Marine Pharmacology in 2012–2013: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action †
Abstract
:1. Introduction
2. Marine Compounds with Antibacterial, Antifungal, Antiprotozoal, Antituberculosis, Antiviral and Anthelmintic Activities
2.1. Antibacterial Activity
2.2. Antifungal Activity
2.3. Antiprotozoal and Antituberculosis Activity
2.4. Antiviral Activity
2.5. Anthelmintic Activity
3. Marine Compounds with Antidiabetic and Anti-Inflammatory Activity, and Affecting the Immune and Nervous System
3.1. Antidiabetic Activity
3.2. Anti-Inflammatory Activity
3.3. Marine Compounds with Activity on the Immune System
3.4. Marine Compounds Affecting the Nervous System
4. Marine Compounds with Miscellaneous Mechanisms of Action
5. Reviews on Marine Pharmacology
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mayer, A.M.S.; Lehmann, V.K.B. Marine pharmacology in 1998: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, antiplatelet, antiprotozoal, and antiviral activities;with actions on the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. Pharmacologist 2000, 42, 62–69. [Google Scholar]
- Mayer, A.M.S.; Hamann, M.T. Marine pharmacology in 1999: Compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, anti-inflammatory, antiplatelet, antiprotozoal and antiviral activities; affecting the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 2002, 132, 315–339. [Google Scholar]
- Mayer, A.M.S.; Hamann, M.T. Marine pharmacology in 2000: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Mar. Biotechnol. 2004, 6, 37–52. [Google Scholar] [PubMed]
- Mayer, A.M.S.; Hamann, M.T. Marine pharmacology in 2001–2002: Marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 140, 265–286. [Google Scholar] [PubMed]
- Mayer, A.M.S.; Rodriguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2003-4: Marine compounds with anthelmintic antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 553–581. [Google Scholar] [PubMed]
- Mayer, A.M.S.; Rodriguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2005-6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta 2009, 1790, 283–308. [Google Scholar] [PubMed]
- Mayer, A.M.S.; Rodriguez, A.D.; Berlinck, R.G.; Fusetani, N. Marine pharmacology in 2007-8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 191–222. [Google Scholar] [PubMed]
- Mayer, A.M.S.; Rodriguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine Pharmacology in 2009–2011: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous Systems, and other Miscellaneous Mechanisms of Action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar] [PubMed]
- Schmitz, F.J.; Bowden, B.F.; Toth, S.I. Antitumor and Cytotoxic Compounds from Marine Organisms. In Marine Biotechnology, Pharmaceutical and Bioactive Natural Products; Attaway, D.H., Zaborsky, O.R., Eds.; Plenum Press: New York, NY, USA; London, UK, 1993; pp. 197–308. [Google Scholar]
- Cervantes, S.; Stout, E.P.; Prudhomme, J.; Engel, S.; Bruton, M.; Cervantes, M.; Carter, D.; Tae-Chang, Y.; Hay, M.E.; Aalbersberg, W.; et al. High content live cell imaging for the discovery of new antimalarial marine natural products. BMC Infect. Dis. 2012, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Spavieri, J.; Allmendinger, A.; Kaiser, M.; Itoe, M.A.; Blunden, G.; Mota, M.M.; Tasdemir, D. Assessment of dual life stage antiplasmodial activity of british seaweeds. Mar. Drugs 2013, 11, 4019–4034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, A.; Salam, K.A.; Furuta, A.; Matsuda, Y.; Fujita, O.; Tani, H.; Fujita, Y.; Fujimoto, Y.; Ikeda, M.; Kato, N.; et al. Inhibition of hepatitis C virus replication and viral helicase by ethyl acetate extract of the marine feather star Alloeocomatella polycladia. Mar. Drugs 2012, 10, 744–761. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.M.; Sassaki, G.L.; Romanos, M.T.; Barreto-Bergter, E. Structural characterization and anti-HSV-1 and HSV-2 activity of glycolipids from the marine algae Osmundaria obtusiloba isolated from Southeastern Brazilian coast. Mar. Drugs 2012, 10, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, I.R.; Cordeiro, S.L.; Gomes, D.L.; Dreyfuss, J.L.; Filgueira, L.G.; Leite, E.L.; Nader, H.B.; Rocha, H.A. Evaluation of Anti-Nociceptive and Anti-Inflammatory Activities of a Heterofucan from Dictyota menstrualis. Mar. Drugs 2013, 11, 2722–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalcante-Silva, L.H.; da Matta, C.B.; de Araujo, M.V.; Barbosa-Filho, J.M.; de Lira, D.P.; de Oliveira Santos, B.V.; de Miranda, G.E.; Alexandre-Moreira, M.S. Antinociceptive and anti-inflammatory activities of crude methanolic extract of red alga Bryothamnion triquetrum. Mar. Drugs 2012, 10, 1977–1992. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.S.; Nicolau, L.A.; Silva, R.O.; Barros, F.C.; Freitas, A.L.; Aragao, K.S.; Ribeiro, R.A.; Souza, M.H.; Barbosa, A.L.; Medeiros, J.V. Antiinflammatory and antinociceptive effects in mice of a sulfated polysaccharide fraction extracted from the marine red algae Gracilaria caudata. Immunopharmacol. Immunotoxicol. 2013, 35, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, M.S.; Choi, H.J.; Choi, J.W.; Shin, T.; Woo, H.C.; Kim, J.I.; Kim, H.R. Hexane fraction from Laminaria japonica exerts anti-inflammatory effects on lipopolysaccharide-stimulated RAW 264.7 macrophages via inhibiting NF-kappa B pathway. Eur. J. Nutr. 2013, 52, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Rezende, B.M.; Bernardes, P.T.; Resende, C.B.; Arantes, R.M.; Souza, D.G.; Braga, F.C.; Castor, M.G.; Teixeira, M.M.; Pinho, V. Lithothamnion muelleri controls inflammatory responses, target organ injury and lethality associated with graft-versus-host disease in mice. Mar. Drugs 2013, 11, 2595–2615. [Google Scholar] [CrossRef] [PubMed]
- Zawadzki, M.; Janosch, C.; Szechinski, J. Perna canaliculus lipid complex PCSO-524 demonstrated pain relief for osteoarthritis patients benchmarked against fish oil, a randomized trial, without placebo control. Mar. Drugs 2013, 11, 1920–1935. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.S.; Liu, L.J.; Qu, Y.L.; Ouyang, X.K.; Yang, L.Y.; Xu, Z.R. Chitosan nanoparticles attenuate hydrogen peroxide-induced stress injury in mouse macrophage RAW264.7 cells. Mar. Drugs 2013, 11, 3582–3600. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, B.; Wang, Q.; Zhang, Z.; Nie, F.; Liu, G.; Zheng, J.; Xiao, L.; Zhang, L. Pharmacological studies of tentacle extract from the jellyfish Cyanea capillata in isolated rat aorta. Mar. Drugs 2013, 11, 3335–3349. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.O.; Santana, A.P.; Carvalho, N.S.; Bezerra, T.S.; Oliveira, C.B.; Damasceno, S.R.; Chaves, L.S.; Freitas, A.L.; Soares, P.M.; Souza, M.H.; et al. A sulfated-polysaccharide fraction from seaweed Gracilaria birdiae prevents naproxen-induced gastrointestinal damage in rats. Mar. Drugs 2012, 10, 2618–2633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Wu, W.H.; Wang, J.; Lan, M.B. Antioxidant properties of polysaccharide from the brown seaweed Sargassum graminifolium (Turn.), and its effects on calcium oxalate crystallization. Mar. Drugs 2012, 10, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Kelman, D.; Posner, E.K.; McDermid, K.J.; Tabandera, N.K.; Wright, P.R.; Wright, A.D. Antioxidant activity of Hawaiian marine algae. Mar. Drugs 2012, 10, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.C.; Giao, M.S.; Seabra, R.; Ferreira, A.C.; Tamagnini, P.; Moradas-Ferreira, P.; Malcata, F.X. Evaluation of the antioxidant activity of cell extracts from microalgae. Mar. Drugs 2013, 11, 1256–1270. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, N.; Desor, F.; Gleizes, C.; Denis, F.M.; Arab-Tehrany, E.; Soulimani, R.; Linder, M. Anxiolytic-like effect of a salmon phospholipopeptidic complex composed of polyunsaturated fatty acids and bioactive peptides. Mar. Drugs 2013, 11, 4294–4317. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.; Fernandes, L.; Conde, T.; Zamith, H.; Silva, R.; Surrage, A.; Frutuoso, V.; Castro-Faria-Neto, H.; Amendoeira, F. Antinociceptive activity of Stephanolepis hispidus skin aqueous extract depends partly on opioid system activation. Mar. Drugs 2013, 11, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Turk, T.; Ambrozic, A.J.; Batista, U.; Strugar, G.; Kosmina, R.; Civovic, S.; Janussen, D.; Kauferstein, S.; Mebs, D.; Sepcic, K. Biological activities of ethanolic extracts from deep-sea Antarctic marine sponges. Mar. Drugs 2013, 11, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Han, D.; Kim, S.B.; Yoon, M.; Yang, H.; Jin, Y.H.; Jo, J.; Yong, H.; Lee, S.H.; Jeon, Y.J.; et al. Depressive Effects on the Central Nervous System and Underlying Mechanism of the Enzymatic Extract and Its Phlorotannin-Rich Fraction from Ecklonia cava Edible Brown Seaweed. Biosci. Biotechnol. Biochem. 2012, 76, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Christopeit, T.; Overbo, K.; Danielson, U.H.; Nilsen, I.W. Efficient screening of marine extracts for protease inhibitors by combining FRET based activity assays and surface plasmon resonance spectroscopy based binding assays. Mar. Drugs 2013, 11, 4279–4293. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.H.; Nam, S.J.; Locke, J.B.; Kauffman, C.A.; Beatty, D.S.; Paul, L.A.; Fenical, W. Anthracimycin, a potent anthrax antibiotic from a marine-derived actinomycete. Angew. Chem. Int. Ed. Engl. 2013, 52, 7822–7824. [Google Scholar] [CrossRef] [PubMed]
- Keffer, J.L.; Huecas, S.; Hammill, J.T.; Wipf, P.; Andreu, J.M.; Bewley, C.A. Chrysophaentins are competitive inhibitors of FtsZ and inhibit Z-ring formation in live bacteria. Bioorg. Med. Chem. 2013, 21, 5673–5678. [Google Scholar] [CrossRef] [PubMed]
