Microbiol. 2007, 54, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hou, Y.; Miranda, L.; Campbell, D.A.; Sturm, N.R.; Gaasterland, T.; Lin, S. Spliced leader RNA trans-splicing in dinoflagellates. Proc. Natl. Acad. Sci. USA 2007, 104, 4618–4623. [Google Scholar] [CrossRef] [PubMed]
- Mašek, T.; Valášek, L.; Pospíšek, M. Polysome analysis and RNA purification from sucrose gradients. Methods Mol. Biol. 2011, 703, 293–309. [Google Scholar] [PubMed]
- Pestova, T.V.; Hellen, C.U. Translation elongation after assembly of ribosomes on the Cricket paralysis virus internal ribosomal entry site without initiation factors or initiator tRNA. Genes Dev. 2003, 17, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Poetsch, T.; Ju, J.; Eyler, D.E.; Dang, Y.; Bhat, S.; Merrick, W.C.; Green, R.; Shen, B.; Liu, J.O. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat. Chem. Biol. 2010, 6, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Ingolia, N.T.; Brar, G.A.; Rouskin, S.; McGeachy, A.M.; Weissman, J.S. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat. Protoc. 2012, 7, 1534–1550. [Google Scholar] [CrossRef] [PubMed]
- Ingolia, N.T.; Brar, G.A.; Rouskin, S.; McGeachy, A.M.; Weissman, J.S. Genome-wide annotation and quantitation of translation by ribosome profiling. Curr. Protoc. Mol. Biol. 2013. [Google Scholar] [CrossRef]
- Wolin, S.L.; Walter, P. Ribosome pausing and stacking during translation of a eukaryotic mRNA. EMBO J. 1988, 7, 3559–3569. [Google Scholar] [PubMed]
- Huang, M.T. Harringtonine, an inhibitor of initiation of protein biosynthesis. Mol. Pharmacol. 1975, 11, 511–519. [Google Scholar] [PubMed]
- Guillard, R. Algal Culturing Techniques; Academic Press: San Diego, CA, USA, 2005; pp. 117–132. [Google Scholar]
- Kodama, M.; Doucette, G.; Green, D. Ecology of Harmful Algae; Springer: Berlin, Germany, 2006; pp. 243–255. [Google Scholar]
- Curtis, T.P.; Sloan, W.T. Prokaryotic diversity and its limits: Microbial community structure in nature and implications for microbial ecology. Curr. Opin. Microbiol. 2004, 7, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Green, D.H.; Llewellyn, L.E.; Negri, A.P.; Blackburn, S.I.; Bolch, C.J. Phylogenetic and functional diversity of the cultivable bacterial community associated with the paralytic shellfish poisoning dinoflagellate Gymnodinium catenatum. FEMS Microbiol. Ecol. 2004, 47, 345–357. [Google Scholar] [CrossRef]
- Hold, G.; Smith, E.; Raape, M.; Maas, E.; Moore, E.; Stroempl, C.; Stephen, J.; Prosser, J.; Birkbeck, T.; Gallacher, S. Characterization of bacterial communities associated with toxic and non-toxic dinoflagellates. FEMS Microbiol. Ecol. 2001, 37, 161–173. [Google Scholar] [CrossRef]
- Allgaier, M.; Uphoff, H.; Felske, A.; Wagner-Dobler, I. Aerobic anoxygenic photosynthesis in Roseobacter clade bacteria from diverse marine habitats. Appl. Environ. Microbiol. 2003, 69, 5051–5059. [Google Scholar] [CrossRef] [PubMed]
- Ferrier, M.; Martin, J.L.; Rooney-Varga, J.N. Stimulation of Alexandrium fundyense growth by bacterial assemblages from the Bay of Fundy. J. Appl. Microbiol. 2002, 92, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Provasoli, L.; Carlucci, A. Algal Physiology and Biochemistry; Blackwell Scientific Publications: Oxford, UK, 1974; pp. 741–787. [Google Scholar]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Croft, M.T.; Warren, M.J.; Smith, A.G. Algae need their vitamins. Eukaryot. Cell 2006, 5, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.; Keller, M. Dinoflagellates; Elsevier: Amsterdam, The Netherlands, 1962; pp. 229–239. [Google Scholar]
- Harrison, P.; Waters, R.; Taylor, F. A broad spectrum artificial seawater medium for coastal and open ocean phytoplankton. J. Phycol. 1980, 16, 28–35. [Google Scholar] [CrossRef]
- Cruz-López, R.; Maske, H. The Vitamin B1 and B12 required by the marine dinoflagellate Lingulodinium polyedrum can be provided by its associated bacterial community in culture. Front. Microbiol. 2016, 7, 560. [Google Scholar] [CrossRef] [PubMed]
