New Marine Antifouling Compounds from the Red Alga Laurencia sp.
Abstract
:1. Introduction
2. Results
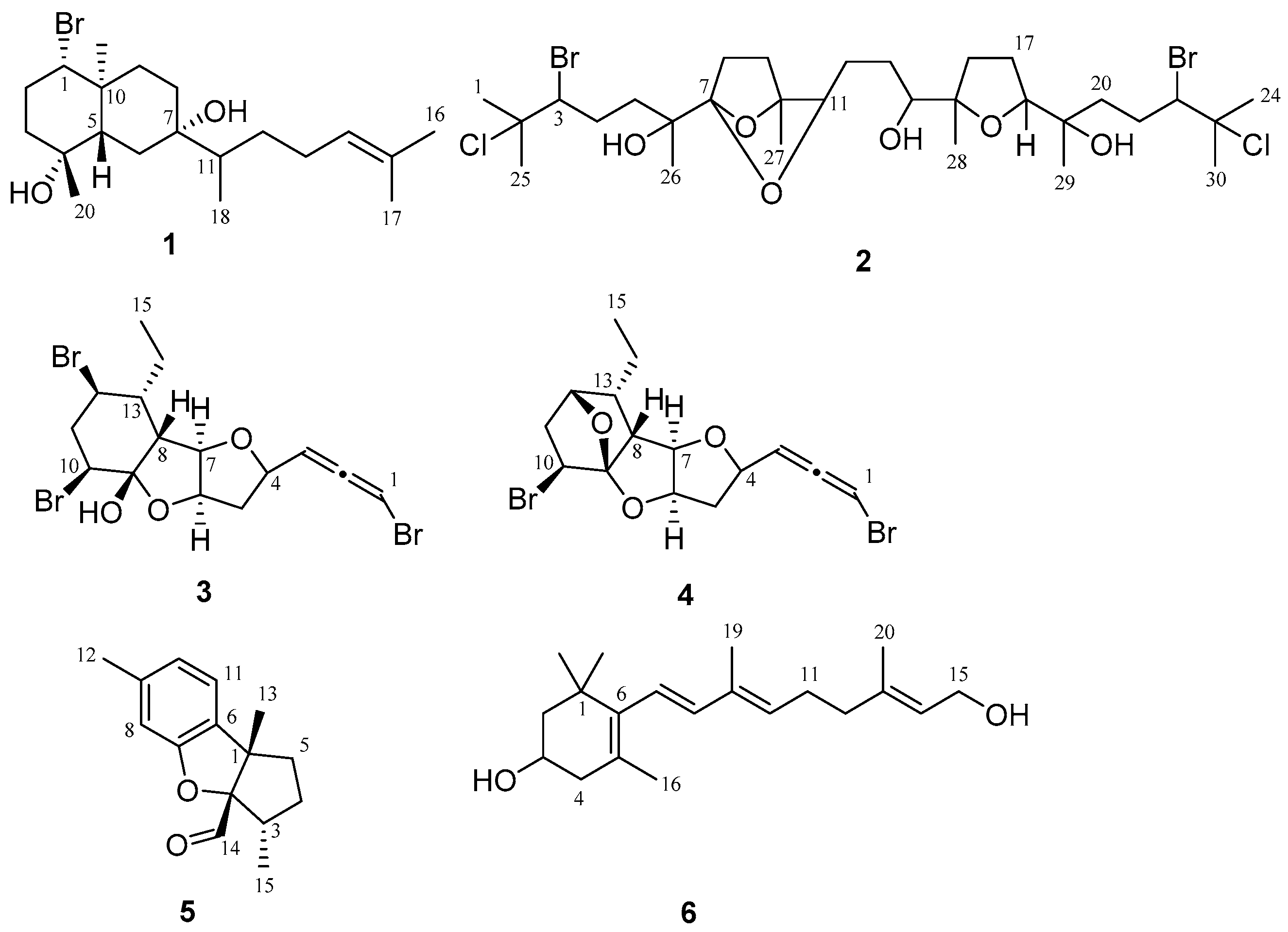
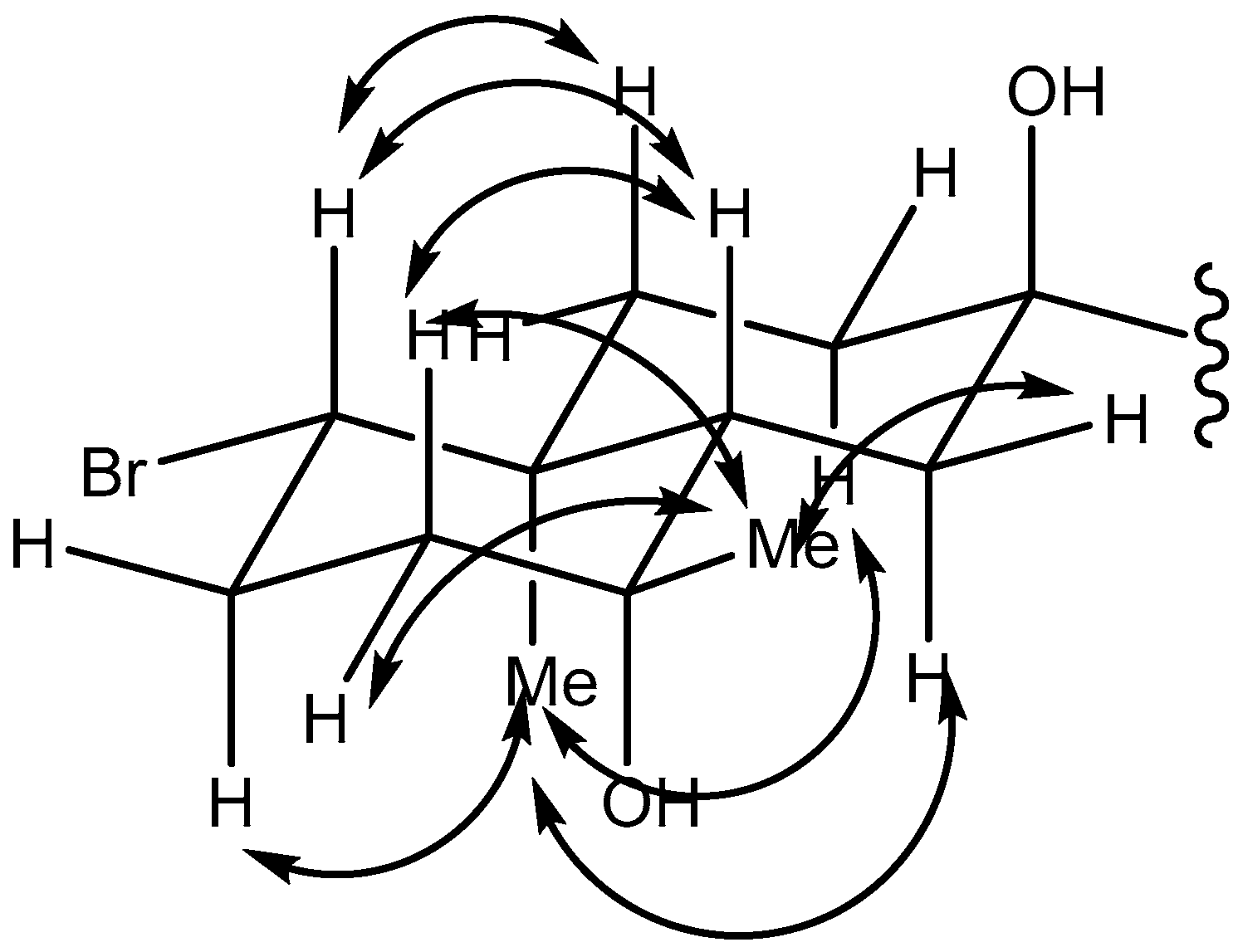
2.1. Omaezol (1) and Intricatriol (2)
2.2. Hachijojimallenes A (3) and B (4)
2.3. Debromoaplysinal (5)
2.4. 11,12-Dihydro-3-hydroxyretinol (6)
2.5. Antifouling Activity
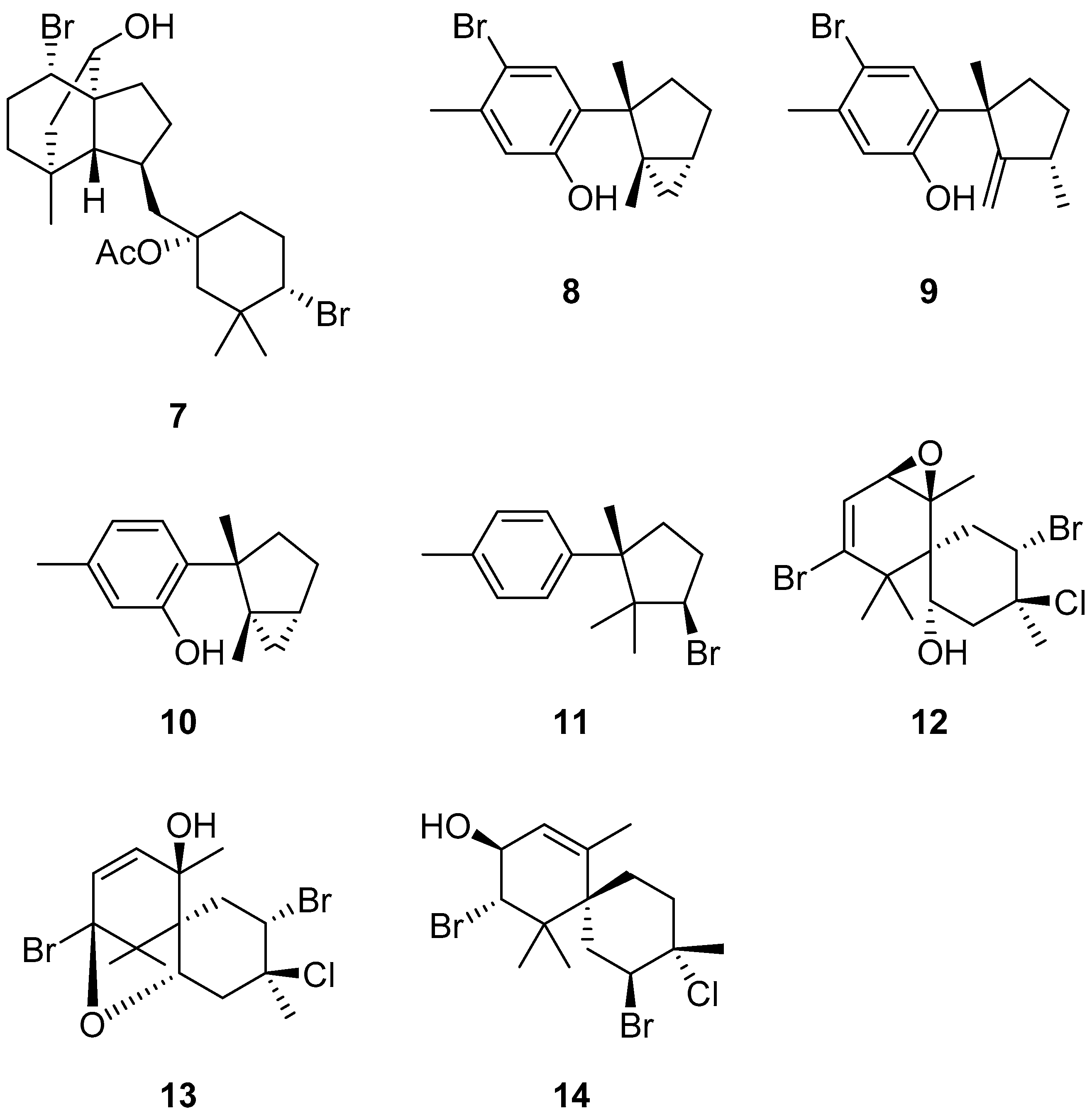
3. Discussion
4. Materials and Methods
4.1. General Procedures
4.2. Plant Material
4.3. Omaezol and Intricatriol
4.4. Hachijojimallenes A and B
4.5. Debromoaplysinal
4.6. 11,12-Dihydro-3-hydroxyretinol
4.7. Antifouling Assay
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Davidson, I.; Scianni, C.; Hewitt, C.; Everett, R.; Holm, E.; Tamburri, M.; Ruiz, G. Assessing the drivers of ship biofouling management—Aligning industry and biosecurity goals. Biofouling 2016, 32, 411–428. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.A.; Santos, L.; Vance, T.; Fileman, T.; Smith, D.; Bishop, J.D.D.; Viard, F.; Queirós, A.M.; Merino, G.; Vuisman, E.; et al. Costs and benefits to European shipping of ballast-water and hull-fouling treatment: Impacts of native and non-indigenous species. Mar. Policy 2016, 64, 148–155. [Google Scholar] [CrossRef]
- Almeida, J.R.; Vasconcelos, V. Natural antifouling compounds: Effectiveness in preventing invertebrate settlement and adhesion. Biotechnol. Adv. 2015, 33, 343–357. [Google Scholar] [CrossRef]