- Sakoulas, G.; Nam, S.J.; Loesgen, S.; Fenical, W.; Jensen, P.R.; Nizet, V.; Hensler, M. Novel Bacterial Metabolite Merochlorin A Demonstrates in vitro Activity against Multi-Drug Resistant Methicillin-Resistant Staphylococcus aureus. PLoS ONE 2012, 7, e29439. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Z.; Qu, H.J.; Liu, P.; Miao, C.; Zhu, T.; Li, J.; Hong, K.; Zhu, W. Antimicrobial aflatoxins from the marine-derived fungus Aspergillus flavus 092008. Arch. Pharm. Res. 2012, 35, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hamann, M.T.; Zou, Y.; Zhang, M.Y.; Gong, X.B.; Xiao, J.R.; Chen, W.S.; Lin, H.W. Antimicrobial metabolites from the Paracel Islands sponge Agelas mauritiana. J. Nat. Prod. 2012, 75, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Shao, C.L.; Guo, Z.Y.; Chen, J.F.; Deng, D.S.; Yang, K.L.; Chen, Y.Y.; Fu, X.M.; She, Z.G.; Lin, Y.C.; et al. Bioactive hydroanthraquinones and anthraquinone dimers from a soft coral-derived Alternaria sp. fungus. J. Nat. Prod. 2012, 75, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Xu, Y.; McConnell, O.; Liu, L.; Li, Y.; Qi, S.; Huang, X.; Qian, P. Two antimycin A analogues from marine-derived actinomycete Streptomyces lusitanus. Mar. Drugs 2012, 10, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, Y.; Shao, C.L.; Yang, R.Y.; Zheng, C.J.; Chen, Y.Y.; Fu, X.M.; Qian, P.Y.; She, Z.G.; de Voogd, N.J.; et al. Antibacterial bisabolane-type sesquiterpenoids from the sponge-derived fungus Aspergillus sp. Mar. Drugs 2012, 10, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Tang, Y.; Zhang, Q.; Bourne, G.T.; Arm, C.A.; Leet, J.E.; Knight, J.C.; Pettit, R.K.; Chapuis, J.C.; Doubek, D.L.; et al. Isolation and structures of axistatins 1–3 from the Republic of Palau marine sponge Agelas axifera Hentschel. J. Nat. Prod. 2013, 76, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, M.E.; Shearer, T.L.; Engel, S.; Alexander, T.S.; Fairchild, C.R.; Prudhomme, J.; Torres, M.; Le, R.K.; Aalbersberg, W.; Hay, M.E.; et al. Bromophycoic acids: Bioactive natural products from a Fijian red alga Callophycus sp. J. Org. Chem. 2012, 77, 8000–8006. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kim, H.; Nam, S.J.; Rho, B.J.; Kang, H. Antibacterial butenolides from the Korean tunicate Pseudodistoma antinboja. J. Nat. Prod. 2012, 75, 2049–2054. [Google Scholar] [CrossRef] [PubMed]
- Won, T.H.; Jeon, J.E.; Kim, S.H.; Lee, S.H.; Rho, B.J.; Oh, D.C.; Oh, K.B.; Shin, J. Brominated aromatic furanones and related esters from the ascidian Synoicum sp. J. Nat. Prod. 2012, 75, 2055–2061. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Xu, Y.; Han, Z.; Li, Y.X.; Lu, L.; Lai, P.Y.; Zhong, J.L.; Guo, X.R.; Zhang, X.X.; Qian, P.Y. Four new antibacterial xanthones from the marine-derived actinomycetes Streptomyces caelestis. Mar. Drugs 2012, 10, 2571–2583. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, P.; Wang, Y.; Wang, H.; Li, J.; Zhuang, Y.; Zhu, W. Antimicrobial Aromatic Polyketides from Gorgonian-Associated Fungus, Penicillium commune 518. Chin. J. Chem. 2012, 30, 1236–1242. [Google Scholar] [CrossRef]
- Ioannou, E.; Quesada, A.; Rahman, M.M.; Gibbons, S.; Vagias, C.; Roussis, V. Structures and Antibacterial Activities of Minor Dolabellanes from the Brown Alga Dilophus spiralis. Eur. J. Org. Chem. 2012, 2012, 5177–5186. [Google Scholar] [CrossRef]
- Felder, S.; Kehraus, S.; Neu, E.; Bierbaum, G.; Schaberle, T.F.; Konig, G.M. Salimyxins and enhygrolides: Antibiotic, sponge-related metabolites from the obligate marine myxobacterium Enhygromyxa salina. Chembiochem 2013, 14, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Won, T.H.; Jeon, J.E.; Lee, S.H.; Rho, B.J.; Oh, K.B.; Shin, J. Beta-carboline alkaloids derived from the ascidian Synoicum sp. Bioorg. Med. Chem. 2012, 20, 4082–4087. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Ye, X.; Yu, S.; Lian, X.Y.; Zhang, Z. New capoamycin-type antibiotics and polyene acids from marine Streptomyces fradiae PTZ0025. Mar. Drugs 2012, 10, 2388–2402. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jang, K.H.; Jeon, J.E.; Yang, W.Y.; Sim, C.J.; Oh, K.B.; Shin, J. Cyclic Bis-1,3-dialkylpyridiniums from the sponge Haliclona sp. Mar. Drugs 2012, 10, 2126–2137. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Momose, R.; Takahashi, Y.; Kubota, T.; Takahashi-Nakaguchi, A.; Gonoi, T.; Fromont, J.; Kobayashi, J. Hyrtimomines D and E, bisindole alkaloids from a marine sponge Hyrtios sp. Tetrahedron Lett. 2013, 54, 4038–4040. [Google Scholar] [CrossRef]
- Xu, M.; Davis, R.A.; Feng, Y.; Sykes, M.L.; Shelper, T.; Avery, V.M.; Camp, D.; Quinn, R.J. Ianthelliformisamines A–C, antibacterial bromotyrosine-derived metabolites from the marine sponge Suberea ianthelliformis. J. Nat. Prod. 2012, 75, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; da S Sousa, T.; Crespo, G.; Palomo, S.; Gonzalez, I.; Tormo, J.R.; de la Cruz, M.; Anderson, M.; Hill, R.T.; Vicente, F.; et al. Kocurin, the true structure of PM181104, an anti-methicillin-resistant Staphylococcus aureus (MRSA) thiazolyl peptide from the marine-derived bacterium Kocuria palustris. Mar. Drugs 2013, 11, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Conte, M.M.; Huang, X.C.; Khalil, Z.; Capon, R.J. A search for BACE inhibitors reveals new biosynthetically related pyrrolidones, furanones and pyrroles from a southern Australian marine sponge, Ianthella sp. Org. Biomol. Chem. 2012, 10, 2656–2663. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Miao, F.P.; Li, K.; Ji, N.Y. Sesquiterpenes and acetogenins from the marine red alga Laurencia okamurai. Fitoterapia 2012, 83, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.Q.; Zhang, S.Y.; Wang, N.; Li, Z.L.; Hua, H.M.; Hu, J.C.; Wang, S.J. New spirotetronate antibiotics, lobophorins H and I, from a South China Sea-derived Streptomyces sp. 12A35. Mar. Drugs 2013, 11, 3891–3901. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Huang, H.; Chen, Y.; Tan, J.; Song, Y.; Zou, J.; Tian, X.; Hua, Y.; Ju, J. Marthiapeptide A, an anti-infective and cytotoxic polythiazole cyclopeptide from a 60 L scale fermentation of the deep sea-derived Marinactinospora thermotolerans SCSIO 00652. J. Nat. Prod. 2012, 75, 2251–2255. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, S.; Li, J.; Chen, Y.; Saurav, K.; Zhang, Q.; Zhang, H.; Zhang, W.; Zhang, W.; Zhang, S.; et al. Antibacterial and cytotoxic new napyradiomycins from the marine-derived Streptomyces sp. SCSIO 10428. Mar. Drugs 2013, 11, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.B.; Jensen, P.R.; Fenical, W. Cytotoxic and Antimicrobial Napyradiomycins from Two Marine-Derived Streptomyces Strains. Eur. J. Org. Chem. 2013, 3751–3757. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.L.; Wei, M.Y.; Shao, C.L.; Fu, X.M.; Guo, Z.Y.; Xu, R.F.; Zheng, C.J.; She, Z.G.; Lin, Y.C.; Wang, C.Y. Antibacterial anthraquinone derivatives from a sea anemone-derived fungus Nigrospora sp. J. Nat. Prod. 2012, 75, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Um, S.; Choi, T.J.; Kim, H.; Kim, B.Y.; Kim, S.H.; Lee, S.K.; Oh, K.B.; Shin, J.; Oh, D.C. Ohmyungsamycins A and B: Cytotoxic and Antimicrobial Cyclic Peptides Produced by Streptomyces sp. from a Volcanic Island. J. Org. Chem. 2013, 78, 12321–12329. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Shao, C.L.; Li, Z.Y.; Gan, L.S.; Fu, X.M.; Bian, W.T.; Zhao, H.Y.; Wang, C.Y. Isocoumarin derivatives and benzofurans from a sponge-derived Penicillium sp. fungus. J. Nat. Prod. 2013, 76, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Rubiolo, J.A.; Ternon, E.; Lopez-Alonso, H.; Thomas, O.P.; Vega, F.V.; Vieytes, M.R.; Botana, L.M. Crambescidin-816 acts as a fungicidal with more potency than crambescidin-800 and -830, inducing cell cycle arrest, increased cell size and apoptosis in Saccharomyces cerevisiae. Mar. Drugs 2013, 11, 4419–4434. [Google Scholar] [CrossRef] [PubMed]
- Yibmantasiri, P.; Leahy, D.C.; Busby, B.P.; Angermayr, S.A.; Sorgo, A.G.; Boeger, K.; Heathcott, R.; Barber, J.M.; Moraes, G.; Matthews, J.H.; et al. Molecular basis for fungicidal action of neothyonidioside, a triterpene glycoside from the sea cucumber, Australostichopus mollis. Mol. Biosyst. 2012, 8, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Subramani, R.; Feussner, K.D.; Aalbersberg, W. Aurantoside K, a new antifungal tetramic acid glycoside from a Fijian marine sponge of the genus Melophlus. Mar. Drugs 2012, 10, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.H.; Liu, D.Q.; Liang, T.J.; Yu, X.Q.; Feng, M.T.; Yao, L.G.; Fang, Y.; Wang, B.; Feng, L.H.; Zhang, M.X.; et al. Caulerprenylols A and B, two rare antifungal prenylated para-xylenes from the green alga Caulerpa racemosa. Bioorg. Med. Chem. Lett. 2013, 23, 2491–2494. [Google Scholar] [CrossRef] [PubMed]
- Haga, A.; Tamoto, H.; Ishino, M.; Kimura, E.; Sugita, T.; Kinoshita, K.; Takahashi, K.; Shiro, M.; Koyama, K. Pyridone alkaloids from a marine-derived fungus, Stagonosporopsis cucurbitacearum, and their activities against azole-resistant Candida albicans. J. Nat. Prod. 2013, 76, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.J.; Song, Y.L.; Jiao, W.H.; Yang, F.; Liu, X.F.; Chen, W.S.; Han, B.N.; Lin, H.W. Hippolachnin A, a New Antifungal Polyketide from the South China Sea Sponge Hippospongia lachne. Org. Lett. 2013, 15, 3526–3529. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H.; Yuan, W.; Gong, W.; Tang, H.; Liu, B.; Krohn, K.; Li, L.; Yi, Y.; Zhang, W. Antifungal nortriterpene and triterpene glycosides from the sea cucumber Apostichopus japonicus Selenka. Food Chem. 2013, 132, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Kusama, T.; Takahashi-Nakaguchi, A.; Gonoi, T.; Fromont, J.; Kobayashi, J. Nagelamides X–Z, dimeric bromopyrrole alkaloids from a marine sponge Agelas sp. Org. Lett. 2013, 15, 3262–3265. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.B.; Liu, X.F.; Xu, Y.; Gan, J.H.; Jiao, W.H.; Shen, Y.; Lin, H.W. Woodylides A–C, new cytotoxic linear polyketides from the South China Sea sponge Plakortis simplex. Mar. Drugs 2012, 10, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Mani, L.; Jullian, V.; Mourkazel, B.; Valentin, A.; Dubois, J.; Cresteil, T.; Folcher, E.; Hooper, J.N.; Erpenbeck, D.; Aalbersberg, W.; et al. New antiplasmodial bromotyrosine derivatives from Suberea ianthelliformis Lendenfeld, 1888. Chem. Biodivers. 2012, 9, 1436–1451. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.F.; Pearce, A.N.; Tan, S.H.; Kaiser, M.; Copp, B.R. Discovery and evaluation of thiazinoquinones as anti-protozoal agents. Mar. Drugs 2013, 11, 3472–3499. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, F.; Grellier, P.; Clement, M.; Roussakis, C.; Loiseau, P.M.; Genin-Seward, E.; Kornprobst, J.M.; Barnathan, G.; Wielgosz-Collin, G. Antimalarial activity of axidjiferosides, new beta-galactosylceramides from the African sponge Axinyssa djiferi. Mar. Drugs 2013, 11, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Beau, J.; Mahid, N.; Burda, W.N.; Harrington, L.; Shaw, L.N.; Mutka, T.; Kyle, D.E.; Barisic, B.; van, O.A.; Baker, B.J. Epigenetic tailoring for the production of anti-infective cytosporones from the marine fungus Leucostoma persoonii. Mar. Drugs 2012, 10, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Calcul, L.; Waterman, C.; Ma, W.S.; Lebar, M.D.; Harter, C.; Mutka, T.; Morton, L.; Maignan, P.; van, O.A.; Kyle, D.E.; et al. Screening mangrove endophytic fungi for antimalarial natural products. Mar. Drugs 2013, 11, 5036–5050. [Google Scholar] [CrossRef] [PubMed]
