Upregulation and Identification of Antibiotic Activity of a Marine-Derived Streptomyces sp. via Co-Cultures with Human Pathogens
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Identification of Streptomyces sp. PTY087I2
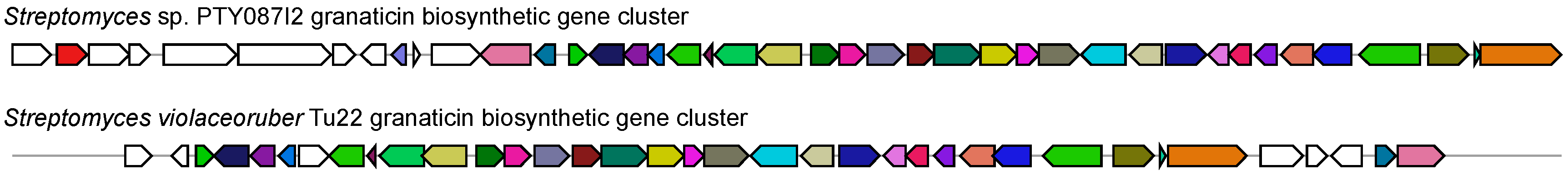
2.2. Secondary Metabolite Biosynthetic Potential of Streptomyces sp. PTY087I2
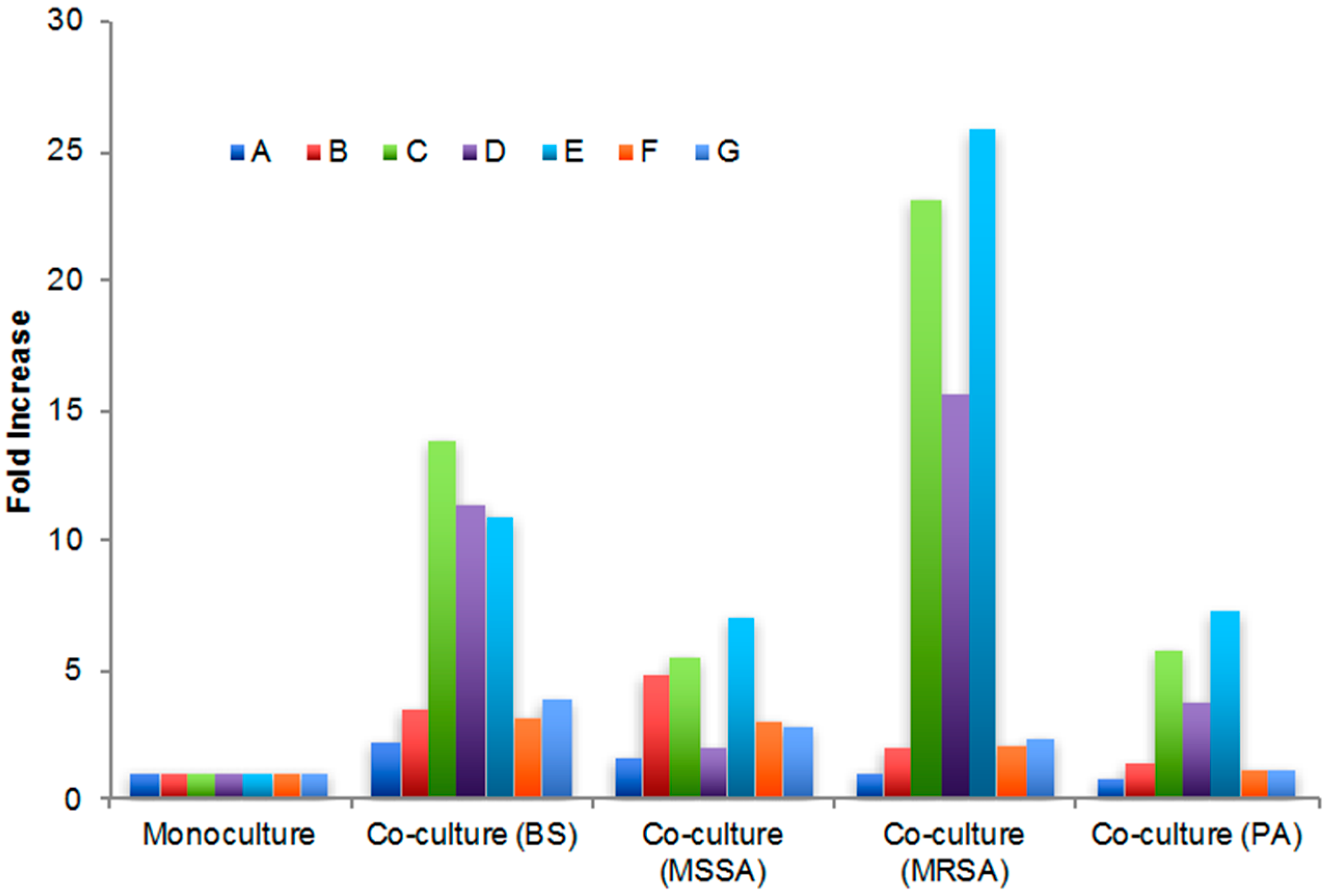
2.3. Secondary Metabolite Production by Streptomyces sp. PTY087I2 Mono- and Co-Cultures
2.4. Antibacterial Activity against Challenge Pathogens
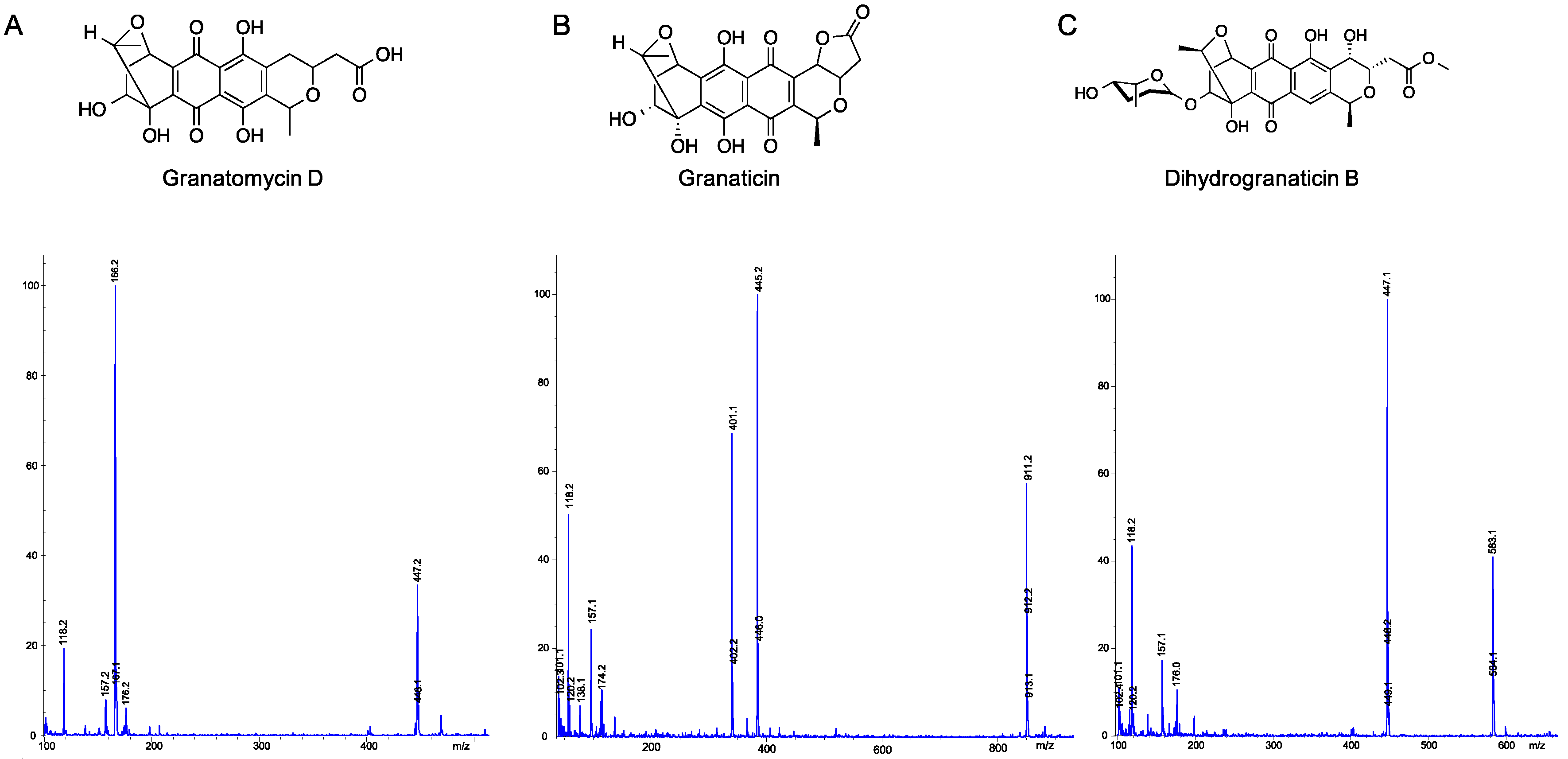
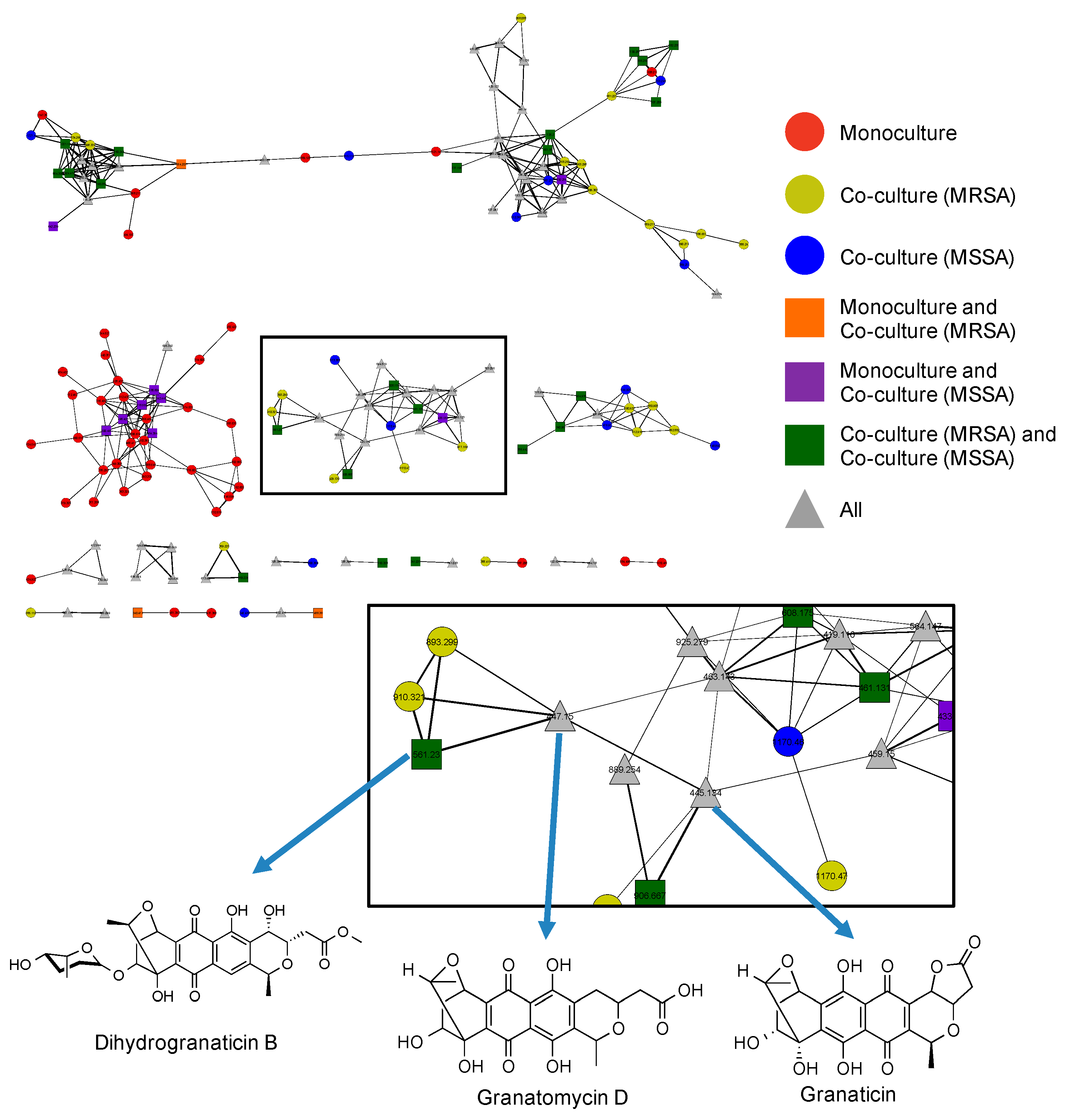
2.5. Utilization of Molecular Networks to Identify Other Naphthoquinone Derivatives
3. Materials and Methods
3.1. Tunicate Collection and Bacterial Isolation
3.2. Genomic Sequencing and antiSMASH Analysis of Streptomyces sp. PTY087I2
3.3. Streptomyces sp. PTY087I2 Mono- and Co-Cultures
3.4. Extraction of Streptomyces sp. PTY087I2 Mono- and Co-Cultures
3.5. LC–MS and HRMS of Streptomyces sp. PTY087I2 Extracts
3.6. Antibacterial Bioassays
3.7. Molecular Networking of Streptomyces sp. PTY087I2 Extracts
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chin, Y.W.; Balunas, M.J.; Chai, H.B.; Kinghorn, A.D. Drug discovery from natural sources. AAPS J. 2006, 8, E239–E253. [Google Scholar] [CrossRef] [PubMed]
- Nett, M.; Ikeda, H.; Moore, B.S. Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat. Prod. Rep. 2009, 26, 1362–1384. [Google Scholar] [CrossRef] [PubMed]
- Pettit, R.K. Mixed fermentation for natural product drug discovery. Appl. Microbiol. Biotechnol. 2009, 83, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Wright, D.L. The challenge of resistance in antimicrobial drug development. Future Microbiol. 2015, 10, 1709–1710. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.J. Antibiotic use and its consequences for the normal microbiome. Science 2016, 352, 544–545. [Google Scholar] [CrossRef] [PubMed]