- Qian, P.; Li, Z.; Xu, Y.; Fusetani, N. Marine natural products and their synthetic analogs as antifouling compounds: 2009–2014. Biofouling 2015, 31, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.; Chen, L.; Xu, Y. Molecular mechanisms of antifouling compounds. Biofouling 2013, 29, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Okino, T.; Yoshimura, E.; Hirota, H.; Fusetani, N. New antifouling sesquiterpenes from four nudibranchs of the family Phyllidiidae. Tetrahedron 1996, 52, 9447–9454. [Google Scholar] [CrossRef]
- Tan, L.T.; Goh, B.P.L.; Tripathi, A.; Lim, M.G.; Dickinson, G.H.; Lee, S.S.C.; Teo, S.L.M. Natural antifoulants from the marine cyanobacterium Lyngbya majuscula. Biofouling 2010, 26, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Casalme, L.O.; Yamauchi, A.; Sato, A.; Petitbois, J.G.; Nogata, Y.; Yoshimura, E.; Okino, T.; Umezawa, T.; Matsuda, F. Total synthesis and biological activity of dolastatin 16. Org. Biomol. Chem. 2017, 15, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Nakahara, H.; Shirokura, Y.; Nogata, Y.; Yoshimura, E.; Umezawa, T.; Okino, T.; Matsuda, F. Total synthesis of 10-isocyano-4-cadinene and its stereoisomers and evaluations of antifouling activities. J. Org. Chem. 2011, 76, 6558–6573. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Vairappan, C.S. Halogenated secondary metabolites from Japanese species of the red algal genus Laurencia (Rhodomelaceae, Ceramiales). Curr. Top. Phytochem. 2005, 7, 1–34. [Google Scholar]
- Wang, B.G.; Gloer, J.B.; Ji, N.Y.; Zhao, J.C. Halogenated organic molecules of Rohodomelaceae origin: Chemistry and biology. Chem. Rev. 2013, 113, 3632–3685. [Google Scholar] [CrossRef] [PubMed]
- König, G.M.; Wright, A.D. Laurencia rigida: Chemical investigation of its antifouling dichloromethane extract. J. Nat. Prod. 1997, 60, 967–970. [Google Scholar] [CrossRef]