- Ilias, M.; Ibrahim, M.A.; Khan, S.I.; Jacob, M.R.; Tekwani, B.L.; Walker, L.A.; Samoylenko, V. Pentacyclic ingamine alkaloids, a new antiplasmodial pharmacophore from the marine sponge Petrosid Ng5 Sp5. Planta Med. 2012, 78, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Mudianta, I.W.; Skinner-Adams, T.; Andrews, K.T.; Davis, R.A.; Hadi, T.A.; Hayes, P.Y.; Garson, M.J. Psammaplysin derivatives from the Balinese marine sponge Aplysinella strongylata. J. Nat. Prod. 2012, 75, 2132–2143. [Google Scholar] [CrossRef] [PubMed]
- Sirirak, T.; Brecker, L.; Plubrukarn, A. Kabiramide L, a new antiplasmodial trisoxazole macrolide from the sponge Pachastrissa nux. Nat. Prod. Res. 2013, 27, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.B.; Rammohan, R.Y.; Khan, S.I.; Tekwani, B.L.; Jacob, M.R.; Khan, I.A.; Vishwakarma, R.A. Meridianin G and its analogs as antimalarial agents. Med. Chem. Commun. 2013, 4, 1042–1048. [Google Scholar] [CrossRef]
- Liew, L.P.; Kaiser, M.; Copp, B.R. Discovery and preliminary structure-activity relationship analysis of 1,14-sperminediphenylacetamides as potent and selective antimalarial lead compounds. Bioorg. Med. Chem. Lett. 2013, 23, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Festa, C.; De Marino, S.; D’Auria, M.V.; Taglialatela-Scafati, O.; Deharo, E.; Petek, S.; Zampella, A. New antimalarial polyketide endoperoxides from the marine sponge Plakinastrella mamillaris collected at Fiji Islands. Tetrahedron 2013, 69, 3706–3713. [Google Scholar] [CrossRef]
- Davis, R.A.; Duffy, S.; Fletcher, S.; Avery, V.M.; Quinn, R.J. Thiaplakortones A-D: Antimalarial thiazine alkaloids from the Australian marine sponge Plakortis lita. J. Org. Chem. 2013, 78, 9608–9613. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.A.; Buchanan, M.S.; Duffy, S.; Avery, V.M.; Charman, S.A.; Charman, W.N.; White, K.L.; Shackleford, D.M.; Edstein, M.D.; Andrews, K.T.; et al. Antimalarial activity of pyrroloiminoquinones from the Australian marine sponge Zyzzya sp. J. Med. Chem. 2012, 55, 5851–5858. [Google Scholar] [CrossRef] [PubMed]
- Supong, K.; Thawai, C.; Suwanborirux, K.; Choowong, W.; Supothina, S.; Pittayakhajonwut, P. Antimalarial and antitubercular C-glycosylated benz[a]anthraquinones from the marine-derived Streptomyces sp. BCC45596. Phytochem. Lett. 2012, 5, 651–656. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Knudsen, G.M.; Helbig, C.; De, M.G.; Mascuch, S.M.; Mackey, Z.B.; Gerwick, L.; Clayton, C.; McKerrow, J.H.; Linington, R.G. Examination of the mode of action of the almiramide family of natural products against the kinetoplastid parasite Trypanosoma brucei. J. Nat. Prod. 2013, 76, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Szesny, M.; Othman, E.M.; Schirmeister, T.; Grond, S.; Stopper, H.; Hentschel, U. Antioxidant and anti-protease activities of diazepinomicin from the sponge-associated Micromonospora strain RV115. Mar. Drugs 2012, 10, 2208–2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desoti, V.C.; Lazarin-Bidoia, D.; Sudatti, D.B.; Pereira, R.C.; Alonso, A.; Ueda-Nakamura, T.; Dias Filho, B.P.; Nakamura, C.V.; Silva, S.O. Trypanocidal action of (−)-elatol involves an oxidative stress triggered by mitochondria dysfunction. Mar. Drugs 2012, 10, 1631–1646. [Google Scholar] [CrossRef] [PubMed]
- Balunas, M.J.; Grosso, M.F.; Villa, F.A.; Engene, N.; McPhail, K.L.; Tidgewell, K.; Pineda, L.M.; Gerwick, L.; Spadafora, C.; Kyle, D.E.; et al. Coibacins A–D, antileishmanial marine cyanobacterial polyketides with intriguing biosynthetic origins. Org. Lett. 2012, 14, 3878–3881. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, S.T.; Goto, Y.; Inoue, N.; Kawazu, S.; Matsumoto, Y.; Imahara, Y.; Tarumi, M.; Nakai, H.; Fusetani, N.; Nakao, Y. Cristaxenicin A, an antiprotozoal xenicane diterpenoid from the deep sea gorgonian Acanthoprimnoa cristata. J. Org. Chem. 2012, 77, 10962–10966. [Google Scholar] [CrossRef] [PubMed]
- Chianese, G.; Scala, F.; Calcinai, B.; Cerrano, C.; Dien, H.A.; Kaiser, M.; Tasdemir, D.; Taglialatela-Scafati, O. Natural and semisynthetic analogues of manadoperoxide B reveal new structural requirements for trypanocidal activity. Mar. Drugs 2013, 11, 3297–3308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Huang, H.; Li, H.; Sun, X.; Huang, H.; Lu, Y.; Lin, Y.; Long, Y.; She, Z. Asperterpenoid A, a new sesterterpenoid as an inhibitor of Mycobacterium tuberculosis protein tyrosine phosphatase B from the culture of Aspergillus sp. 16–5c. Org. Lett. 2013, 15, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Liu, X.; Guo, H.; Ren, B.; Chen, C.; Piggott, A.M.; Yu, K.; Gao, H.; Wang, Q.; Liu, M.; et al. Brevianamides with antitubercular potential from a marine-derived isolate of Aspergillus versicolor. Org. Lett. 2012, 14, 4770–4773. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, J.; Guo, H.; Hou, W.; Yang, N.; Ren, B.; Liu, M.; Dai, H.; Liu, X.; Song, F.; et al. Three antimycobacterial metabolites identified from a marine-derived Streptomyces sp. MS100061. Appl. Microbiol. Biotechnol. 2013, 97, 3885–3892. [Google Scholar] [CrossRef] [PubMed]
- Yamano, Y.; Arai, M.; Kobayashi, M. Neamphamide B, new cyclic depsipeptide, as an anti-dormant mycobacterial substance from a Japanese marine sponge of Neamphius sp. Bioorg. Med. Chem. Lett. 2012, 22, 4877–4881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aviles, E.; Rodriguez, A.D.; Vicente, J. Two rare-class tricyclic diterpenes with antitubercular activity from the Caribbean sponge Svenzea flava. Application of vibrational circular dichroism spectroscopy for determining absolute configuration. J. Org. Chem. 2013, 78, 11294–11301. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa Guimaraes, T.; Quiroz, C.G.; Borges, C.R.; de Oliveira, S.Q.; de Almeida, M.T.; Bianco, E.M.; Moritz, M.I.; Carraro, J.L.; Palermo, J.A.; Cabrera, G.; et al. Anti HSV-1 activity of halistanol sulfate and halistanol sulfate C isolated from Brazilian marine sponge Petromica citrina (Demospongiae). Mar. Drugs 2013, 11, 4176–4192. [Google Scholar] [CrossRef] [PubMed]
- Ellithey, M.S.; Lall, N.; Hussein, A.A.; Meyer, D. Cytotoxic, cytostatic and HIV-1 PR inhibitory activities of the soft coral Litophyton arboreum. Mar. Drugs 2013, 11, 4917–4936. [Google Scholar] [CrossRef] [PubMed]
- Salam, K.A.; Furuta, A.; Noda, N.; Tsuneda, S.; Sekiguchi, Y.; Yamashita, A.; Moriishi, K.; Nakakoshi, M.; Tsubuki, M.; Tani, H.; et al. Inhibition of hepatitis C virus NS3 helicase by manoalide. J. Nat. Prod. 2012, 75, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Song, J.H.; Kim, T.; Shin, W.S.; Park, G.M.; Lee, S.; Kim, Y.J.; Choi, P.; Kim, H.; Kim, H.S.; et al. Anti-human rhinoviral activity of polybromocatechol compounds isolated from the rhodophyta, Neorhodomela aculeata. Mar. Drugs 2012, 10, 2222–2233. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, L.; Zhu, T.; Ba, M.; Li, G.; Gu, Q.; Guo, Y.; Li, D. Phenylspirodrimanes with anti-HIV activity from the sponge-derived fungus Stachybotrys chartarum MXH-X73. J. Nat. Prod. 2013, 76, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.H.; Xu, H.; Cui, J.T.; Ge, H.M.; Tan, R.X. Neuraminidase Inhibitors from marine-derived actinomycete Streptomyces seoulensis. J. Appl. Microbiol. 2013, 114, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Bao, J.; Zhang, X.Y.; Tu, Z.C.; Shi, Y.M.; Qi, S.H. Asperterrestide A, a cytotoxic cyclic tetrapeptide from the marine-derived fungus Aspergillus terreus SCSGAF0162. J. Nat. Prod. 2013, 76, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Lin, T.; Wang, W.; Xin, Z.; Zhu, T.; Gu, Q.; Li, D. Antiviral alkaloids produced by the mangrove-derived fungus Cladosporium sp. PJX-41. J. Nat. Prod. 2013, 76, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Hawas, U.W.; El-Beih, A.A.; El-Halawany, A.M. Bioactive anthraquinones from endophytic fungus Aspergillus versicolor isolated from red sea algae. Arch. Pharm. Res. 2012, 35, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.F.; Han, W.B.; Cui, J.T.; Ng, S.W.; Guo, Z.K.; Tan, R.X.; Ge, H.M. Neuraminidase inhibitory polyketides from the marine-derived fungus Phoma herbarum. Planta Med. 2012, 78, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Jiao, J.; Li, J.; Wang, W.; Gu, Q.; Zhu, T.; Li, D. Pyronepolyene C-glucosides with NF-kappaB inhibitory and anti-influenza A viral (H1N1) activities from the sponge-associated fungus Epicoccum sp. JJY40. Bioorg. Med. Chem. Lett. 2012, 22, 3188–3190. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ibrahim, A.; Satar Arafa, A. Anti-H5N1 virus metabolites from the Red Sea soft coral, Sinularia candidula. Tetrahedron Lett. 2013, 54, 2377–2381. [Google Scholar] [CrossRef]
- Plouguerne, E.; de Souza, L.M.; Sassaki, G.L.; Cavalcanti, J.F.; Villela Romanos, M.T.; da Gama, B.A.; Pereira, R.C.; Barreto-Bergter, E. Antiviral Sulfoquinovosyldiacylglycerols (SQDGs) from the Brazilian brown seaweed Sargassum vulgare. Mar. Drugs 2013, 11, 4628–4640. [Google Scholar] [CrossRef] [PubMed]