- Chellat, M.F.; Raguz, L.; Riedl, R. Targeting antibiotic resistance. Angew. Chem. Int. Ed. Engl. 2016, 55, 6600–6626. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Dai, M.; Ahmed, S.; Hao, H.; Wang, X.; Yuan, Z. Antimicrobial drugs in fighting against antimicrobial resistance. Front. Microbiol. 2016, 7, 470. [Google Scholar] [CrossRef] [PubMed]
- Erwin, P.M.; Olson, J.B.; Thacker, R.W. Phylogenetic diversity, host-specificity and community profiling of sponge-associated bacteria in the northern Gulf of Mexico. PLoS ONE 2011, 6, e26806. [Google Scholar] [CrossRef] [PubMed]
- Inbaneson, S.J.; Ravikumar, S. In vitro antiplasmodial activity of bacterium RJAUTHB 14 associated with marine sponge Haliclona Grant against Plasmodium falciparum. Parasitol. Res. 2012, 110, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, E.; Coates, C.; Simmons, L.; Gonzalez, D.; Gerwick, W.H.; Dorrestein, P.C. Visualizing the spatial distribution of secondary metabolites produced by marine cyanobacteria and sponges via MALDI-TOF imaging. Mol. Biosyst. 2008, 4, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.D. Early central nervous system evolution: An era of skin brains? Nat. Rev. Neurosci. 2003, 4, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Brown, F.D.; Prendergast, A.; Swalla, B.J. Man is but a worm: Chordate origins. Genesis 2008, 46, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Hirose, E.; Hirose, M. Morphological process of vertical transmission of photosymbionts in the colonial ascidian Trididemnum miniatum Kott, 1977. Mar. Biol. 2007, 150, 359–367. [Google Scholar] [CrossRef]