- Umezawa, T.; Oguri, Y.; Matsuura, H.; Yamazaki, S.; Suzuki, M.; Yoshimura, E.; Furuta, T.; Nogata, Y.; Serisawa, Y.; Matsuyama-Serisawa, K.; et al. Omaezallene from red alga Laurencia sp.: Structure elucidation, total synthesis, and antifouling activity. Angew. Chem. Int. Ed. 2014, 53, 3909–3912. [Google Scholar] [CrossRef] [PubMed]
- Al-Lihaibi, S.S.; Abdel-Lateff, A.; Alarif, W.M.; Nogata, Y.; Ayyad, S.N.; Okino, T. Potent antifouling metabolites form Red Sea organisms. Asian J. Chem. 2015, 27, 2252–2256. [Google Scholar] [CrossRef]
- Suzuki, M.; Matsuo, Y.; Takeda, S.; Suzuki, T. Intricatetraol, a halogenated triterpene alcohol from the red alga Laurencia intricata. Phytochemistry 1993, 33, 651–656. [Google Scholar] [CrossRef]
- Morimoto, Y.; Okita, T.; Takaishi, M.; Tanaka, T. Total synthesis and determination of the absolute configuration of (+)-intricatetraol. Angew. Chem. Int. Ed. 2007, 46, 1132–1135. [Google Scholar] [CrossRef]
- Carter, G.T.; Rimehart, K.L., Jr.; Li, L.H.; Kuentzel, S.L.; Connor, J.L. Brominated indoles from Laurencia brongniartii. Tetrahedron Lett. 1978, 19, 4479–4482. [Google Scholar] [CrossRef]
- Fukuzawa, A.; Kumagai, Y.; Masamune, T.; Furusaki, A.; Matsumoto, T.; Katayama, C. Pinnaterpenes A, B, and C, new dibromoditerpenes from the red alga Laurencia pinnata Yamada. Chem. Lett. 1982, 11, 1389–1392. [Google Scholar] [CrossRef]
- Suzuki, M.; Kurosawa, E.; Furusaki, A. The structure and absolute stereochemistry of a halogenated chamigrene derivative from the red alga Laurencia species. Bull. Chem. Soc. Jpn. 1988, 61, 3371–3373. [Google Scholar] [CrossRef]