- Melek, F.R.; Tadros, M.M.; Yousif, F.; Selim, M.A.; Hassan, M.H. Screening of marine extracts for schistosomicidal activity in vitro. Isolation of the triterpene glycosides echinosides A and B with potential activity from the Sea Cucumbers Actinopyga echinites and Holothuria polii. Pharm. Biol. 2012, 50, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yu, S.; Van, O.L.; Proksch, P.; Lin, W. Anthogorgienes A–O, new guaiazulene-derived terpenoids from a Chinese gorgonian Anthogorgia species, and their antifouling and antibiotic activities. J. Agric. Food Chem. 2012, 60, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, G.; Ciavatta, M.L.; Villani, G.; Manzo, E.; Zanfardino, A.; Varcamonti, M.; Gavagnin, M. Fulvynes, antimicrobial polyoxygenated acetylenes from the Mediterranean sponge Haliclona fulva. Tetrahedron 2012, 68, 754–760. [Google Scholar] [CrossRef]
- Cheng, Z.B.; Xiao, H.; Fan, C.Q.; Lu, Y.N.; Zhang, G.; Yin, S. Bioactive polyhydroxylated sterols from the marine sponge Haliclona crassiloba. Steroids 2013, 78, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, K.; MacMillan, J.B. Hunanamycin A, an antibiotic from a marine-derived Bacillus hunanensis. Org. Lett. 2013, 15, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Khamthong, N.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Bioactive polyketides from the sea fan-derived fungus Penicillium citrinum PSU-F51. Tetrahedron 2012, 68, 8245–8250. [Google Scholar] [CrossRef]
- Wei, M.Y.; Li, D.; Shao, C.L.; Deng, D.S.; Wang, C.Y. (+/−)-Pestalachloride D, an antibacterial racemate of chlorinated benzophenone derivative from a soft coral-derived fungus Pestalotiopsis sp. Mar. Drugs 2013, 11, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Wyche, T.P.; Hou, Y.; Vazquez-Rivera, E.; Braun, D.; Bugni, T.S. Peptidolipins B–F, antibacterial lipopeptides from an ascidian-derived Nocardia sp. J. Nat. Prod. 2012, 75, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and biological action of cucumariosides I1, I3, I4, three new minor disulfated pentaosides. Nat. Prod. Commun. 2013, 8, 1053–1058. [Google Scholar] [PubMed]
- Sun, H.F.; Li, X.M.; Meng, L.; Cui, C.M.; Gao, S.S.; Li, C.S.; Huang, C.G.; Wang, B.G. Asperolides A–C, tetranorlabdane diterpenoids from the marine alga-derived endophytic fungus Aspergillus wentii EN-48. J. Nat. Prod. 2012, 75, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yin, L.; Gao, L.; Gao, J.; Chen, J.; Li, J.; Song, F. Two new bromophenols with radical scavenging activity from marine red alga Symphyocladia latiuscula. Mar. Drugs 2013, 11, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, S.M.; Ko, S.C.; Lee, D.H.; Jeon, Y.J. Octaphlorethol A, a novel phenolic compound isolated from a brown alga, Ishige foliacea, increases glucose transporter 4-mediated glucose uptake in skeletal muscle cells. Biochem. Biophys. Res. Commun. 2012, 420, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Chae, D.; Manzoor, Z.; Kim, S.C.; Kim, S.; Oh, T.H.; Yoo, E.S.; Kang, H.K.; Hyun, J.W.; Lee, N.H.; Ko, M.H.; et al. Apo-9′-fucoxanthinone, isolated from Sargassum muticum, inhibits CpG-induced inflammatory response by attenuating the mitogen-activated protein kinase pathway. Mar. Drugs 2013, 11, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; Pesce, M.; Patruno, A.; Franceschelli, S.; de Lutiis, M.A.; Grilli, A.; Felaco, M. Astaxanthin treatment reduced oxidative induced pro-inflammatory cytokines secretion in U937: SHP-1 as a novel biological target. Mar. Drugs 2012, 10, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.A.; Sohn, J.; Vaske, Y.M.; White, K.N.; Cohen, T.L.; Vervoort, H.C.; Tenney, K.; Valeriote, F.A.; Bjeldanes, L.F.; Crews, P. Myxobacteria versus sponge-derived alkaloids: The bengamide family identified as potent immune modulating agents by scrutiny of LC-MS/ELSD libraries. Bioorg. Med. Chem. 2012, 20, 4348–4355. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Dou, H.; Gong, W.; Liu, X.; Yu, Z.; Li, E.; Tan, R.; Hou, Y. Bis-N-norgliovictin, a small-molecule compound from marine fungus, inhibits LPS-induced inflammation in macrophages and improves survival in sepsis. Eur. J. Pharmacol. 2013, 705, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.I.; Shin, H.C.; Kim, S.H.; Park, W.Y.; Lee, K.T.; Choi, J.H. 6,6′-Bieckol, isolated from marine alga Ecklonia cava, suppressed LPS-induced nitric oxide and PGE(2) production and inflammatory cytokine expression in macrophages: The inhibition of NFkappaB. Int. Immunopharmacol. 2012, 12, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.L.; Chiu, S.J.; Tsai, Y.T.; Chang, C.M.; Wang, J.Y.; Wang, E.T.; Hou, M.F.; Huang, C.Y.; Sheu, J.H.; Chang, W.C. A soft coral natural product, 11-episinulariolide acetate, inhibits gene expression of cyclooxygenase-2 and interleukin-8 through attenuation of calcium signaling. Molecules 2013, 18, 7023–7034. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Mascuch, S.J.; Villa, F.A.; Byrum, T.; Teasdale, M.E.; Smith, J.E.; Preskitt, L.B.; Rowley, D.C.; Gerwick, L.; Gerwick, W.H. Honaucins A–C, potent inhibitors of inflammation and bacterial quorum sensing: Synthetic derivatives and structure-activity relationships. Chem. Biol. 2012, 19, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Aviles, E.; Rodriguez, A.D. Marine sponge Hymeniacidon sp. amphilectane metabolites potently inhibit rat brain microglia thromboxane B2 generation. Bioorg. Med. Chem. 2012, 20, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Riegsecker, S.; Beamer, M.; Rahman, A.; Bellini, J.V.; Bhansali, P.; Tillekeratne, L.M. Largazole, a class I histone deacetylase inhibitor, enhances TNF-alpha-induced ICAM-1 and VCAM-1 expression in rheumatoid arthritis synovial fibroblasts. Toxicol. Appl. Pharmacol. 2013, 270, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Huang, S.Y.; Lin, Y.Y.; Wang, H.M.; Jean, Y.H.; Wu, S.F.; Duh, C.Y.; Wen, Z.H. Soft coral-derived lemnalol alleviates monosodium urate-induced gouty arthritis in rats by inhibiting leukocyte infiltration and iNOS, COX-2 and c-Fos protein expression. Mar. Drugs 2013, 11, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Cui, X.; Lee, D.S.; Sohn, J.H.; Yim, J.H.; Kim, Y.C.; Oh, H. Anti-inflammatory effect of neoechinulin a from the marine fungus Eurotium sp. SF-5989 through the suppression of NF-small ka, CyrillicB and p38 MAPK Pathways in lipopolysaccharide-stimulated RAW264.7 macrophages. Molecules 2013, 18, 13245–13259. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Jang, J.H.; Ko, W.; Kim, K.S.; Sohn, J.H.; Kang, M.S.; Ahn, J.S.; Kim, Y.C.; Oh, H. PTP1B inhibitory and anti-inflammatory effects of secondary metabolites isolated from the marine-derived fungus Penicillium sp. JF-55. Mar. Drugs 2013, 11, 1409–1426. [Google Scholar] [CrossRef] [PubMed]
- Vilasi, A.; Monti, M.C.; Tosco, A.; De Marino, S.; Margarucci, L.; Riccio, R.; Casapullo, A. Differential in gel electrophoresis (DIGE) comparative proteomic analysis of macrophages cell cultures in response to perthamide C treatment. Mar. Drugs 2013, 11, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Reina, E.; Ramos, F.A.; Castellanos, L.; Aragon, M.; Ospina, L.F. Anti-inflammatory R-prostaglandins from Caribbean Colombian soft coral Plexaura homomalla. J. Pharm. Pharmacol. 2013, 65, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Chen, N.F.; Chen, W.F.; Hung, H.C.; Lee, H.P.; Lin, Y.Y.; Wang, H.M.; Sung, P.J.; Sheu, J.H.; Wen, Z.H. Sinularin from indigenous soft coral attenuates nociceptive responses and spinal neuroinflammation in carrageenan-induced inflammatory rat model. Mar. Drugs 2012, 10, 1899–1919. [Google Scholar] [CrossRef] [PubMed]
- De, M.S.; Ummarino, R.; D’Auria, M.V.; Chini, M.G.; Bifulco, G.; D’Amore, C.; Renga, B.; Mencarelli, A.; Petek, S.; Fiorucci, S.; et al. 4-Methylenesterols from Theonella swinhoei sponge are natural pregnane-X-receptor agonists and farnesoid-X-receptor antagonists that modulate innate immunity. Steroids 2012, 77, 485–495. [Google Scholar]
- Thao, N.P.; Cuong, N.X.; Luyen, B.T.; Quang, T.H.; Hanh, T.T.; Kim, S.; Koh, Y.S.; Nam, N.H.; Van, K.P.; Van, M.C.; et al. Anti-inflammatory components of the starfish Astropecten polyacanthus. Mar. Drugs 2013, 11, 2917–2926. [Google Scholar] [CrossRef] [PubMed]
- Lind, K.F.; Hansen, E.; Osterud, B.; Eilertsen, K.E.; Bayer, A.; Engqvist, M.; Leszczak, K.; Jorgensen, T.O.; Andersen, J.H. Antioxidant and anti-inflammatory activities of barettin. Mar. Drugs 2013, 11, 2655–2666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, P.H.; Su, Y.D.; Su, J.H.; Chen, Y.H.; Hwang, T.L.; Weng, C.F.; Lee, C.H.; Wen, Z.H.; Sheu, J.H.; Lin, N.C.; et al. Briarenolides F and G, new briarane diterpenoids from a Briareum sp. octocoral. Mar. Drugs 2012, 10, 1156–1168. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Lan, X.P.; Liu, Y.; Jia, A.Q. The effects of diketopiperazines from Callyspongia sp. on release of cytokines and chemokines in cultured J774A.1 macrophages. Bioorg. Med. Chem. Lett. 2012, 22, 3177–3180. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Hwang, T.L.; Su, Y.D.; Chang, Y.C.; Chen, Y.H.; Hong, P.H.; Hu, L.C.; Yen, W.H.; Hsu, H.Y.; Huang, S.J.; et al. New 6-hydroxyeunicellins from a soft coral Cladiella sp. Chem. Pharm. Bull. 2012, 60, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H.; Chou, K.J.; Huang, C.Y.; Wen, Z.H.; Hsu, C.H.; Wu, Y.C.; Dai, C.F.; Sheu, J.H. Steroids from the soft coral Sinularia crassa. Mar. Drugs 2012, 10, 439–450. [Google Scholar] [CrossRef] [PubMed]
- De Los, R.C.; Zbakh, H.; Motilva, V.; Zubia, E. Antioxidant and anti-inflammatory meroterpenoids from the brown alga Cystoseira usneoides. J. Nat. Prod. 2013, 76, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.S.; Oh, J.S.; Jeong, E.J.; Sim, C.J.; Rho, J.R. Densanins A and B, new macrocyclic pyrrole alkaloids isolated from the marine sponge Haliclona densaspicula. Org. Lett. 2012, 14, 6154–6157. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.P.; Nam, N.H.; Cuong, N.X.; Tai, H.; Quang, T.H.; Ngan, N.T.; Luyen, B.T.T.; Yang, S.Y.; Choi, C.H.; Kim, S.; et al. Steroidal Constituents from the Soft Coral Sinularia dissecta and Their Inhibitory Effects on Lipopolysaccharide-Stimulated Production of Pro-inflammatory Cytokines in Bone Marrow-Derived Dendritic Cells. Bull. Korean Chem. Soc. 2013, 34, 949–952. [Google Scholar]