- Hirose, E.; Hirose, M.; Neilan, B.A. Localization of symbiotic cyanobacteria in the colonial ascidian Trididemnum miniatum (Didemnidae, Ascidiacea). Zool. Sci. 2006, 23, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Hirose, E.; Uchida, H.; Murakami, A. Ultrastructural and microspectrophotometric characterization of multiple species of cyanobacterial photosymbionts coexisting in the colonial ascidian Trididemnum clinides (Tunicata, Ascidiacea, Didemnidae). Eur. J. Phycol. 2009, 44, 365–375. [Google Scholar] [CrossRef]
- Schmidt, E.W.; Donia, M.S. Life in cellulose houses: Symbiotic bacterial biosynthesis of ascidian drugs and drug leads. Curr. Opin. Biotechnol. 2010, 21, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.; Diaz-Valdes, M.; Wanner, G.; Ramos-Espla, A.; Anton, J. Microbial community associated with the colonial ascidian Cystodytes dellechiajei. Environ. Microbiol. 2007, 9, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Konig, G.M.; Kehraus, S.; Seibert, S.F.; Abdel-Lateff, A.; Muller, D. Natural products from marine organisms and their associated microbes. ChemBioChem 2006, 7, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Gontang, E.A.; Gaudencio, S.P.; Fenical, W.; Jensen, P.R. Sequence-based analysis of secondary-metabolite biosynthesis in marine actinobacteria. Appl. Environ. Microbiol. 2010, 76, 2487–2499. [Google Scholar] [CrossRef] [PubMed]
- Shank, E.A.; Kolter, R. New developments in microbial interspecies signaling. Curr. Opin. Microbiol. 2009, 12, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, S.; Wakimoto, T.; Onaka, H.; Abe, I. Chojalactones a–c, cytotoxic butanolides isolated from Streptomyces sp. cultivated with mycolic acid containing bacterium. Org. Lett. 2015, 17, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.C.