- Abe, T.; Masuda, M.; Suzuki, T.; Suzuki, M. Chemical races in the red alga Laurencia nipponica (Rhodomelaeae, Ceramiales). Phycol. Res. 1999, 47, 87–95. [Google Scholar] [CrossRef]
- Suzuki, M.; Kurosawa, E. Halogenated and non-halogenated aromatic sesquiterpenes from the red algae Laurencia okamurae Yamada. Bull. Chem. Soc. Jpn. 1979, 52, 3352–3354. [Google Scholar] [CrossRef]
- Takahashi, Y.; Suzuki, M.; Abe, T.; Masuda, M. Anhydroaplysiadiol from Laurencia japonensis. Phytochemistry 1998, 48, 987–990. [Google Scholar] [CrossRef]
- Kaneko, K.; Washio, K.; Umezawa, T.; Matsuda, F.; Morikawa, M.; Okino, T. cDNA cloning and characterization of vanadium-dependent bromoperoxidases from the red alga Laurencia nipponica. Biosci. Biotechnol. Biochem. 2014, 78, 1310–1319. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Daitoh, M.; Suzuki, M.; Abe, T.; Masuda, M. Antibacterial halogenated metabolites from the Malaysian Laurencia species. Phytochemistry 2001, 58, 291–297. [Google Scholar] [CrossRef]
- Blackman, A.J.; Wells, R.J. Caulerpol, a diterpene alcohol, related to vitamin A, from Caulerpa brownii (algae). Tetrahedron Lett. 1976, 17, 2729–2730. [Google Scholar] [CrossRef]
| Carbon Number | Compound 1 | Compound 2 | Carbon Number | Compound 2 | |||
|---|---|---|---|---|---|---|---|
| 13C | 1H | 13C | 1H | 13C | 1H | ||
| 1 | 67.6 | 3.91 | 27.8 | 1.689 | 24 | 27.5 | 1.695 |
| 2 | 30.2 | 2.44, 2.01 | 72.0 | 23 | 72.0 | ||
| 3 | 41.9 | 1.55, 1.70 | 67.1 | 4.07 | 22 | 67.1 | 4.08 |
| 4 | 71.4 | 28.7 | 1.85, 2.61 | 21 | 28.9 | 1.75, 2.45 | |