- Chung, H.M.; Hu, L.C.; Yen, W.H.; Su, J.H.; Lu, M.C.; Hwang, T.L.; Wang, W.H.; Sung, P.J. Echinohalimane A, a bioactive halimane-type diterpenoid from a Formosan gorgonian Echinomuricea sp. (Plexauridae). Mar. Drugs 2012, 10, 2246–2253. [Google Scholar] [CrossRef] [PubMed]
- Marchbank, D.H.; Berrue, F.; Kerr, R.G. Eunicidiol, an anti-inflammatory dilophol diterpene from Eunicea fusca. J. Nat. Prod. 2012, 75, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.J.; Tseng, Y.J.; Huang, C.Y.; Wen, Z.H.; Dai, C.F.; Sheu, J.H. Cytotoxic and anti-inflammatory diterpenoids from the Dongsha Atoll soft coral Sinularia flexibilis. Tetrahedron 2013, 68, 244–249. [Google Scholar] [CrossRef]
- Lin, Y.F.; Kuo, C.Y.; Wen, Z.H.; Lin, Y.Y.; Wang, W.H.; Su, J.H.; Sheu, J.H.; Sung, P.J. Flexibilisquinone, a new anti-inflammatory quinone from the cultured soft coral Sinularia flexibilis. Molecules 2013, 18, 8160–8167. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kao, C.Y.; Kao, S.Y.; Chang, C.H.; Su, J.H.; Hwang, T.L.; Kuo, Y.H.; Wen, Z.H.; Sung, P.J. Terpenoids from the octocorals Menella sp. (Plexauridae) and Lobophytum crassum (Alcyonacea). Mar. Drugs 2012, 10, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Festa, C.; De Marino, S.; D’Auria, M.V.; Monti, M.C.; Bucci, M.; Vellecco, V.; Debitus, C.; Zampella, A. Anti-inflammatory cyclopeptides from the marine sponge Theonella swinhoei. Tetrahedron 2012, 68, 2851–2857. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lu, M.C.; Chang, Y.C.; Hwang, T.L.; Wang, W.H.; Weng, C.F.; Kuo, J.; Sung, P.J. Pseudoalteromone A: A novel bioactive ubiquinone from a marine bacterium Pseudoalteromonas sp. CGH2XX (Pseudoalteromonadaceae). Tetrahedron Lett. 2012, 53, 1675–1677. [Google Scholar] [CrossRef]
- Lin, W.Y.; Lu, Y.; Chen, B.W.; Huang, C.Y.; Su, J.H.; Wen, Z.H.; Dai, C.F.; Kuo, Y.H.; Sheu, J.H. Sarcocrassocolides M–O, bioactive cembranoids from the Dongsha Atoll soft coral Sarcophyton crassocaule. Mar. Drugs 2012, 10, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.Y.; Hsu, C.H.; Chao, C.H.; Wen, Z.H.; Wu, Y.C.; Dai, C.F.; Sheu, J.H. Cytotoxic and anti-inflammatory metabolites from the soft coral Scleronephthya gracillimum. Mar. Drugs 2013, 11, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Reyes, J.F.; Salazar, A.; Guzman, H.M.; Gonzalez, Y.; Fernandez, P.L.; Ariza-Castolo, A.; Gutierrez, M. Seco-Briarellinone and briarellin S, two new eunicellin-based diterpenoids from the Panamanian octocoral Briareum asbestinum. Mar. Drugs 2012, 10, 2608–2617. [Google Scholar] [CrossRef] [PubMed]
- Putra, M.Y.; Ianaro, A.; Panza, E.; Bavestrello, G.; Cerrano, C.; Fattorusso, E.; Taglialatela-Scafati, O. Sinularioside, a triacetylated glycolipid from the Indonesian soft coral Sinularia sp., is an inhibitor of NO release. Bioorg. Med. Chem. Lett. 2012, 22, 2723–2725. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Lu, M.C.; Su, J.H.; Chu, C.L.; Shiuan, D.; Weng, C.F.; Sung, P.J.; Huang, K.J. Immunomodulatory effect of marine cembrane-type diterpenoids on dendritic cells. Mar. Drugs 2013, 11, 1336–1350. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zheng, Z.; Huang, H.; Song, Y.; Zhang, X.; Ma, J.; Wang, B.; Zhang, C.; Ju, J. Penicacids A–C, three new mycophenolic acid derivatives and immunosuppressive activities from the marine-derived fungus Penicillium sp. SOF07. Bioorg. Med. Chem. Lett. 2012, 22, 3332–3335. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.E.; Mobli, M.; Brust, A.; Alewood, P.F.; King, G.F.; Rash, L.D. Cyclisation increases the stability of the sea anemone peptide APETx2 but decreases its activity at acid-sensing ion channel 3. Mar. Drugs 2012, 10, 1511–1527. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bowling, J.J.; Fronczek, F.R.; Hong, J.; Jabba, S.V.; Murray, T.F.; Ha, N.C.; Hamann, M.T.; Jung, J.H. Asteropsin A: An unusual cystine-crosslinked peptide from porifera enhances neuronal Ca2+ influx. Biochim. Biophys. Acta 2013, 1830, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Orts, D.J.; Peigneur, S.; Madio, B.; Cassoli, J.S.; Montandon, G.G.; Pimenta, A.M.; Bicudo, J.E.; Freitas, J.C.; Zaharenko, A.J.; Tytgat, J. Biochemical and electrophysiological characterization of two sea anemone type 1 potassium toxins from a geographically distant population of Bunodosoma caissarum. Mar. Drugs 2013, 11, 655–679. [Google Scholar] [CrossRef] [PubMed]
- Favreau, P.; Benoit, E.; Hocking, H.G.; Carlier, L.; D’ hoedt, D.; Leipold, E.; Markgraf, R.; Schlumberger, S.; Cordova, M.A.; Gaertner, H.; et al. A novel micro-conopeptide, CnIIIC, exerts potent and preferential inhibition of NaV1.2/1.4 channels and blocks neuronal nicotinic acetylcholine receptors. Br. J. Pharmacol. 2012, 166, 1654–1668. [Google Scholar] [CrossRef] [PubMed]
- Vetter, I.; Dekan, Z.; Knapp, O.; Adams, D.J.; Alewood, P.F.; Lewis, R.J. Isolation, characterization and total regioselective synthesis of the novel muO-conotoxin MfVIA from Conus magnificus that targets voltage-gated sodium channels. Biochem. Pharmacol. 2012, 84, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Kompella, S.N.; Akondi, K.B.; Melaun, C.; Daly, N.L.; Luetje, C.W.; Alewood, P.F.; Craik, D.J.; Adams, D.J.; Mari, F. RegIIA: An α4/7-conotoxin from the venom of Conus regius that potently blocks α3β4 nAChRs. Biochem. Pharmacol. 2012, 83, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Bernaldez, J.; Roman-Gonzalez, S.A.; Martinez, O.; Jimenez, S.; Vivas, O.; Arenas, I.; Corzo, G.; Arreguin, R.; Garcia, D.E.; Possani, L.D.; et al. A Conus regularis conotoxin with a novel eight-cysteine framework inhibits CaV2.2 channels and displays an anti-nociceptive activity. Mar. Drugs 2013, 11, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, G.S.; Zardo, R.S.; Silva, B.V.; Violante, F.A.; Pinto, A.C.; Fernandes, P.D. Convolutamydine A and synthetic analogues have antinociceptive properties in mice. Pharmacol. Biochem. Behav. 2013, 103, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Andreev, Y.A.; Kozlov, S.A.; Korolkova, Y.V.; Dyachenko, I.A.; Bondarenko, D.A.; Skobtsov, D.I.; Murashev, A.N.; Kotova, P.D.; Rogachevskaja, O.A.; Kabanova, N.V.; et al. Polypeptide modulators of TRPV1 produce analgesia without hyperthermia. Mar. Drugs 2013, 11, 5100–5115. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Bowden, B.F.; Kapoor, V. Ianthellamide A, a selective kynurenine-3-hydroxylase inhibitor from the Australian marine sponge Ianthella quadrangulata. Bioorg. Med. Chem. Lett. 2012, 22, 3398–3401. [Google Scholar] [CrossRef] [PubMed]
- Burgy, G.; Tahtouh, T.; Durieu, E.; Foll-Josselin, B.; Limanton, E.; Meijer, L.; Carreaux, F.; Bazureau, J.P. Chemical synthesis and biological validation of immobilized protein kinase inhibitory Leucettines. Eur. J. Med. Chem. 2013, 62, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Guzii, A.G.; Makarieva, T.N.; Korolkova, Y.V.; Andreev, Y.A.; Mosharova, I.V.; Tabakmaher, K.M.; Denisenko, V.A.; Dmitrenok, P.S.; Ogurtsova, E.L.; Antonov, A.S.; et al. Pulchranin A, isolated from the Far-Eastern marine sponge, Monanchora pulchra: The first marine non-peptide inhibitor of TRPV-1 channels. Tetrahedron Lett. 2013, 54, 1247–1250. [Google Scholar] [CrossRef]
- Montaser, R.; Paul, V.J.; Luesch, H. Marine cyanobacterial fatty acid amides acting on cannabinoid receptors. Chembiochem 2012, 13, 2676–2681. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, X.; Ding, B.; Lin, M.; Liu, L.; Huang, H.; She, Z. A new anti-acetylcholinesterase alpha-pyrone meroterpene, arigsugacin I, from mangrove endophytic fungus Penicillium sp. sk5GW1L of Kandelia candel. Planta Med. 2013, 79, 1572–1575. [Google Scholar] [PubMed]
- Xiao, Z.; Huang, H.; Shao, C.; Xia, X.; Ma, L.; Huang, X.; Lu, Y.; Lin, Y.; Long, Y.; She, Z. Asperterpenols A and B, new sesterterpenoids isolated from a mangrove endophytic fungus Aspergillus sp. 085242. Org. Lett. 2013, 15, 2522–2525. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Proteau, P.J.; Byrum, T.; Pereira, A.R.; Gerwick, W.H. Cymatherelactone and cymatherols A–C, polycyclic oxylipins from the marine brown alga Cymathere triplicata. Phytochemistry 2012, 73, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Conte, M.M.; Khalil, Z.; Huang, X.C.; Capon, R.J. New dictyodendrins as BACE inhibitors from a southern Australian marine sponge, Ianthella sp. RSC Adv. 2012, 2, 4209–4214. [Google Scholar] [CrossRef]
- Ohlendorf, B.; Schulz, D.; Erhard, A.; Nagel, K.; Imhoff, J.F. Geranylphenazinediol, an acetylcholinesterase inhibitor produced by a Streptomyces species. J. Nat. Prod. 2012, 75, 1400–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyche, T.P.; Standiford, M.; Hou, Y.; Braun, D.; Johnson, D.A.; Johnson, J.A.; Bugni, T.S. Activation of the nuclear factor e2-related factor 2 pathway by novel natural products halomadurones a–d and a synthetic analogue. Mar. Drugs 2013, 11, 5089–5099. [Google Scholar] [CrossRef] [PubMed]
- Balansa, W.; Islam, R.; Fontaine, F.; Piggott, A.M.; Zhang, H.; Xiao, X.; Webb, T.I.; Gilbert, D.F.; Lynch, J.W.; Capon, R.J. Sesterterpene glycinyl-lactams: A new class of glycine receptor modulator from Australian marine sponges of the genus Psammocinia. Org. Biomol. Chem. 2013, 11, 4695–4701. [Google Scholar] [CrossRef] [PubMed]