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Induced production of emericellamides A and B from the marine-derived fungus Emericella sp. in competing co-culture. J. Nat. Prod. 2007, 70, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.C.; Jensen, P.R.; Kauffman, C.A.; Fenical, W. Libertellenones A-D: Induction of cytotoxic diterpenoid biosynthesis by marine microbial competition. Bioorg. Med. Chem. 2005, 13, 5267–5273. [Google Scholar] [CrossRef] [PubMed]
- Valliappan, K.; Sun, W.; Li, Z. Marine actinobacteria associated with marine organisms and their potentials in producing pharmaceutical natural products. Appl. Microbiol. Biotechnol. 2014, 98, 7365–7377. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Kang, K.H.; Sivakumar, K.; Li-Chan, E.C.; Oh, H.M.; Kim, S.K. Marine actinobacteria: An important source of bioactive natural products. Environ. Toxicol. Pharmacol. 2014, 38, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Fenical, W.; Jensen, P.R. Developing a new resource for drug discovery: Marine actinomycete bacteria. Nat. Chem. Biol. 2006, 2, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Gromek, S.M.; Sung, A.A.; Klassen, J.L.; Balunas, M.J. Draft genome sequence of Streptomyces sp. strain PTY087I2 isolated from Styela canopus, a Panamanian tunicate. Genome Announc. 2016, 4, e00874-16. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Blin, K.; Duddela, S.; Krug, D.; Kim, H.U.; Bruccoleri, R.; Lee, S.Y.; Fischbach, M.A.; Muller, R.; Wohlleben, W.; et al. antiSMASH 3.0-a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015, 43, W237–W243. [Google Scholar] [CrossRef] [PubMed]