| 5 | 48.2 | 1.10 | 36.8 | 1.72, 1.89 | 20 | 36.9 | 1.47, 1.72 |
| 6 | 32.1 | 1.48, 2.00 | 72.2 | 19 | 73.3 | ||
| 7 | 74.6 | 113.4 | 18 | 84.3 | 3.85 | ||
| 8 | 31.8 | 1.49, 1.90 | 27.9 | 1.60, 1.70 | 17 | 26.4 | 1.93 |
| 9 | 38.7 | 1.05, 1.76 | 32.8 | 1.91 | 16 | 32.7 | 1.57, 2.19 |
| 10 | 39.4 | 86.7 | 15 | 85.6 | |||
| 11 | 33.6 | 1.67 | 84.7 | 3.61 | 14 | 76.0 | 3.57 |
| 12 | 30.2 | 1.52 | 29.6 | 1.55, 2.40 | 13 | 29.4 | 1.74 |
| 13 | 26.5 | 1.12 | 25 | 33.1 | 1.785 | ||
| 14 | 124.3 | 5.10 | 30 | 32.9 | 1.793 | ||
| 15 | 131.7 | 26 | 21.2 | 1.28 | |||
| 16 | 25.5 | 1.70 | 29 | 24.5 | 1.26 | ||
| 17 | 17.6 | 1.62 | 27 | 17.6 | 1.45 | ||
| 18 | 12.3 | 0.90 | 28 | 23.0 | 1.19 | ||
| 19 | 14.4 | 1.26 | |||||
| 20 | 29.7 | 1.18 | |||||
| Carbon Number | Compound 3 | Compound 4 | Compound 5 | |||
|---|---|---|---|---|---|---|
| 13C | 1H | 13C | 1H | 13C | 1H | |
| 1 | 74.1 | 6.08 | 74.0 | 3.09 | 58.7 | |
| 2 | 201.9 | 201.9 | 103.4 | |||
| 3 | 99.4 | 5.45 | 100.0 | 5.48 | 42.4 | 2.59 |
| 4 | 73.8 | 4.73 | 74.1 | 4.72 | 31.7 | 1.26, 1.74 |
| 5 | 39.3 | 1.81, 2.26 | 39.6 | 1.82, 2.35 | 43.0 | 1.74, 1.92 |
| 6 | 79.4 | 4.56 | 88.6 | 5.11 | 131.6 | |
| 7 | 81.4 | 4.61 | 82.8 | 4.77 | 159.4 | |
| 8 | 50.5 | 2.77 | 51.0 | 3.19 | 110.0 | 6.72 |
| 9 | 102.1 | 119.7 | 138.8 | |||
| 10 | 53.6 | 4.07 | 42.8 | 4.13 | 122.1 | 6.74 |
| 11 | 44.3 | 2.50, 2.76 | 40.6 | 1.74, 2.70 | 122.4 | 6.92 |
| 12 | 51.3 | 3.79 | 75.6 | 4.03 | 21.5 | 2.33 |
| 13 | 44.7 | 1.97 | 50.2 | 2.13 | 24.2 | 1.31 |
| 14 | 24.7 | 1.47, 2.11 | 23.3 | 1.44, 1.60 | 203.5 | 9.78 |
| 15 | 11.6 | 1.03 | 12.5 | 0.99 | 13.1 | 1.04 |
| 9-OH | 3.65 | |||||