- Palyanova, N.V.; Pankova, T.M.; Starostina, M.V.; Kicha, A.A.; Ivanchina, N.V.; Stonik, V.A. Neuritogenic and neuroprotective effects of polar steroids from the Far East starfishes Patiria pectinifera and Distolasterias nipon. Mar. Drugs 2013, 11, 1440–1455. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Murphy, J.; Macadam, D.; Osterbauer, C.; Baseer, I.; Hall, M.L.; Feher, D.; Williams, P. Classical and Alternative Activation of Cyanobacterium Oscillatoria sp. Lipopolysaccharide-Treated Rat Microglia in vitro. Toxicol. Sci. 2016, 149, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Urbarova, I.; Karlsen, B.O.; Okkenhaug, S.; Seternes, O.M.; Johansen, S.D.; Emblem, A. Digital marine bioprospecting: Mining new neurotoxin drug candidates from the transcriptomes of cold-water sea anemones. Mar. Drugs 2012, 10, 2265–2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dona, G.; Kozuh, I.; Brunati, A.M.; Andrisani, A.; Ambrosini, G.; Bonanni, G.; Ragazzi, E.; Armanini, D.; Clari, G.; Bordin, L. Effect of astaxanthin on human sperm capacitation. Mar. Drugs 2013, 11, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, T.; Mase, N.; Sasaki, H.; Teruya, T.; Hasegawa, S.; Cha, B.Y.; Yagasaki, K.; Suenaga, K.; Nagai, K.; Woo, J.T. Biselyngbyaside, isolated from marine cyanobacteria, inhibits osteoclastogenesis and induces apoptosis in mature osteoclasts. J. Cell. Biochem. 2012, 113, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Shirouzu, T.; Watari, K.; Ono, M.; Koizumi, K.; Saiki, I.; Tanaka, C.; van Soest, R.W.; Miyamoto, T. Structure, synthesis, and biological activity of a C-20 bisacetylenic alcohol from a marine sponge Callyspongia sp. J. Nat. Prod. 2013, 76, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Sepe, V.; Ummarino, R.; D’Auria, M.V.; Chini, M.G.; Bifulco, G.; Renga, B.; D’Amore, C.; Debitus, C.; Fiorucci, S.; Zampella, A. Conicasterol E, a small heterodimer partner sparing farnesoid X receptor modulator endowed with a pregnane X receptor agonistic activity, from the marine sponge Theonella swinhoei. J. Med. Chem. 2012, 55, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.D.; Cheong, O.J.; Bae, S.Y.; Shin, J.; Lee, S.K. 6′′-Debromohamacanthin A, a bis (indole) alkaloid, inhibits angiogenesis by targeting the VEGFR2-mediated PI3K/AKT/mTOR signaling pathways. Mar. Drugs 2013, 11, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Heo, S.J.; Kim, K.N.; Lee, S.H.; Yang, H.M.; Kim, A.D.; Jeon, Y.J. Molecular docking studies of a phlorotannin, dieckol isolated from Ecklonia cava with tyrosinase inhibitory activity. Bioorg. Med. Chem. 2012, 20, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.H.; Lee, Y.R.; Lee, D.S.; Kim, Y.C.; Oh, H. PTP1B inhibitory secondary metabolites from marine-derived fungal strains Penicillium spp. and Eurotium sp. J. Microbiol. Biotechnol. 2013, 23, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Cheung, F.W.; Guo, J.; Ling, Y.H.; Che, C.T.; Liu, W.K. Anti-melanogenic property of geoditin A in murine B16 melanoma cells. Mar. Drugs 2012, 10, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Putra, M.Y.; Bavestrello, G.; Cerrano, C.; Renga, B.; D’Amore, C.; Fiorucci, S.; Fattorusso, E.; Taglialatela-Scafati, O. Polyhydroxylated sterols from the Indonesian soft coral Sinularia sp. and their effect on farnesoid X-activated receptor. Steroids 2012, 77, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Festa, C.; Lauro, G.; De Marino, S.; D’Auria, M.V.; Monti, M.C.; Casapullo, A.; D’Amore, C.; Renga, B.; Mencarelli, A.; Petek, S.; et al. Plakilactones from the marine sponge Plakinastrella mamillaris. Discovery of a new class of marine ligands of peroxisome proliferator-activated receptor gamma. J. Med. Chem. 2012, 55, 8303–8317. [Google Scholar] [CrossRef] [PubMed]
- Festa, C.; D’Amore, C.; Renga, B.; Lauro, G.; De Marino, S.; D’Auria, M.V.; Bifulco, G.; Zampella, A.; Fiorucci, S. Oxygenated polyketides from Plakinastrella mamillaris as a new chemotype of PXR agonists. Mar. Drugs 2013, 11, 2314–2327. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Xiao, B.; Park, M.; Yoo, E.S.; Shin, S.; Hong, J.; Chung, H.Y.; Kim, H.S.; Jung, J.H. PPAR-gamma agonistic metabolites from the ascidian Herdmania momus. J. Nat. Prod. 2012, 75, 2082–2087. [Google Scholar] [CrossRef] [PubMed]
- Yamanokuchi, R.; Imada, K.; Miyazaki, M.; Kato, H.; Watanabe, T.; Fujimuro, M.; Saeki, Y.; Yoshinaga, S.; Terasawa, H.; Iwasaki, N.; et al. Hyrtioreticulins A–E, indole alkaloids inhibiting the ubiquitin-activating enzyme, from the marine sponge Hyrtios reticulatus. Bioorg. Med. Chem. 2012, 20, 4437–4442. [Google Scholar] [CrossRef] [PubMed]
- Gladkikh, I.; Monastyrnaya, M.; Leychenko, E.; Zelepuga, E.; Chausova, V.; Isaeva, M.; Anastyuk, S.; Andreev, Y.; Peigneur, S.; Tytgat, J.; et al. Atypical reactive center Kunitz-type inhibitor from the sea anemone Heteractis crispa. Mar. Drugs 2012, 10, 1545–1565. [Google Scholar] [CrossRef] [PubMed]
- Schweikart, K.; Guo, L.; Shuler, Z.; Abrams, R.; Chiao, E.T.; Kolaja, K.L.; Davis, M. The effects of jaspamide on human cardiomyocyte function and cardiac ion channel activity. Toxicol. In Vitro 2013, 27, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Carlile, G.W.; Keyzers, R.A.; Teske, K.A.; Robert, R.; Williams, D.E.; Linington, R.G.; Gray, C.A.; Centko, R.M.; Yan, L.; Anjos, S.M.; et al. Correction of F508del-CFTR trafficking by the sponge alkaloid latonduine is modulated by interaction with PARP. Chem. Biol. 2012, 19, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Fujiwara, Y.; Hayashida, A.; Horlad, H.; Kato, H.; Rotinsulu, H.; Losung, F.; Mangindaan, R.E.; de Voogd, N.J.; Takeya, M.; et al. Manzamine A, a marine-derived alkaloid, inhibits accumulation of cholesterol ester in macrophages and suppresses hyperlipidemia and atherosclerosis in vivo. Bioorg. Med. Chem. 2013, 21, 3831–3838. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E.; Dalisay, D.S.; Li, F.; Amphlett, J.; Maneerat, W.; Chavez, M.A.; Wang, Y.A.; Matainaho, T.; Yu, W.; Brown, P.J.; et al. Nahuoic acid A produced by a Streptomyces sp. isolated from a marine sediment is a selective SAM-competitive inhibitor of the histone methyltransferase SETD8. Org. Lett. 2013, 15, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Cheruku, P.; Plaza, A.; Lauro, G.; Keffer, J.; Lloyd, J.R.; Bifulco, G.; Bewley, C.A. Discovery and synthesis of namalide reveals a new anabaenopeptin scaffold and peptidase inhibitor. J. Med. Chem. 2012, 55, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Plisson, F.; Conte, M.; Khalil, Z.; Huang, X.C.; Piggott, A.M.; Capon, R.J. Kinase inhibitor scaffolds against neurodegenerative diseases from a Southern Australian ascidian, Didemnum sp. ChemMedChem 2012, 7, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Margarucci, L.; Tosco, A.; De Simone, R.; Riccio, R.; Monti, M.C.; Casapullo, A. Modulation of proteasome machinery by natural and synthetic analogues of the marine bioactive compound petrosaspongiolide M. Chembiochem 2012, 13, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.K.; Cha, B.Y.; Yagyu, T.; Woo, J.T.; Ojika, M. Sponge-derived acetylenic alcohols, petrosiols, inhibit proliferation and migration of platelet-derived growth factor (PDGF)-induced vascular smooth muscle cells. Bioorg. Med. Chem. 2013, 21, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Vitale, R.M.; Gatti, M.; Carbone, M.; Barbieri, F.; Felicita, V.; Gavagnin, M.; Florio, T.; Amodeo, P. Minimalist hybrid ligand/receptor-based pharmacophore model for CXCR4 applied to a small-library of marine natural products led to the identification of phidianidine a as a new CXCR4 ligand exhibiting antagonist activity. ACS Chem. Biol. 2013, 8, 2762–2770. [Google Scholar] [CrossRef] [PubMed]
- Lunder, M.; Drevensek, G.; Hawlina, S.; Sepcic, K.; Ziberna, L. Cardiovascular effects induced by polymeric 3-alkylpyridinium salts from the marine sponge Reniera sarai. Toxicon 2012, 60, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Su, J.H.; Chen, Y.C.; El-Shazly, M.; Du, Y.C.; Su, C.W.; Tsao, C.W.; Liu, L.L.; Chou, Y.; Chang, W.B.; Su, Y.D.; et al. Towards the small and the beautiful: A small dibromotyrosine derivative from Pseudoceratina sp. sponge exhibits potent apoptotic effect through targeting IKK/NFkappaB signaling pathway. Mar. Drugs 2013, 11, 3168–3185. [Google Scholar] [CrossRef] [PubMed]
- Day, D.R.; Jabaiah, S.; Jacobs, R.S.; Little, R.D. Cyclodextrin formulation of the marine natural product pseudopterosin A uncovers optimal pharmacodynamics in proliferation studies of human umbilical vein endothelial cells. Mar. Drugs 2013, 11, 3258–3271. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Kim, K.N.; Heo, S.J.; Han, S.C.; Kim, J.; Ko, Y.J.; Kang, H.K.; Yoo, E.S. Sargachromanol G inhibits osteoclastogenesis by suppressing the activation NF-kappaB and MAPKs in RANKL-induced RAW 264.7 cells. Biochem. Biophys. Res. Commun. 2013, 434, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Kong, T.; Wu, W.H.; Lan, M.B. The protection of polysaccharide from the Brown Seaweed Sargassum graminifolium against ethylene glycol-induced mitochondrial damage. Mar. Drugs 2013, 11, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Kawamura-Konishi, Y.; Watanabe, N.; Saito, M.; Nakajima, N.; Sakaki, T.; Katayama, T.; Enomoto, T. Isolation of a new phlorotannin, a potent inhibitor of carbohydrate-hydrolyzing enzymes, from the brown alga Sargassum patens. J. Agric. Food Chem. 2012, 60, 5565–5570. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.J.; Liu, X.J.; Zhao, Y.L.; Liu, D.; Xu, Z.H.; Lang, X.M.; Ao, P.; Lin, W.H.; Yang, S.L.; Zhang, Z.G.; et al. Identification and characterization of an anti-fibrotic benzopyran compound isolated from mangrove-derived Streptomyces xiamenensis. Mar. Drugs 2012, 10, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Sepe, V.; Ummarino, R.; D’Auria, M.V.; Taglialatela-Scafati, O.; Marino, S.D.; D’Amore, C.; Renga, B.; Chini, M.G.; Bifulco, G.; Nakao, Y.; et al. Preliminary structure-activity relationship on theonellasterol, a new chemotype of FXR antagonist, from the marine sponge Theonella swinhoei. Mar. Drugs 2012, 10, 2448–2466. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Caballero, M.; Mari-Beffa, M.; Canedo, L.; Medina, M.A.; Quesada, A.R. Toluquinol, a marine fungus metabolite, is a new angiosuppresor that interferes with the Akt pathway. Biochem. Pharmacol. 2013, 85, 1727–1740. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Paul, V.J.; Luesch, H. Seaweed extracts and unsaturated fatty acid constituents from the green alga Ulva lactuca as activators of the cytoprotective Nrf2-ARE pathway. Free Radic. Biol. Med. 2013, 57, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Daoust, J.; Chen, M.; Wang, M.; Williams, D.E.; Garcia Chavez, M.A.; Wang, Y.A.; Merchant, C.E.; Fontana, A.; Kieffer, T.J.; Andersen, R.J. Sesterterpenoids isolated from a northeastern Pacific Phorbas sp. J. Org. Chem. 2013, 78, 8267–8273. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.W.; Ji, C.Z.; Gu, Q.Q.; Li, D.H.; Zhu, T.J. Three new polyketides from marine-derived fungus Aspergillus glaucus HB1-19. J. Asian Nat. Prod. Res. 2013, 15, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Haroon, M.H.; Premaratne, S.R.; Choudhry, M.I.; Dharmaratne, H.R. A new beta-glucuronidase inhibiting butyrolactone from the marine endophytic fungus Aspergillus terreus. Nat. Prod. Res. 2013, 27, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante-Silva, L.H.; de Carvalho Correia, A.C.; Barbosa-Filho, J.M.; da Silva, B.A.; de Oliveira Santos, B.V.; de Lira, D.P.; Sousa, J.C.; de Miranda, G.E.; de Andrade, C.F.; Alexandre-Moreira, M.S. Spasmolytic effect of caulerpine involves blockade of Ca2+ influx on guinea pig ileum. Mar. Drugs 2013, 11, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Chini, M.G.; Jones, C.R.; Zampella, A.; D’Auria, M.V.; Renga, B.; Fiorucci, S.; Butts, C.P.; Bifulco, G. Quantitative NMR-derived interproton distances combined with quantum mechanical calculations of 13C chemical shifts in the stereochemical determination of conicasterol F, a nuclear receptor ligand from Theonella swinhoei. J. Org. Chem. 2012, 77, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.N.; Watjen, W.; Schmitz, R.; Chovolou, Y.; Edrada-Ebel, R.; Youssef, D.T.; Kamel, M.S.; Proksch, P. A new bioactive sesquiterpenoid quinone from the Mediterranean Sea marine sponge Dysidea avara. Nat. Prod. Commun. 2013, 8, 289–292. [Google Scholar] [PubMed]
- Shin, K.; Chin, J.; Hahn, D.; Lee, J.; Hwang, H.; Won, D.H.; Ham, J.; Choi, H.; Kang, E.; Kim, H.; et al. Sterols from a soft coral, Dendronephthya gigantea as farnesoid X-activated receptor antagonists. Steroids 2012, 77, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.H.; Huang, X.J.; Yang, J.S.; Yang, F.; Piao, S.J.; Gao, H.; Li, J.; Ye, W.C.; Yao, X.S.; Chen, W.S.; et al. Dysidavarones A–D, new sesquiterpene quinones from the marine sponge Dysidea avara. Org. Lett. 2012, 14, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Martinez, E.D.; MacMillan, J.B. Anthraquinones from a marine-derived Streptomyces spinoverrucosus. J. Nat. Prod. 2012, 75, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E.; Steino, A.; de Voogd, N.J.; Mauk, A.G.; Andersen, R.J. Halicloic acids A and B isolated from the marine sponge Haliclona sp. collected in the Philippines inhibit indoleamine 2,3-dioxygenase. J. Nat. Prod. 2012, 75, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Jansen, N.; Ohlendorf, B.; Erhard, A.; Bruhn, T.; Bringmann, G.; Imhoff, J.F. Helicusin E, isochromophilone X and isochromophilone XI: New chloroazaphilones produced by the fungus Bartalinia robillardoides strain LF550. Mar. Drugs 2013, 11, 800–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chianese, G.; Fattorusso, E.; Putra, M.Y.; Calcinai, B.; Bavestrello, G.; Moriello, A.S.; De Petrocellis, L.; Di Marzo, V.; Taglialatela-Scafati, O. Leucettamols, bifunctionalized marine sphingoids, act as modulators of TRPA1 and TRPM8 channels. Mar. Drugs 2012, 10, 2435–2447. [Google Scholar] [CrossRef] [PubMed]
- Ushiyama, S.; Umaoka, H.; Kato, H.; Suwa, Y.; Morioka, H.; Rotinsulu, H.; Losung, F.; Mangindaan, R.E.; de Voogd, N.J.; Yokosawa, H.; et al. Manadosterols A and B, sulfonated sterol dimers inhibiting the Ubc13-Uev1A interaction, isolated from the marine sponge Lissodendryx fibrosa. J. Nat. Prod. 2012, 75, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Hemberger, Y.; Schmitt, S.M.; Bouhired, S.; Natesan, L.; Kehraus, S.; Dimas, K.; Gutschow, M.; Bringmann, G.; Konig, G.M. Marilines A–C: Novel phthalimidines from the sponge-derived fungus Stachylidium sp. Chemistry 2012, 18, 8827–8834. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.F.; Kurtan, T.; Mandi, A.; Yao, L.G.; Li, J.; Zhang, W.; Guo, Y.W. Unprecedented diterpenoids as a PTP1B inhibitor from the Hainan soft coral Sarcophyton trocheliophorum Marenzeller. Org. Lett. 2013, 15, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Takagi, M.; Shin-Ya, K. JBIR-124: A novel antioxidative agent from a marine sponge-derived fungus Penicillium citrinum SpI080624G1f01. J. Antibiot. 2012, 65, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chin, J.; Choi, H.; Baek, K.; Lee, T.G.; Park, S.E.; Wang, W.; Hahn, D.; Yang, I.; Lee, J.; et al. Phosphoiodyns A and B, unique phosphorus-containing iodinated polyacetylenes from a Korean sponge Placospongia sp. Org. Lett. 2013, 15, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Liu, D.; Wei, C.; Proksch, P.; Lin, W. Purpuroines A–J, halogenated alkaloids from the sponge Iotrochota purpurea with antibiotic activity and regulation of tyrosine kinases. Bioorg. Med. Chem. 2012, 20, 6924–6928. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, C.M.; Wong, C.Y.; Ononye, S.; Lopez, D.D.; Engene, N.; McPhail, K.L.; Gerwick, W.H.; Balunas, M.J. Santacruzamate A, a potent and selective histone deacetylase inhibitor from the Panamanian marine cyanobacterium cf. Symploca sp. J. Nat. Prod. 2013, 76, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.F.; Gao, L.X.; Li, J.; Taglialatela-Scafati, O.; Guo, Y.W. Cembrane diterpenoids from the soft coral Sarcophyton trocheliophorum Marenzeller as a new class of PTP1B inhibitors. Bioorg. Med. Chem. 2013, 21, 5076–5080. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, I.K.; Cho, G.Y.; Oh, K.H.; Lim, Y.W.; Yun, B.S. Sargassumol, a novel antioxidant from the brown alga Sargassum micracanthum. J. Antibiot. 2012, 65, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H.; Lin, W.; Muller, W.E.; Kubbutat, M.; Lai, D.; Proksch, P. Trimeric hemibastadin congener from the marine sponge Ianthella basta. J. Nat. Prod. 2013, 76, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.; Gamal Eldeen, A.M.; Shahat, A.A.; Abdel-Latif, F.F.; Mohamed, T.A.; Whittlesey, B.R.; Pare, P.W. Bioactive hydroperoxyl cembranoids from the Red Sea soft coral Sarcophyton glaucum. Mar. Drugs 2012, 10, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F.; Reynolds, K.A.; Morinaka, B.I. Symplocin A, a linear peptide from the Bahamian cyanobacterium Symploca sp. Configurational analysis of N,N-dimethylamino acids by chiral-phase HPLC of naphthacyl esters. J. Nat. Prod. 2012, 75, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Dolusic, E.; Larrieu, P.; Meinguet, C.; Colette, D.; Rives, A.; Blanc, S.; Ferain, T.; Pilotte, L.; Stroobant, V.; Wouters, J.; et al. Indoleamine 2,3-dioxygenase inhibitory activity of derivatives of marine alkaloid tsitsikammamine A. Bioorg. Med. Chem. Lett. 2013, 23, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.K.; Hansen, E.; Isaksson, J.; Andersen, J.H. Cellular Antioxidant Effect of Four Bromophenols from the Red Algae, Vertebrata lanosa. Mar. Drugs 2013, 11, 2769–2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsunaga, S.; Akiyama, T.; Takada, K.; Oikawa, T.; Matsuura, N.; Ise, Y.; Okada, S.; Matsunaga, S. Stimulators of adipogenesis from the marine sponge Xestospongia testudinaria. Tetrahedron 2013, 69, 6560–6564. [Google Scholar]
- Dong, L.Y.; Jin, J.; Lu, G.; Kang, X.L. Astaxanthin attenuates the apoptosis of retinal ganglion cells in db/db mice by inhibition of oxidative stress. Mar. Drugs 2013, 11, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Camp, D.; Davis, R.A.; Evans-Illidge, E.A.; Quinn, R.J. Guiding principles for natural product drug discovery. Future Med. Chem. 2012, 4, 1067–1084. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.R.; Chavarria, K.L.; Fenical, W.; Moore, B.S.; Ziemert, N. Challenges and triumphs to genomics-based natural product discovery. J. Ind. Microbiol Biotechnol. 2013, 41, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.T.; Lu, X.L.; Liu, X.Y.; Gao, Y.; Hu, B.; Jiao, B.H.; Zheng, H. Bioactive natural products from the antarctic and arctic organisms. Mini Rev. Med. Chem. 2013, 13, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.F.; Guo, Y.W. Terpenes from the soft corals of the genus Sarcophyton: Chemistry and biological activities. Chem. Biodivers. 2013, 10, 2161–2196. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2013, 4, 6–24. [Google Scholar] [CrossRef]
- Berrue, F.; McCulloch, M.W.; Kerr, R.G. Marine diterpene glycosides. Bioorg. Med. Chem. 2011, 19, 6702–6719. [Google Scholar] [CrossRef] [PubMed]
- Morya, V.K.; Kim, J.; Kim, E.K. Algal fucoidan: Structural and size-dependent bioactivities and their perspectives. Appl. Microbiol. Biotechnol. 2012, 93, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Fucanomics and galactanomics: Marine distribution, medicinal impact, conceptions, and challenges. Mar. Drugs 2012, 10, 793–811. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, V.; Seca, A.M.; Barreto, M.C.; Pinto, D.C. Di- and sesquiterpenoids from Cystoseira genus: Structure, intra-molecular transformations and biological activity. Mini Rev. Med. Chem. 2013, 13, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Kim, S.K. Sulfated polysaccharides as bioactive agents from marine algae. Int. J. Biol. Macromol. 2013, 62, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.G.; Gloer, J.B.; Ji, N.Y.; Zhao, J.C. Halogenated organic molecules of Rhodomelaceae origin: Chemistry and biology. Chem. Rev. 2013, 113, 3632–3685. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, M.; Maruthanayagam, V.; Sundararaman, M. A review of pharmacological and toxicological potentials of marine cyanobacterial metabolites. J. Appl. Toxicol. 2012, 32, 153–185. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T. Pharmaceutical agents from filamentous marine cyanobacteria. Drug Discov. Today 2013, 18, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.I.; Ivanchina, N.V.; Krasokhin, V.B.; Makarieva, T.N.; Stonik, V.A. Glycosides from marine sponges (Porifera, Demospongiae): Structures, taxonomical distribution, biological activities and biological roles. Mar. Drugs 2012, 10, 1671–1710. [Google Scholar] [CrossRef] [PubMed]