- Derewacz, D.K.; Covington, B.C.; McLean, J.A.; Bachmann, B.O. Mapping microbial response metabolomes for induced natural product discovery. ACS Chem. Biol. 2015, 10, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Snipes, C.E.; Chang, C.-J.; Floss, H.G. Biosynthesis of the antibiotic granaticin. J. Am. Chem. Soc. 1979, 101, 701–706. [Google Scholar] [CrossRef]
- Ichinose, K.; Bedford, D.J.; Tornus, D.; Bechthold, A.; Bibb, M.J.; Revill, W.P.; Floss, H.G.; Hopwood, D.A. The granaticin biosynthetic gene cluster of Streptomyces violaceoruber TÜ22: Sequence analysis and expression in a heterologous host. Chem. Biol. 1998, 5, 647–659. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto–Knaan, T.; et al. Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Klassen, J.L.; Currie, C.R. ORFcor: Identifying and accommodating ORF prediction inconsistencies for phylogenetic analysis. PLoS ONE 2013, 8, e58387. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. Fasttree 2—approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Gromek, S.M.; Suria, A.; Fullmer, M.S.; Garcia, J.L.; Gogarten, J.P.; Nyholm, S.V.; Balunas, M.J. Leisingera sp. JC1, a bacterial isolate from Hawaiian bobtail squid eggs, produces indigoidine and differentially inhibits vibrios. Front. Microbiol. 2016, 7, 1342. [Google Scholar] [CrossRef] [PubMed]
- Zgoda, J.R.; Porter, J.R. A convenient microdilution method for screening natural products against bacteria and fungi. Pharm. Biol. 2001, 39, 221–225. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Yang, J.Y.; Sanchez, L.M.; Rath, C.M.; Liu, X.; Boudreau, P.D.; Bruns, N.; Glukhov, E.; Wodtke, A.; de Felicio, R.; Fenner, A.; et al. Molecular networking as a dereplication strategy. J. Nat. Prod. 2013, 76, 1686–1699. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