- Bolch, C.J.S.; Bejoy, T.A.; Green, D.H. Bacterial associates modify growth dynamics of the dinoflagellate Gymnodinium catenatum. Front. Microbiol. 2017, 8, 670. [Google Scholar] [CrossRef] [PubMed]
- Aquino-Cruz, A.; Okolodkov, Y. Impact of increasing water temperature on growth, photosynthetic efficiency, nutrient consumption, and potential toxicity of Amphidinium carterae and Coolia monotis (Dinoflagellata). Revista Biología Marina Y Oceanografía 2016, 51, 565–580. [Google Scholar] [CrossRef]
- Hughes, R. Membrane Glycoproteins. A Review of Structure and Function; Elsevier: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Klut, E.; Bisalputra, T.; Antia, N. Some cytochemical studies on the cell surface of Amphidinium carterae. Protoplasma 1985, 129, 93–99. [Google Scholar] [CrossRef]
- Turner, S.; Pryer, K.M.; Miao, V.P.; Palmer, J.D. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J. Eukaryot. Microbiol. 1999, 46, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Lane, D. Nucleic Acid Techniques in Bacterial Systematics; John Wiley and Sons: Hoboken, NJ, USA, 1999; pp. 115–175. [Google Scholar]
- Nash, E.A.; Nisbet, R.E.; Barbrook, A.C.; Howe, C.J. Dinoflagellates: A mitochondrial genome all at sea. Trends Genet. 2008, 24, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Barbrook, A.C.; Howe, C.J.; Kurniawan, D.P.; Tarr, S.J. Organization and expression of organellar genomes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Karunasagar, I.; Karunasagar, I. Influence of bacteria on growth and hemolysin production by the marine dinoflagellate Amphidinium carterae. Mar. Biol. 1997, 130, 35–39. [Google Scholar] [CrossRef]
- Soffer, N.; Gibbs, P.; Baker, A. Practical applications of contaminant-free Symbiodinium cultures grown on solid media. In Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, FL, USA, 7–11 July 2008; pp. 159–163. [Google Scholar]
- Xiang, T.; Hambleton, E.; DeNofrio, J.; Pringle, J.; Grossman, A. Isolation of clonal axenic strains of the symbiotic dinoflagellate Symbiodinium and their growth and host specificity. J. Phycol. 2013, 49, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Rolinson, G.N. Forty years of beta-lactam research. J. Antimicrob. Chemother. 1998, 41, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Galkiewicz, J.P.; Kellogg, A. Cross-Kingdom Amplification Using Bacteria-Specific Primers: Complications for Studies of Coral Microbial Ecology. Appl. Environ. Microbiol. 2008, 74, 7828–7831. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Zhang, Y.; Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397, 271–274. [Google Scholar] [PubMed]
- Markovic, P.; Roenneberg, T.; Morse, D. Phased protein synthesis at several circadian times does not change protein levels in Gonyaulax. J. Biol. Rhythms 1996, 11, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Bachvaroff, T.R.; Concepcion, G.T.; Rogers, C.R.; Herman, E.M.; Delwiche, C.F. Dinoflagellate expressed sequence tag data indicate massive transfer of chloroplast genes to the nuclear genome. Protist 2004, 155, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Hackett, J.D.; Yoon, H.S.; Soares, M.B.; Bonaldo, M.F.; Casavant, T.L.; Scheetz, T.E.; Nosenko, T.; Bhattacharya, D. Migration of the plastid genome to the nucleus in a peridinin dinoflagellate. Curr. Biol. 2004, 14, 213–218. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-L.; Place, A.R.; Jagus, R. Use of Antibiotics for Maintenance of Axenic Cultures of Amphidinium carterae for the Analysis of Translation. Mar. Drugs 2017, 15, 242. https://doi.org/10.3390/md15080242
Liu C-L, Place AR, Jagus R. Use of Antibiotics for Maintenance of Axenic Cultures of Amphidinium carterae for the Analysis of Translation. Marine Drugs. 2017; 15(8):242. https://doi.org/10.3390/md15080242
Chicago/Turabian StyleLiu, Chieh-Lun, Allen R. Place, and Rosemary Jagus. 2017. "Use of Antibiotics for Maintenance of Axenic Cultures of Amphidinium carterae for the Analysis of Translation" Marine Drugs 15, no. 8: 242. https://doi.org/10.3390/md15080242