| Carbon Number | 13C | 1H | Carbon Number | 13C | 1H |
|---|---|---|---|---|---|
| 1 | 37.9 | 11 | 27.5 | 2.29 | |
| 2 | 49.1 | 1.47, 1.76 | 12 | 40.0 | 2.12 |
| 3 | 65.9 | 4.00 | 13 | 140.0 | |
| 4 | 43.2 | 2.02, 2.37 | 14 | 124.2 | 5.44 |
| 5 | 125.9 | 15 | 60.1 | 4.18 | |
| 6 | 138.2 | 16 | 22.4 | 1.71 | |
| 7 | 124.0 | 5.92 | 17 | 31.0 | 1.06 |
| 8 | 139.0 | 6.00 | 18 | 29.5 | 1.06 |
| 9 | 134.6 | 19 | 13.2 | 1.79 | |
| 10 | 131.4 | 5.40 | 20 | 17.2 | 1.71 |
| Compound | EC50 | LC50 |
|---|---|---|
| 1 | 0.59 | 9.6 |
| 2 | — | — |
| 3 | 0.31 | 20 |
| 4 | 0.76 | 17 |
| 5 | 4.3 | — |
| 7 | 1.6 | — |
| 8 | 2.2 | 20 |
| 9 | 1.2 | — |
| 10 | 2.3 | — |
| 11 | — | — |
| 12 | 1.5 | — |
| 13 | 5.5 | — |
| 14 | 6.1 | — |
| CuSO4 | 0.72 | 1.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oguri, Y.; Watanabe, M.; Ishikawa, T.; Kamada, T.; Vairappan, C.S.; Matsuura, H.; Kaneko, K.; Ishii, T.; Suzuki, M.; Yoshimura, E.; et al. New Marine Antifouling Compounds from the Red Alga Laurencia sp. Mar. Drugs 2017, 15, 267. https://doi.org/10.3390/md15090267
Oguri Y, Watanabe M, Ishikawa T, Kamada T, Vairappan CS, Matsuura H, Kaneko K, Ishii T, Suzuki M, Yoshimura E, et al. New Marine Antifouling Compounds from the Red Alga Laurencia sp. Marine Drugs. 2017; 15(9):267. https://doi.org/10.3390/md15090267
Chicago/Turabian StyleOguri, Yuko, Mami Watanabe, Takafumi Ishikawa, Takashi Kamada, Charles S. Vairappan, Hiroshi Matsuura, Kensuke Kaneko, Takahiro Ishii, Minoru Suzuki, Erina Yoshimura, and et al. 2017. "New Marine Antifouling Compounds from the Red Alga Laurencia sp." Marine Drugs 15, no. 9: 267. https://doi.org/10.3390/md15090267