| Cluster | Type | Most Similar Known Cluster | Percent Similarity | Molecular Weight (g/mol) a |
|---|---|---|---|---|
| 1 | Bacteriocin | Tetronasin | 3% | 602.80 |
| 2 | Nrps | Coelibactin | 72% | 391.46 |
| 3 | Nrps-T1pks | SGR PTMs | 100% | |
| 4 | Otherks-Nrps | Calcium-dependent antibiotic | 15% | |
| 5 | Terpene | Hopene | 69% | 410.72 |
| 6 | Bacteriocin | - | - | |
| 7 | Terpene | - | - | |
| 8 | Lantipeptide | - | - | |
| 9 | Melanin | Melanin | 100% | 318.28 |
| 10 | T3pks | Herboxidiene | 8% | 438.60 |
| 11 | Nrps | Herboxidiene | 9% | 438.60 |
| 12 | Nrps-T1pks | Daptomycin | 4% | 1620.67 |
| 13 | Ectoine | Ectoine | 100% | 142.16 |
| 14 | Siderophore | Kinamycin | 22% | 484.50 |
| 15 | T2pks | Granaticin | 83% | 444.39 |
| 16 | Butyrolactone | Oxazolomycin | 6% | 655.78 |
| 17 | Terpene | - | - | |
| 18 | Nrps | Griseobactin | 100% | 1179.50 |
| 19 | Otherks-T1pks | Nataxazole | 59% | 400.38 |
| 20 | T3pks | Herboxidiene | 6% | 438.60 |
| 21 | Nrps | Coelichelin | 72% | 565.58 |
| 22 | Lantipeptide | Guadinomine | 7% | 518.57 |
| 23 | Thiopeptide-Lantipeptide | - | - | |
| 24 | Lassopeptide | - | - | |
| 25 | Nrps | Phosphonoglycans | 3% | |
| 26 | Lassopeptide | SRO15-2005 | 100% | |
| 27 | Nrps | SRO15-2005 | 100% | |
| 28 | Terpene | Steffimycin | 19% | 574.53 |
| 29 | Siderophore | Desferrioxamine B | 80% | 560.68 |
| 30 | Ectoine | Pristinamycin | 23% | IA 866.96; IB 852.93; IIA 525.59; IIB 527.62 |
| 31 | Lantipeptide-Melanin | Labyrinthopeptin A1, A2, A3 | 40% | A1 2073.76; A2 1922.68; A3 2188.78 |
| 32 | T1pks-Nrps | Enduracidin | 10% | 2355.61 |
| 33 | Ladderane-Arylpolyene | Skyllamycin | 26% | 1481.99 |
| 34 | Other | Skyllamycin | 26% | 1481.99 |
| 35 | Lantipeptide | AmfS | 100% | |
| 36 | Nrps | Skyllamycin | 10% | 1481.99 |
| 37 | Nrps | - | - |
| Extract | Peak A (5 min) | Peak B (10 min) | Peak C (11 min) | Peak D (13 min) | Peak E (16 min) | Peak F (34 min) | Peak G (68 min) |
|---|---|---|---|---|---|---|---|
| Monoculture | 930.4 | 231.3 | 15.1 | 13.2 | 5.4 | 27.3 | 29.0 |
| Co-culture (BS) | 2117.8 | 826.0 | 208.4 | 149.7 | 58.7 | 86.6 | 110.7 |
| Co-culture (MSSA) | 1480.5 | 1107.4 | 83.6 | 25.9 | 38.2 | 84.2 | 81.9 |
| Co-culture (MRSA) | 962.2 | 463.2 | 349.3 | 206.2 | 139.6 | 56.4 | 67.1 |
| Co-culture (PA) | 739.0 | 335.1 | 87.4 | 49.7 | 39.3 | 33.3 | 33.8 |
| Extract | Bacillus subtilis | MSSA | MRSA | Pseudomonas aeruginosa |
|---|---|---|---|---|
| Monoculture | 25 | 50 | 50 | >400 |
| Co-culture (BS) | 6.25 | 25 | 25 | >400 |
| Co-culture (MSSA) | 25 | 50 | 50 | >400 |
| Co-culture (MRSA) | 1.56 | 12.5 | 6.25 | >400 |
| Co-culture (PA) | 6.25 | 12.5 | 12.5 | >400 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, A.A.; Gromek, S.M.; Balunas, M.J. Upregulation and Identification of Antibiotic Activity of a Marine-Derived Streptomyces sp. via Co-Cultures with Human Pathogens. Mar. Drugs 2017, 15, 250. https://doi.org/10.3390/md15080250
Sung AA, Gromek SM, Balunas MJ. Upregulation and Identification of Antibiotic Activity of a Marine-Derived Streptomyces sp. via Co-Cultures with Human Pathogens. Marine Drugs. 2017; 15(8):250. https://doi.org/10.3390/md15080250
Chicago/Turabian StyleSung, Anne A., Samantha M. Gromek, and Marcy J. Balunas. 2017. "Upregulation and Identification of Antibiotic Activity of a Marine-Derived Streptomyces sp. via Co-Cultures with Human Pathogens" Marine Drugs 15, no. 8: 250. https://doi.org/10.3390/md15080250