- Kiew, P.L.; Don, M.M. Jewel of the seabed: Sea cucumbers as nutritional and drug candidates. Int. J. Food Sci. Nutr. 2011, 63, 616–636. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Rodriguez, J.; Sanchez-Miron, A.; Garcia-Camacho, F.; Lopez-Rosales, L.; Chisti, Y.; Molina-Grima, E. Bioactives from microalgal dinoflagellates. Biotechnol. Adv. 2012, 30, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Desriac, F.; Jegou, C.; Balnois, E.; Brillet, B.; Le, C.P.; Fleury, Y. Antimicrobial peptides from marine proteobacteria. Mar. Drugs 2013, 11, 3632–3660. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.H.; Kim, Y.M.; Kim, S.K. Marine bacteria: Potential sources for compounds to overcome antibiotic resistance. Appl. Microbiol. Biotechnol. 2013, 97, 4763–4773. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Yi, X.; Huang, R.; Yan, F.; He, B.; Chen, B. Alkaloids from corals. Chem. Biodivers. 2013, 10, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.L.; Kim, S.K. Antimicrobial peptides from marine sources. Curr. Protein Pept. Sci. 2013, 14, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Zotchev, S.B. Marine actinomycetes as an emerging resource for the drug development pipelines. J. Biotechnol. 2012, 158, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Mondol, M.A.; Shin, H.J.; Islam, M.T. Diversity of secondary metabolites from marine Bacillus species: Chemistry and biological activity. Mar. Drugs 2013, 11, 2846–2872. [Google Scholar] [CrossRef] [PubMed]
- Solov’eva, T.; Davydova, V.; Krasikova, I.; Yermak, I. Marine compounds with therapeutic potential in gram-negative sepsis. Mar. Drugs 2013, 11, 2216–2229. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, M.; Robinson, W.E. Structure, biosynthesis and possible function of tunichromes and related compounds. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012, 163, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H.; Fenner, A.M. Drug discovery from marine microbes. Microb. Ecol. 2013, 65, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, J.; Yang, B.; Lin, X.; Yang, X.W.; Liu, Y. Marine natural products with anti-HIV activities in the last decade. Curr. Med. Chem. 2013, 20, 953–973. [Google Scholar] [PubMed]
- Prokofjeva, M.M.; Imbs, T.I.; Shevchenko, N.M.; Spirin, P.V.; Horn, S.; Fehse, B.; Zvyagintseva, T.N.; Prassolov, V.S. Fucoidans as potential inhibitors of HIV-1. Mar. Drugs 2013, 11, 3000–3014. [Google Scholar] [CrossRef] [PubMed]
- Raveh, A.; Delekta, P.C.; Dobry, C.J.; Peng, W.; Schultz, P.J.; Blakely, P.K.; Tai, A.W.; Matainaho, T.; Irani, D.N.; Sherman, D.H.; et al. Discovery of potent broad spectrum antivirals derived from marine actinobacteria. PLoS ONE 2013, 8, e82318. [Google Scholar] [CrossRef] [PubMed]
- Huskens, D.; Schols, D. Algal lectins as potential HIV microbicide candidates. Mar. Drugs 2012, 10, 1476–1497. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.J.; Grkovic, T.; Sykes, M.L.; Avery, V.M. Trypanocidal activity of marine natural products. Mar. Drugs 2013, 11, 4058–4082. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S.; Gohari, A.R.; Haddadi, A. Biogenic trypanocidal sesquiterpenes: Lead compounds to design future trypanocidal drugs—A mini review. J. Fac. Pharm. Tehran Univ. Med. Sci. 2013, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Bladt, T.T.; Frisvad, J.C.; Knudsen, P.B.; Larsen, T.O. Anticancer and antifungal compounds from Aspergillus, Penicillium and other filamentous fungi. Molecules 2013, 18, 11338–11376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, B.F.; Zhu, H.L. The chemistry and biology of the bryostatins: Potential PKC inhibitors in clinical development. Curr. Med. Chem. 2012, 19, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Kim, S.K. Bioactive peptides from marine sources as potential anti-inflammatory therapeutics. Curr. Protein Pept. Sci. 2013, 14, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.C.; Sung, P.J.; Duh, C.Y.; Chen, B.W.; Sheu, J.H.; Yang, N.S. Anti-inflammatory activities of natural products isolated from soft corals of Taiwan between 2008 and 2012. Mar. Drugs 2013, 11, 4083–4126. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, N.; Gammone, M.A.; Gemello, E.; De, G.M.; Cusenza, S.; Riccioni, G. Marine bioactives: Pharmacological properties and potential applications against inflammatory diseases. Mar. Drugs 2012, 10, 812–833. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Conde, E.; Moure, A.; Falque, E.; Dominguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef] [PubMed]
- Albert, B.B.; Cameron-Smith, D.; Hofman, P.L.; Cutfield, W.S. Oxidation of marine omega-3 supplements and human health. Biomed. Res. Int. 2013, 2013, 464921. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.C.; Jeon, Y.J. Marine peptides for preventing metabolic syndrome. Curr. Protein Pept. Sci. 2013, 14, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jeon, Y.J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Kim, S.K. Marine bioactive peptides as potential antioxidants. Curr. Protein Pept. Sci. 2013, 14, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. Cardioprotective peptides from marine sources. Curr. Protein Pept. Sci. 2013, 14, 162–172. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; De Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A treasure from the sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar]
- Nasri, R.; Nasri, M. Marine-derived bioactive peptides as new anticoagulant agents: A review. Curr. Protein Pept. Sci. 2013, 14, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Cusick, K.D.; Sayler, G.S. An overview on the marine neurotoxin, saxitoxin: Genetics, molecular targets, methods of detection and ecological functions. Mar. Drugs 2013, 11, 991–1018. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.; Avila, J.; Villanueva, N. Use of okadaic acid to identify relevant phosphoepitopes in pathology: A focus on neurodegeneration. Mar. Drugs 2013, 11, 1656–1668. [Google Scholar] [CrossRef] [PubMed]
- Molgo, J.; Araoz, R.; Benoit, E.; Iorga, B.I. Physical and virtual screening methods for marine toxins and drug discovery targeting nicotinic acetylcholine receptors. Expert Opin. Drug Discov. 2013, 8, 1203–1223. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, K. Marine-derived bioactive materials for neuroprotection. Food Sci. Biotechnol. 2013, 22, 1175–1186. [Google Scholar] [CrossRef]
- Lin, Z.; Torres, J.P.; Ammon, M.A.; Marett, L.; Teichert, R.W.; Reilly, C.A.; Kwan, J.C.; Hughen, R.W.; Flores, M.; Tianero, M.D.; et al. A bacterial source for mollusk pyrone polyketides. Chem. Biol. 2013, 20, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.B.; Sawant, S.D.; Singh, P.P.; Vishwakarma, R.A. Kinase inhibitors of marine origin. Chem. Rev. 2013, 113, 6761–6815. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, Y.; Waller, D.L.; Wang, J.; Liu, Q. Natural products as kinase inhibitors. Nat. Prod. Rep. 2012, 29, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.S.; Liang, L.F.; Guo, Y.W. Natural products possessing protein tyrosine phosphatase 1B (PTP1B) inhibitory activity found in the last decades. Acta Pharmacol. Sin. 2012, 33, 1217–1245. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.M.; Baell, J.B.; Norton, R.S. Strategies for the development of conotoxins as new therapeutic leads. Mar. Drugs 2013, 11, 2293–2313. [Google Scholar] [CrossRef] [PubMed]
- Essack, M.; Bajic, V.B.; Archer, J.A. Conotoxins that confer therapeutic possibilities. Mar. Drugs 2012, 10, 1244–1265. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Mahdavi, S.; Kuyucak, S. Computational studies of marine toxins targeting ion channels. Mar. Drugs 2013, 11, 848–869. [Google Scholar] [CrossRef] [PubMed]
- Green, D.W.; Padula, M.P.; Santos, J.; Chou, J.; Milthorpe, B.; Ben-Nissan, B. A therapeutic potential for marine skeletal proteins in bone regeneration. Mar. Drugs 2013, 11, 1203–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, N.V.; Kim, S.K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Distrutti, E.; Bifulco, G.; D’Auria, M.V.; Zampella, A. Marine sponge steroids as nuclear receptor ligands. Trends Pharmacol. Sci. 2012, 33, 591–601. [Google Scholar] [CrossRef] [PubMed]
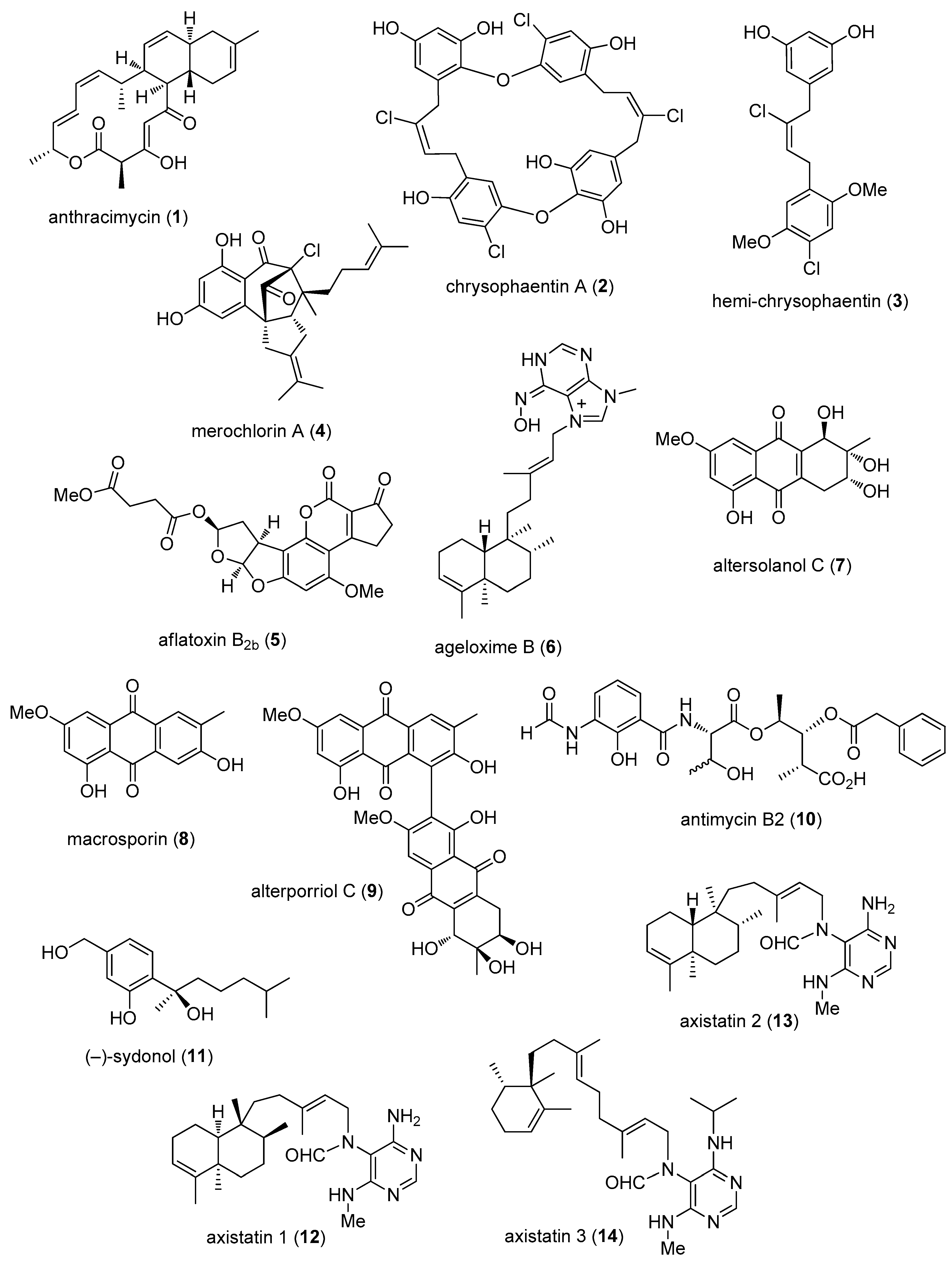
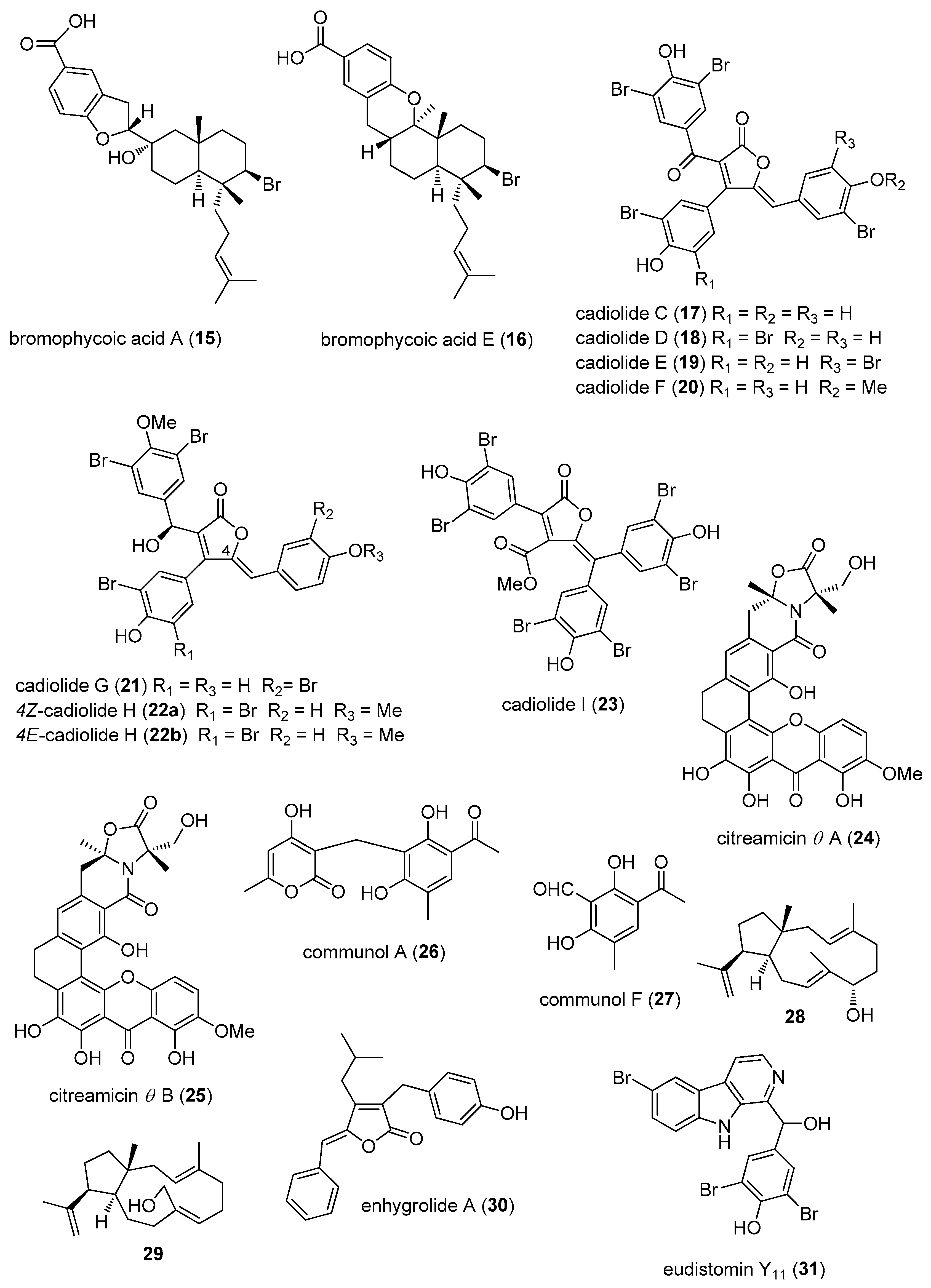

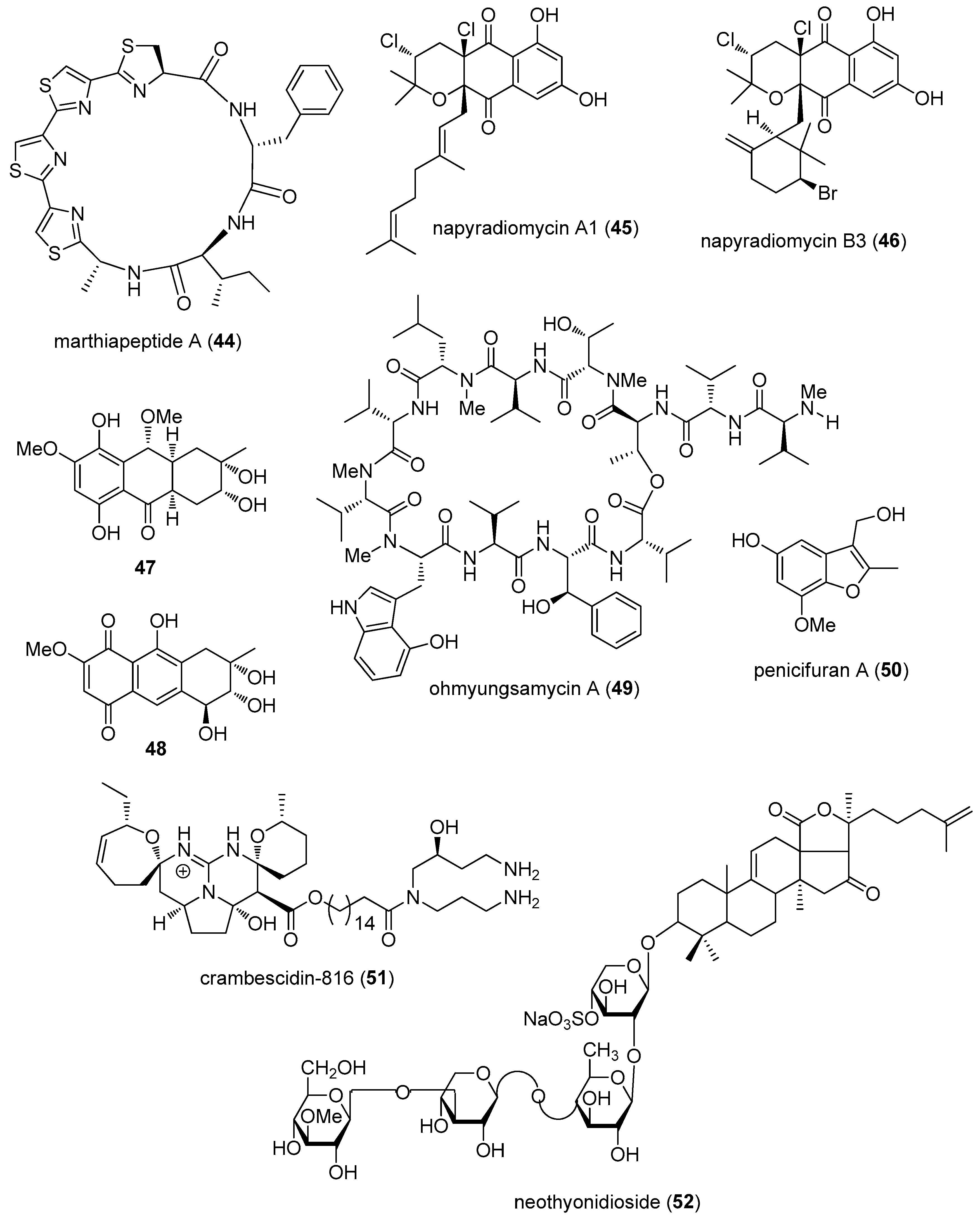
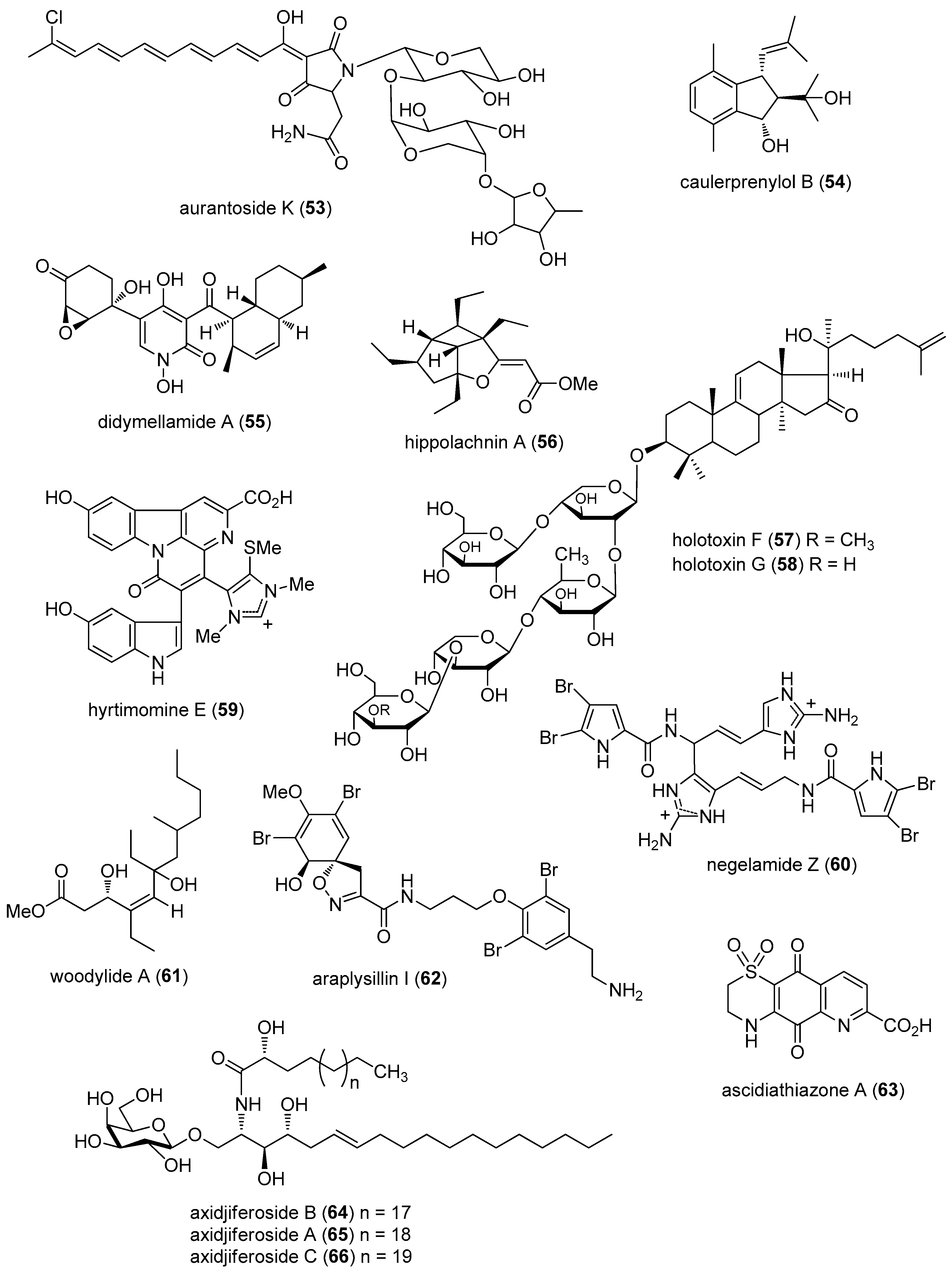
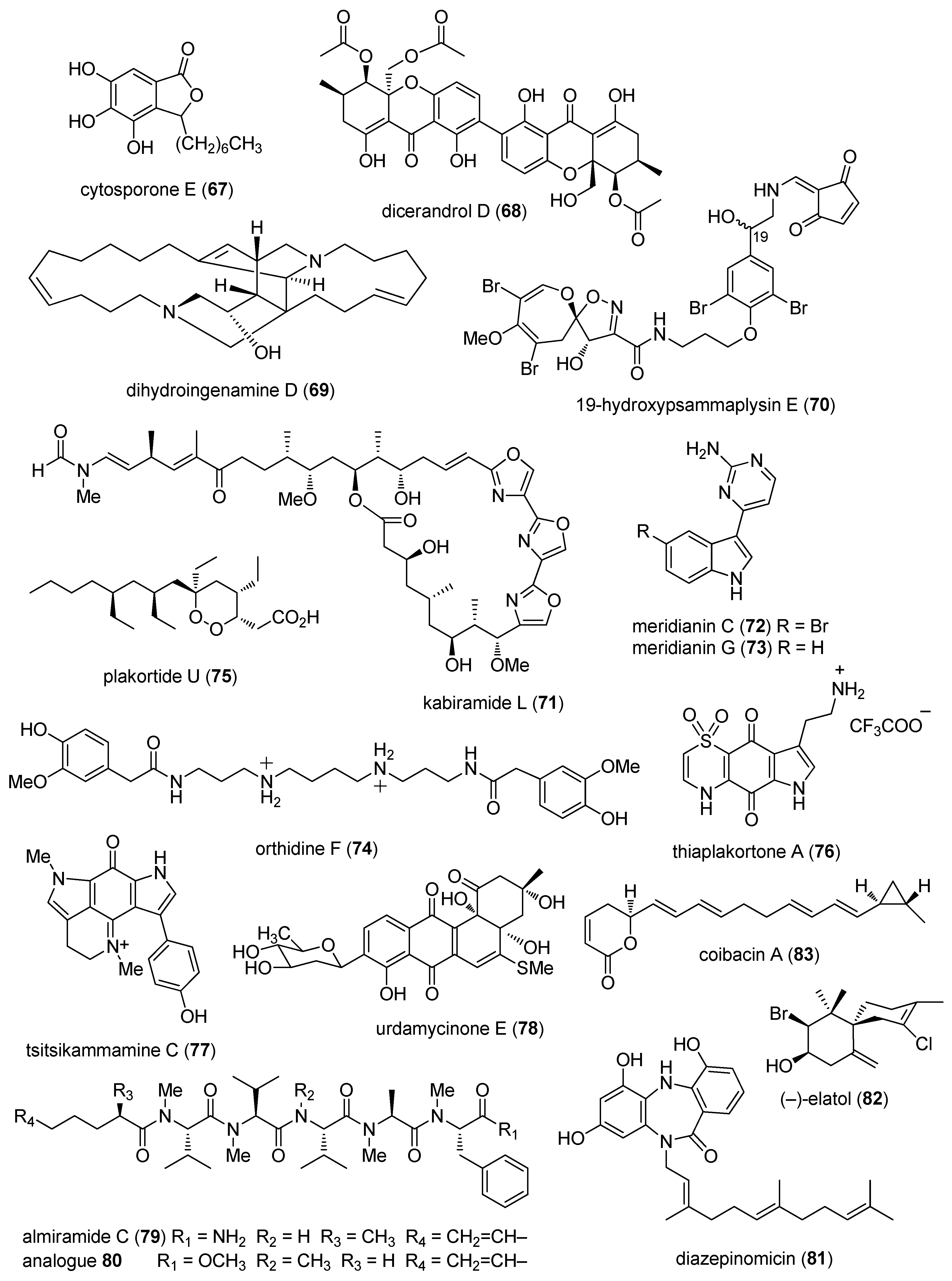
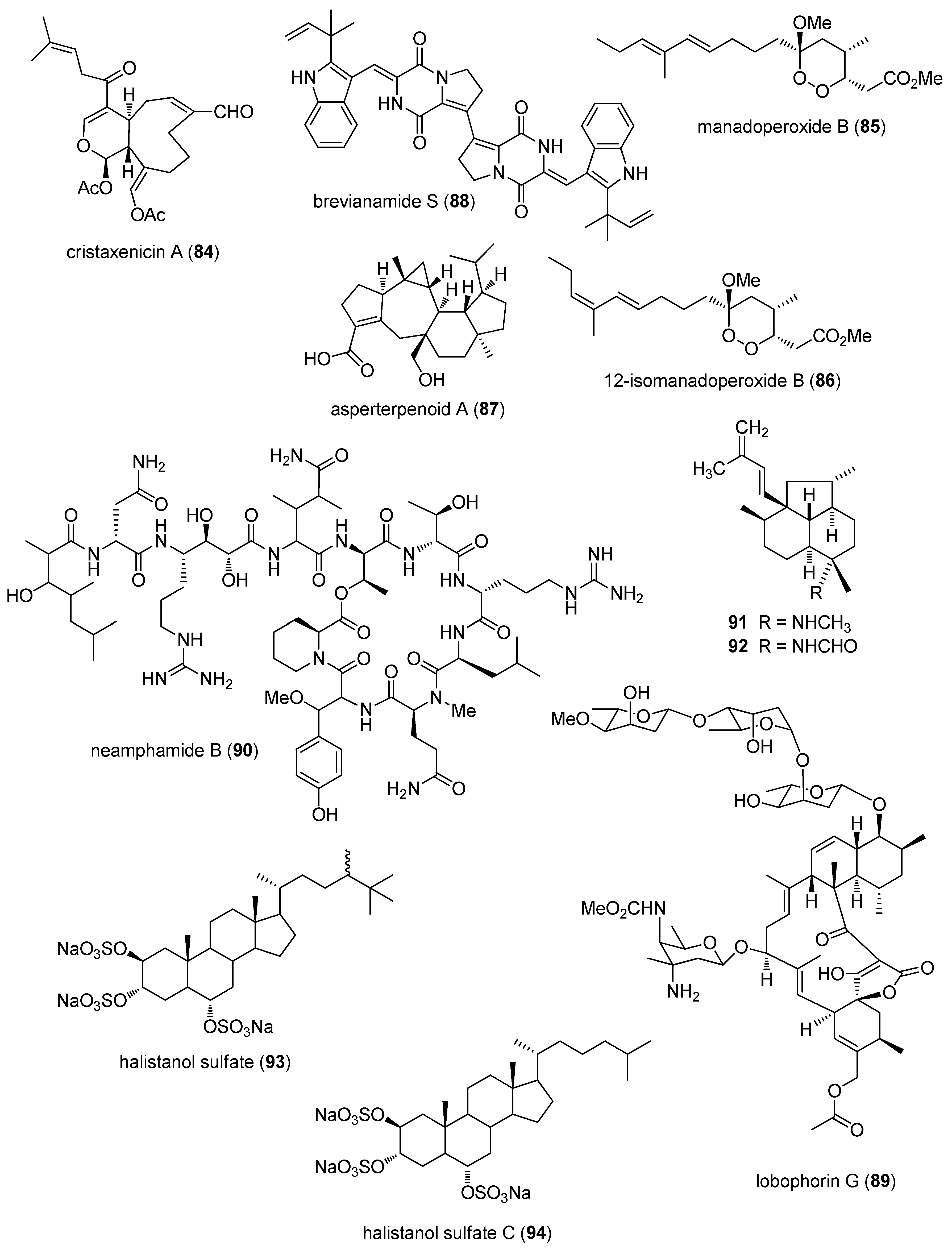
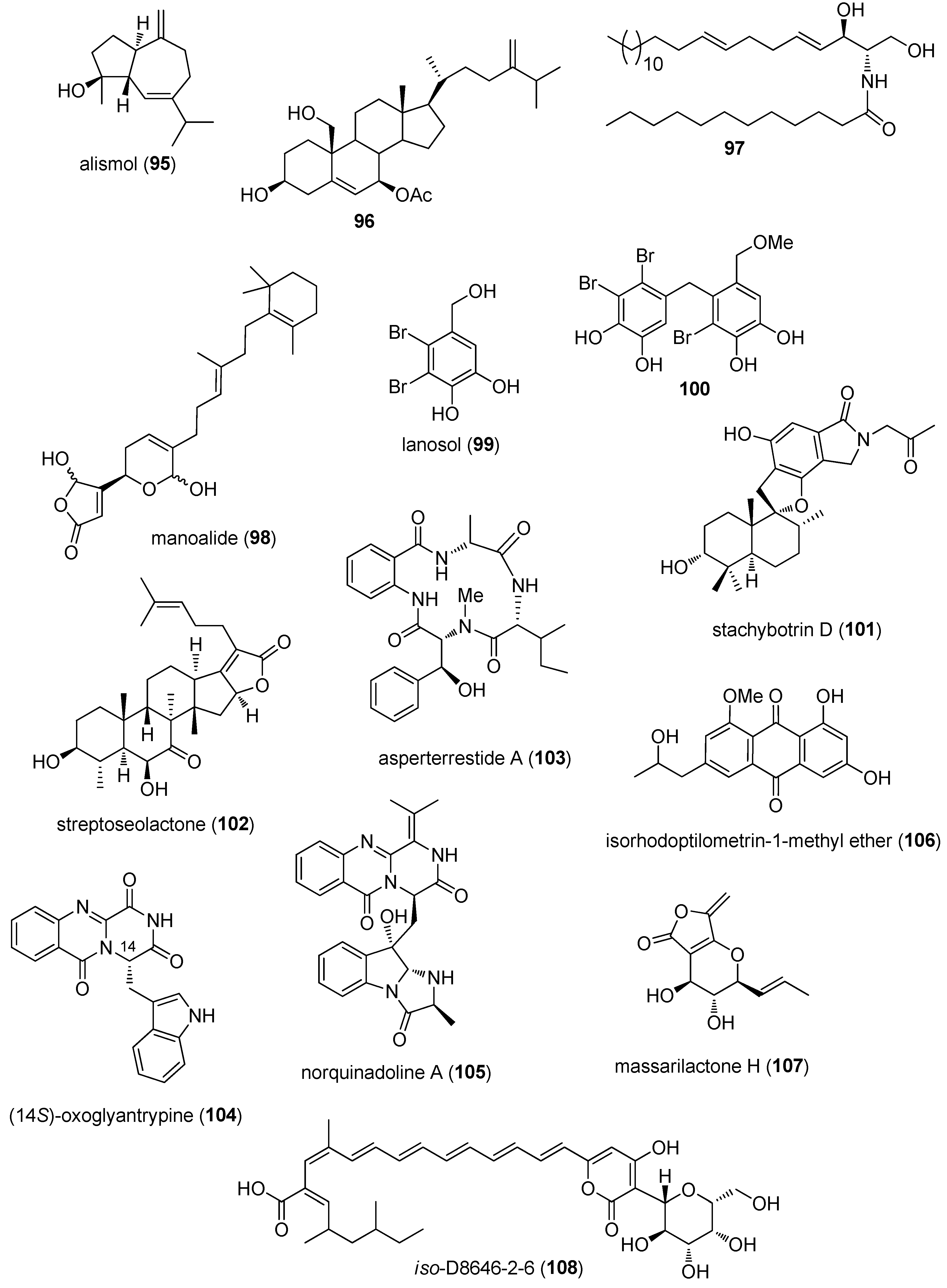
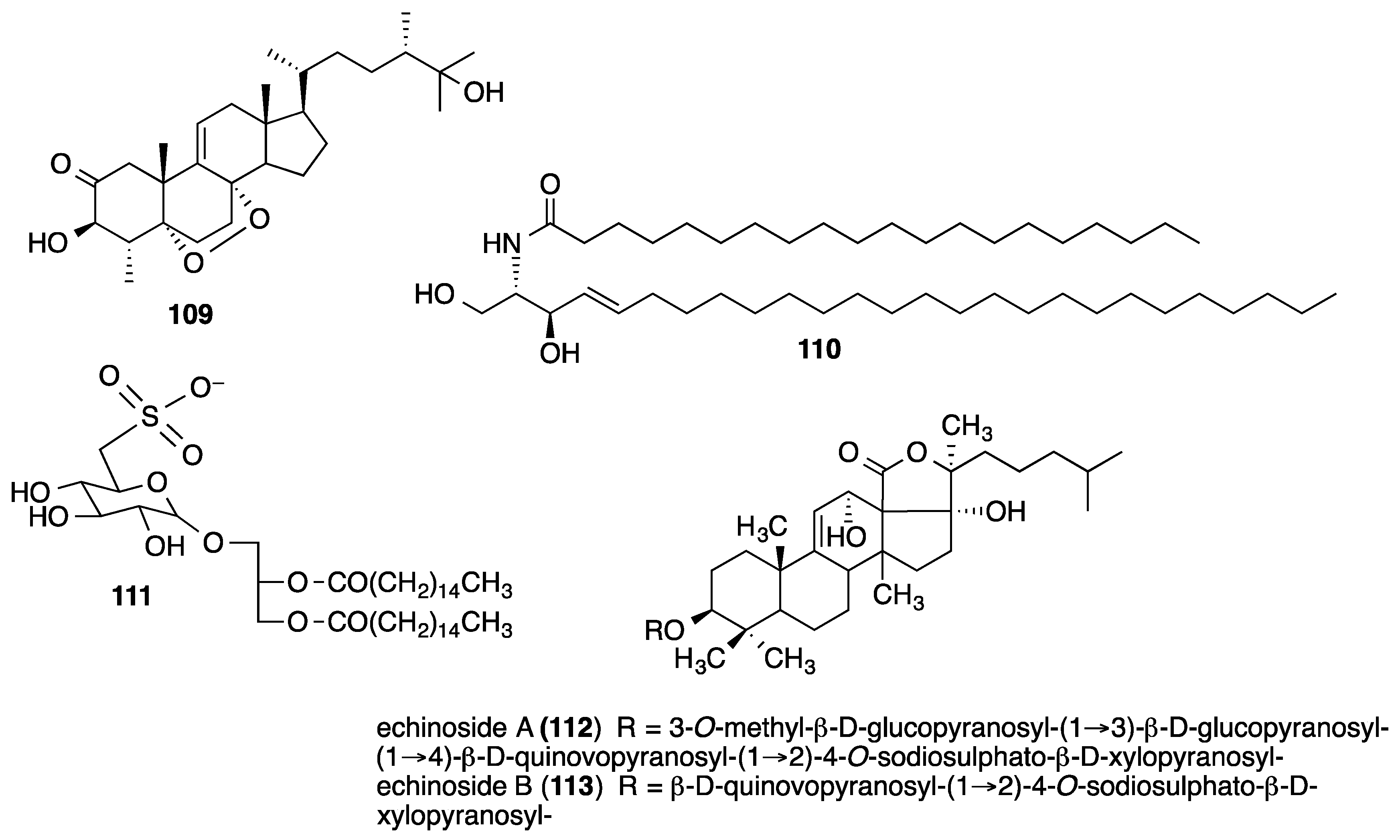
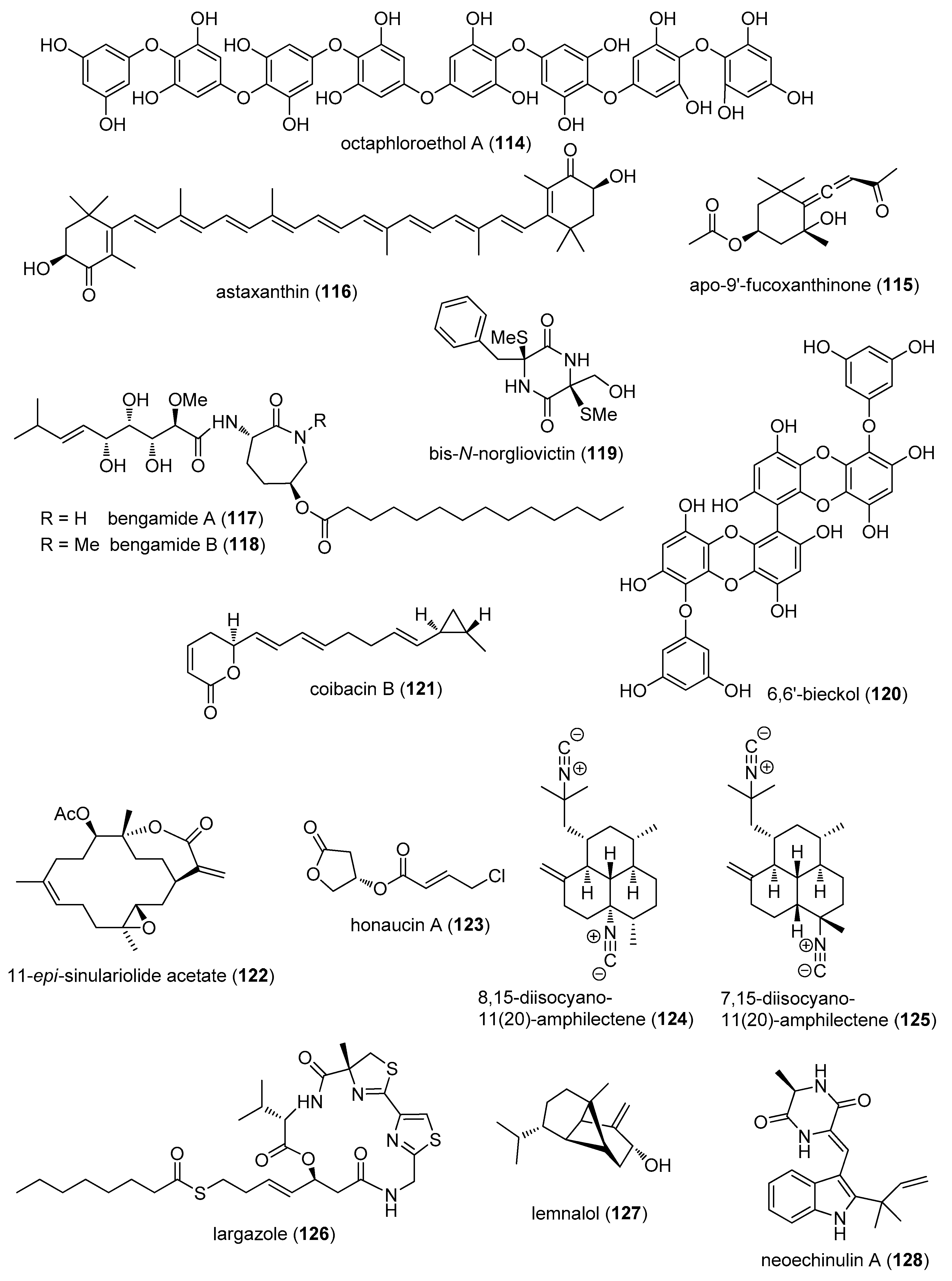
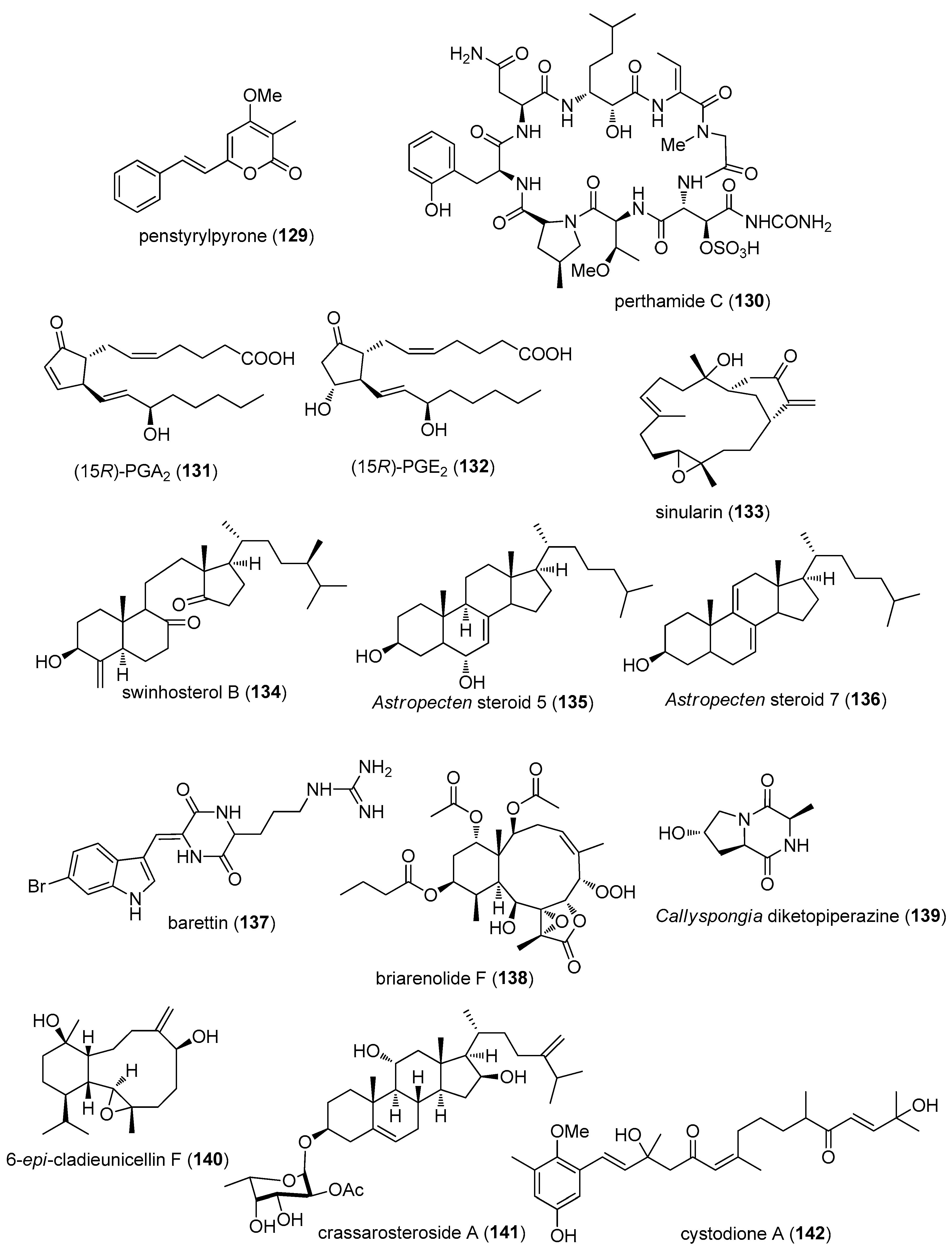
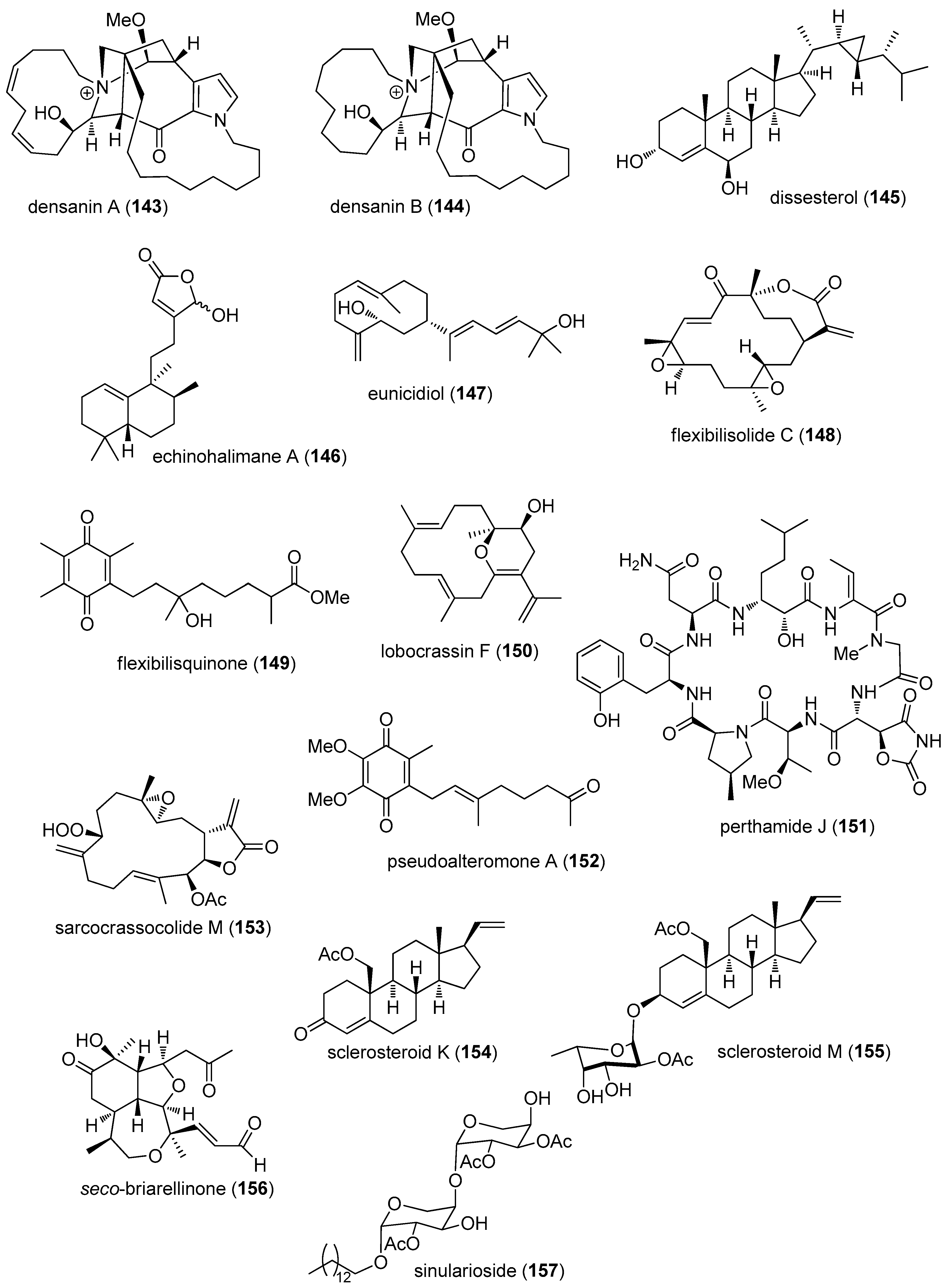
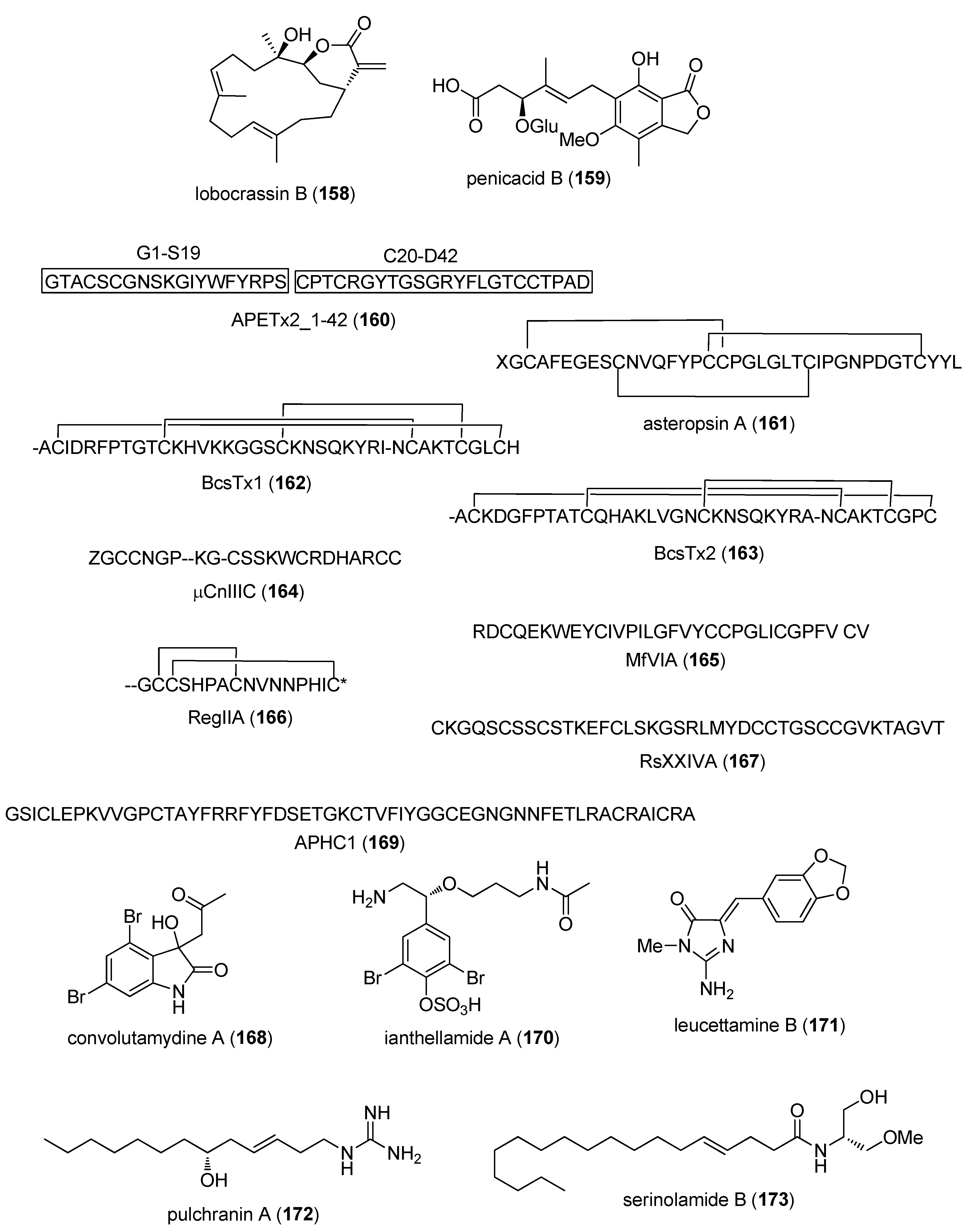
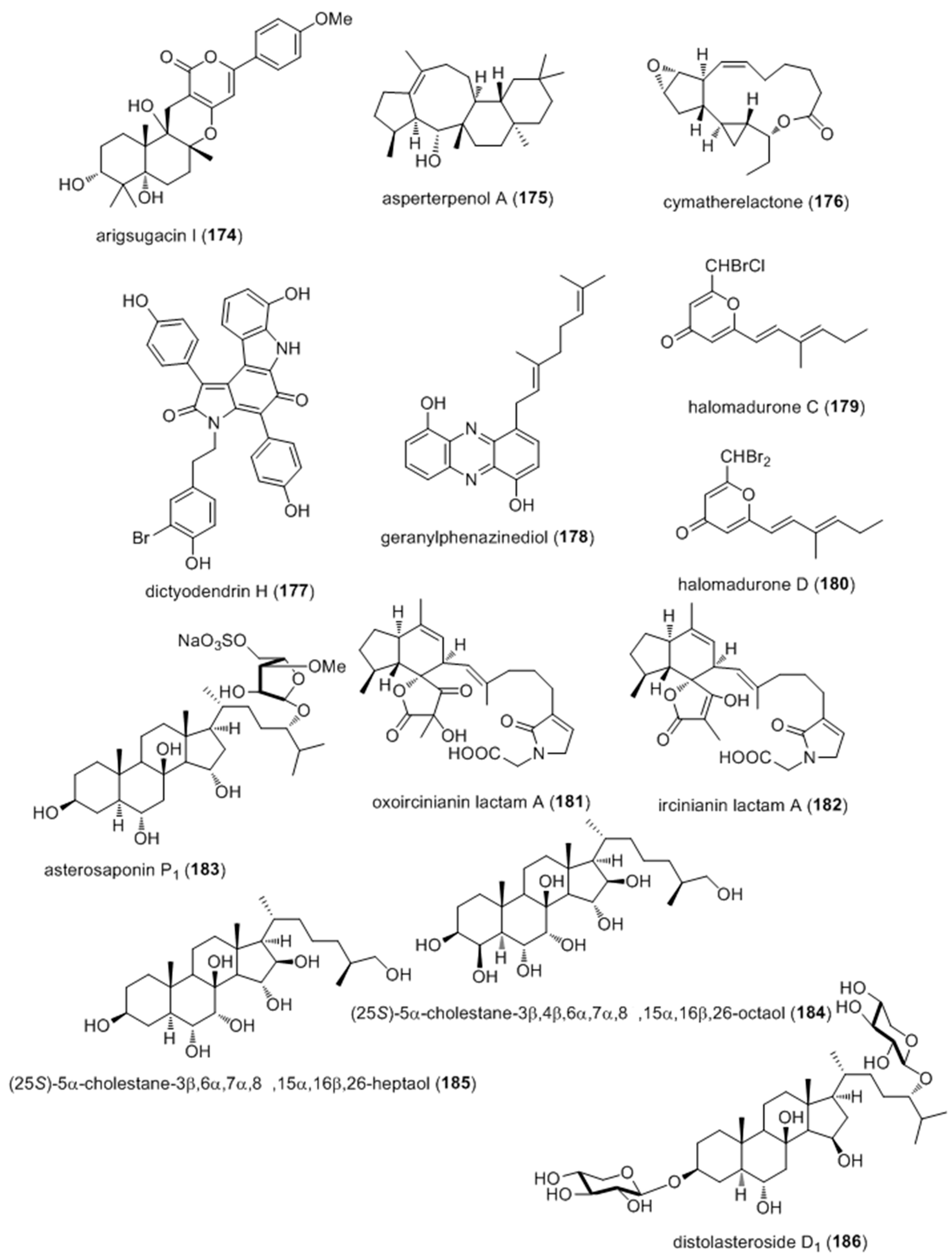
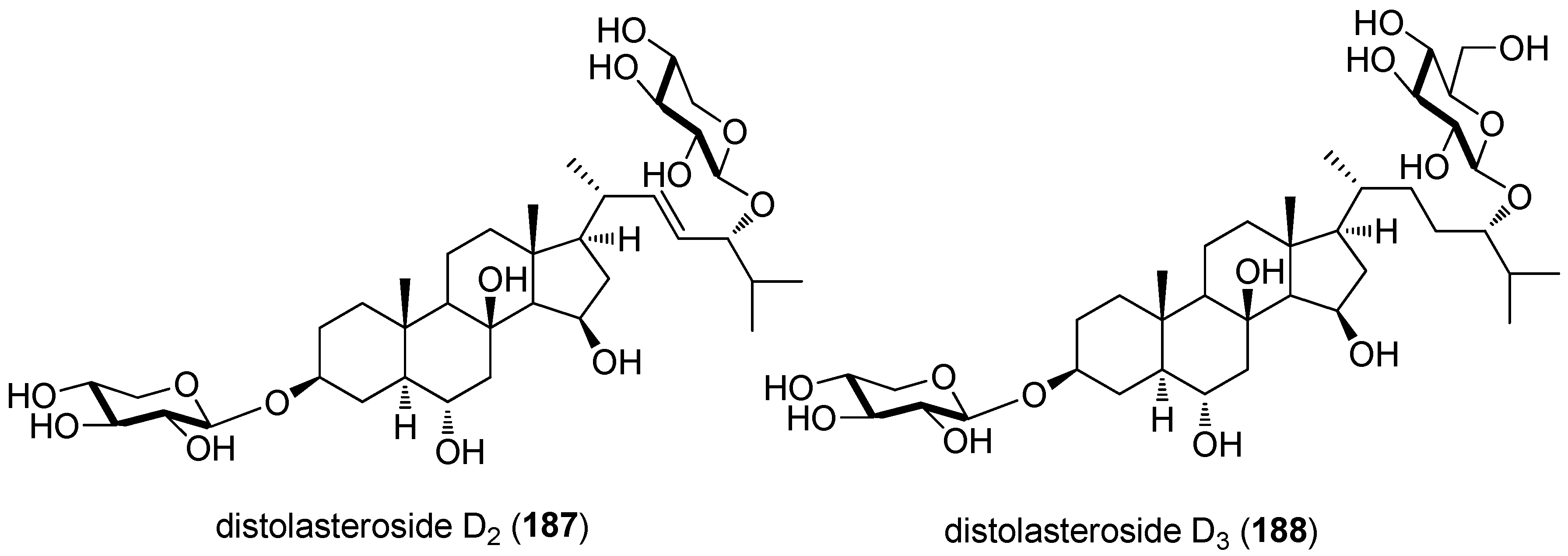
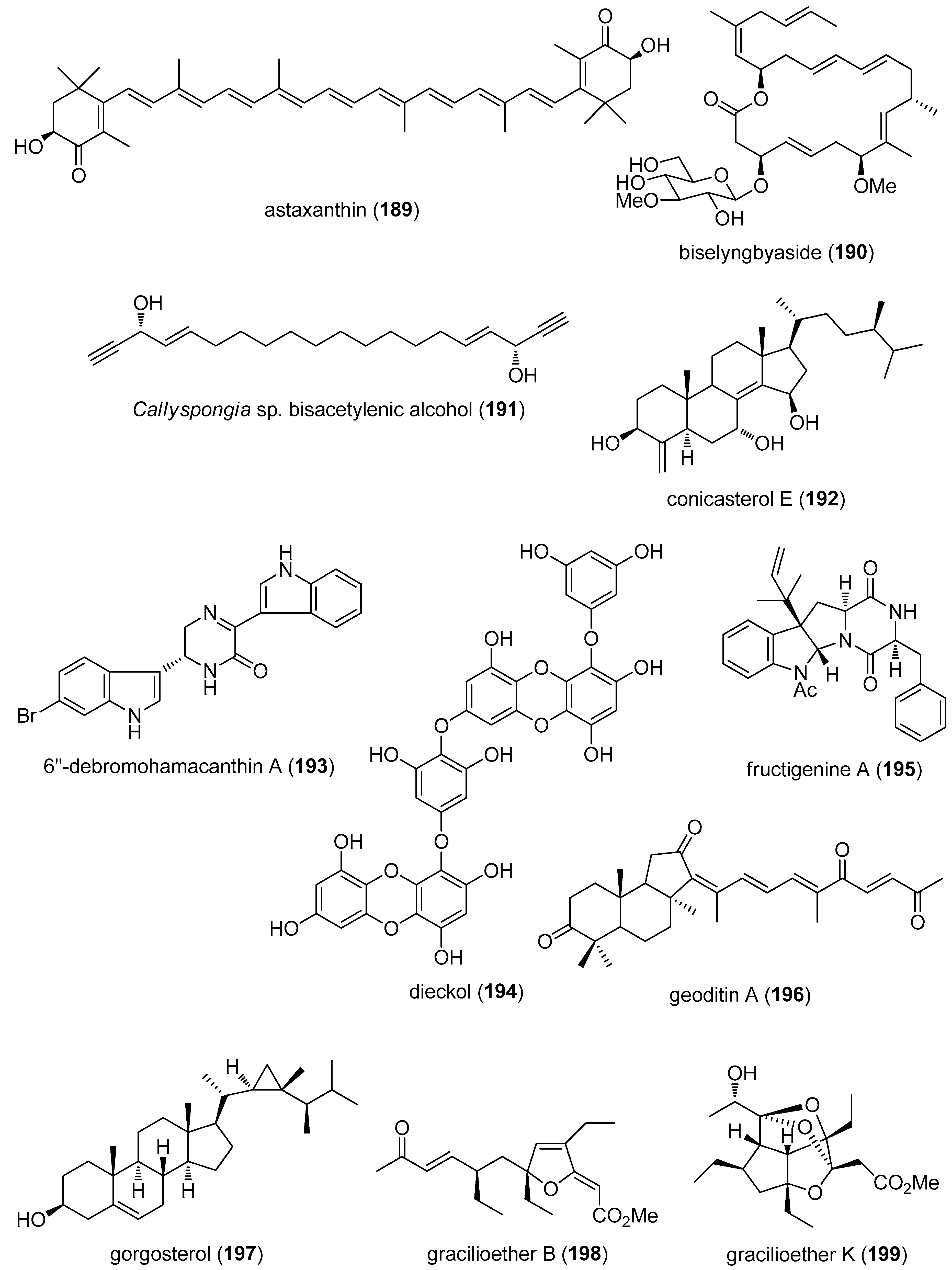
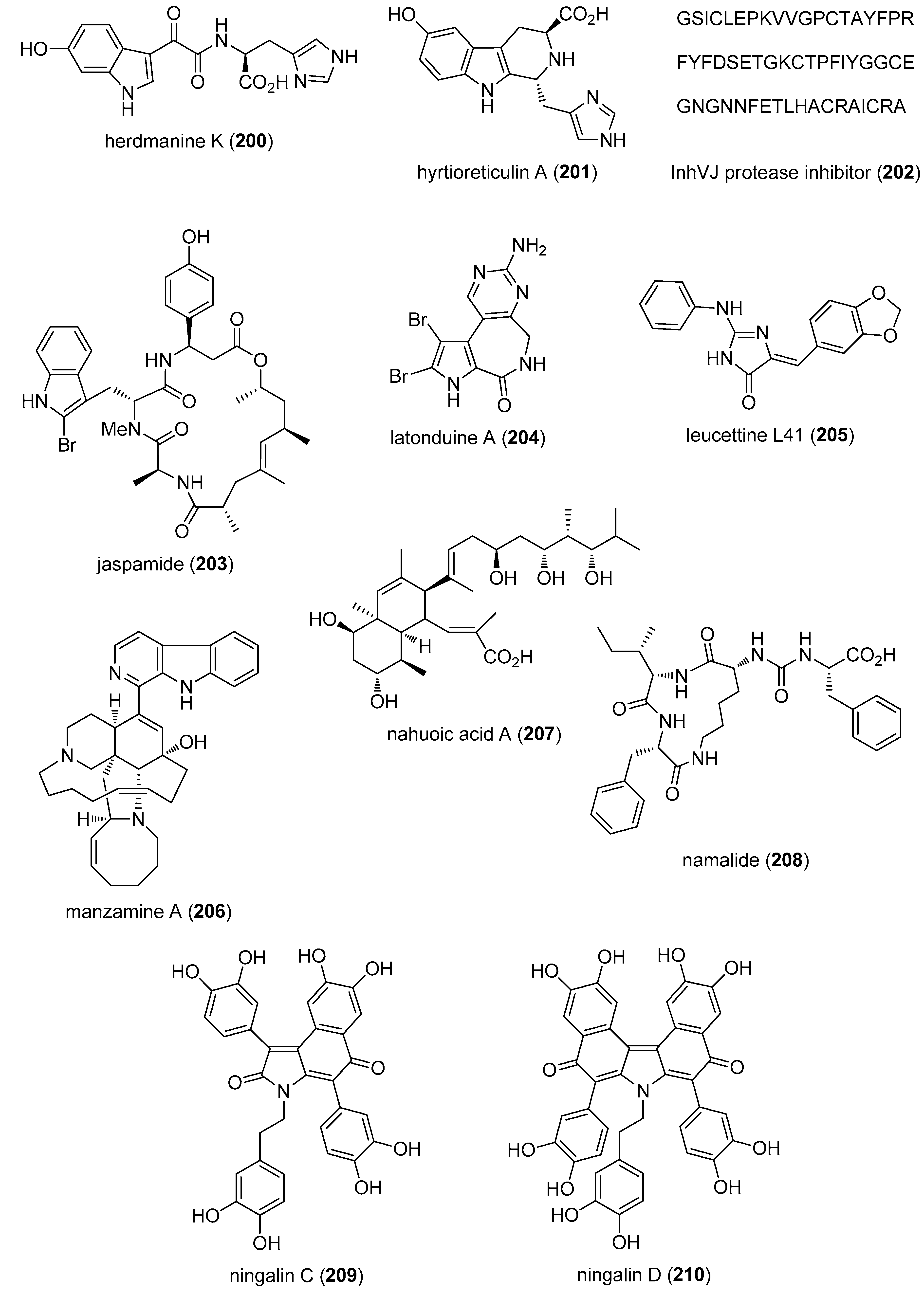
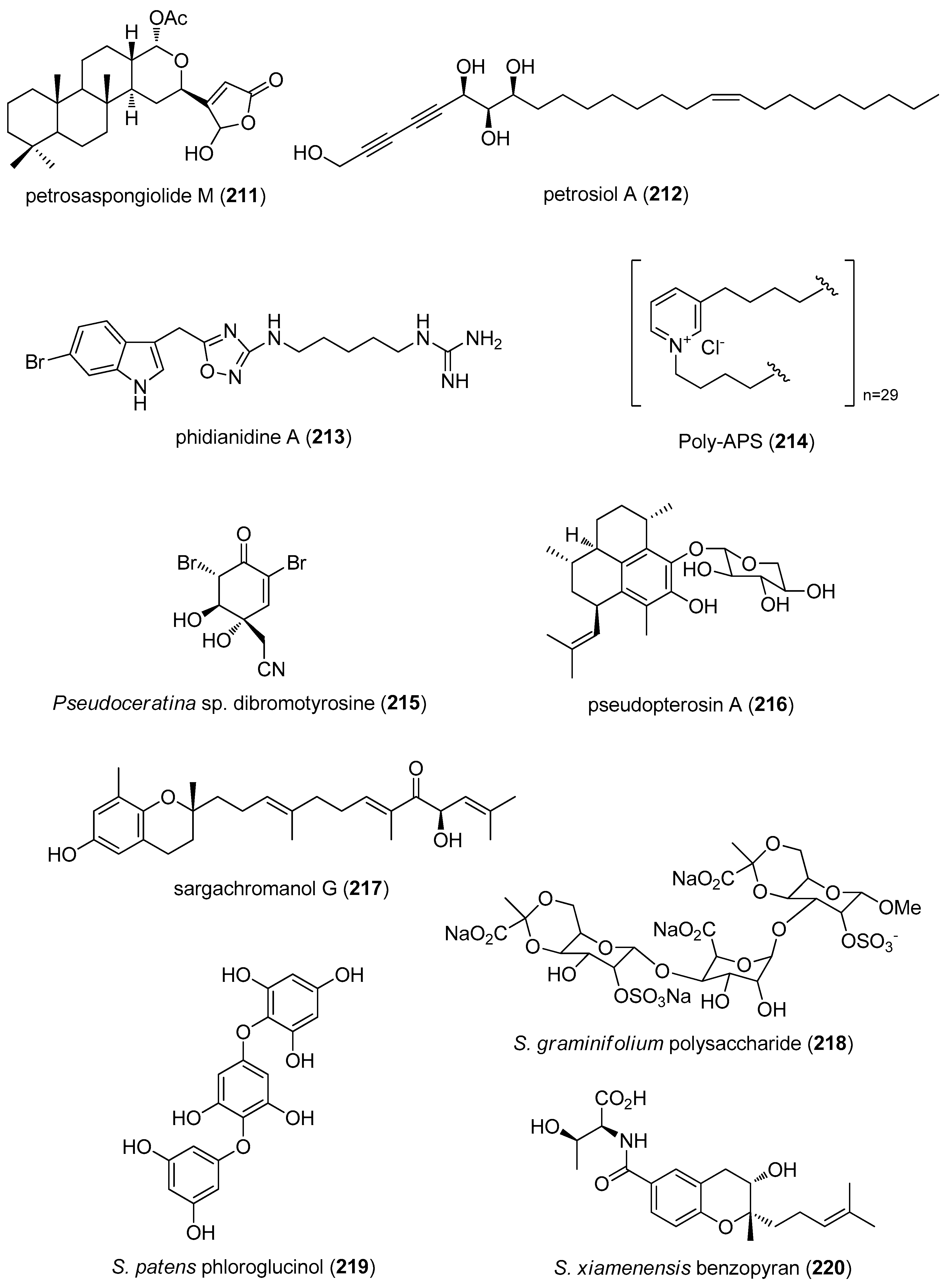

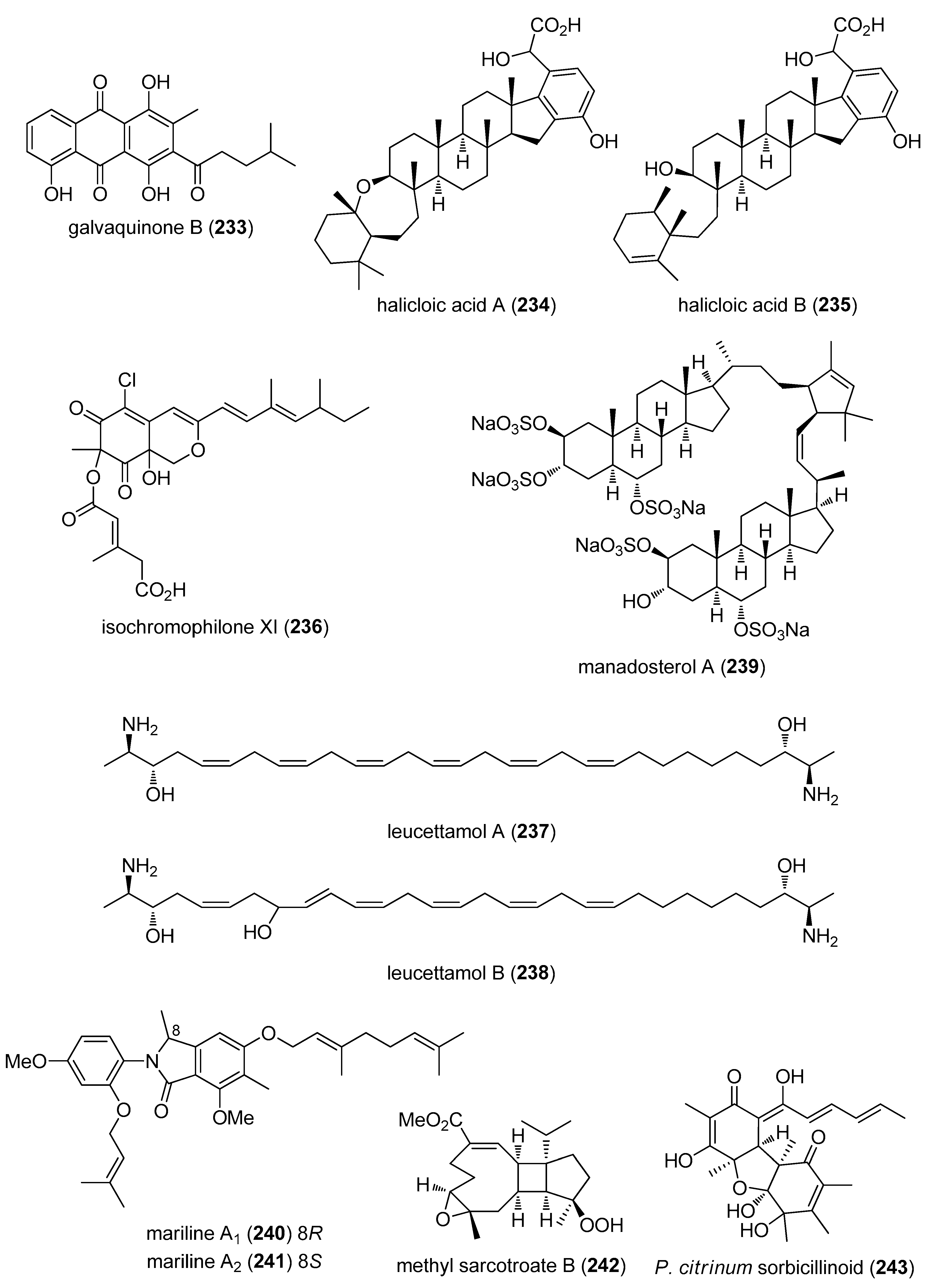
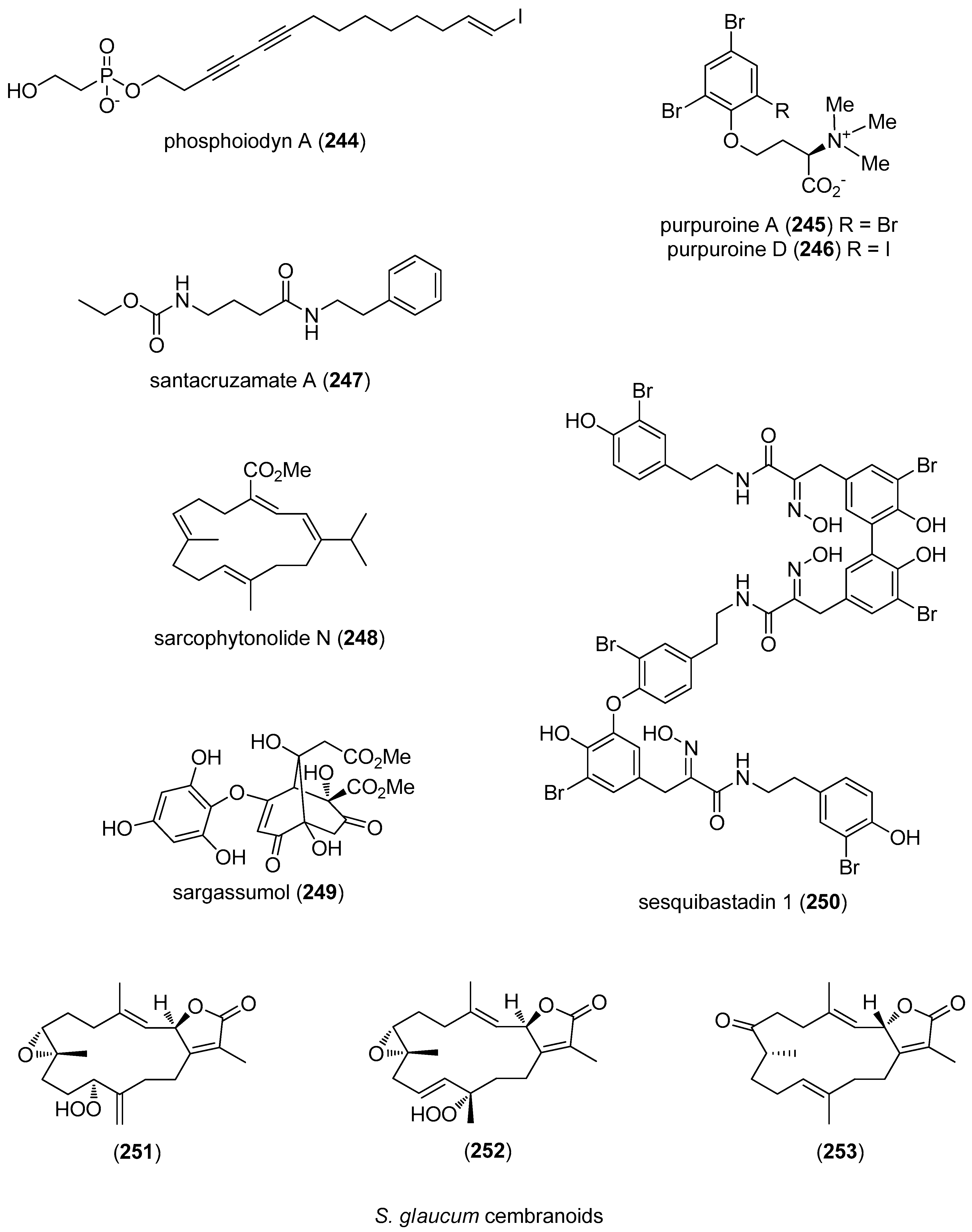

| Drug Class | Compound/Organism a | Chemistry | Pharmacologic Activity | IC50 b | MMOA b | Country c | References |
|---|---|---|---|---|---|---|---|
| Antibacterial | anthracimycin (1)/bacterium | Polyketide d | B. anthracis & S. aureus inhibition | 0.03–0.06 μg/mL + | DNA/RNA inhibition | USA | [31] |
| Antibacterial | chrysophaentins (2,3)/alga | Shikimate h | Gram-negative & -positive bacterial inhibition | 27–84 μM + | Competitive inhibition of FtsZ GTP-binding site | ESP, USA | [32] |
| Antibacterial | merochlorin A (4)/bacterium | Terpenoid e | C. dificile & S. aureus strains inhibition | 0.3–2 μg/mL + | DNA, RNA, protein & cell wall synthesis inhibition | USA | [33] |
| Antibacterial | aflatoxin B2b (5)/fungus | Polyketide d | B. subtilis & E. aerogenes inhibition | 1.7, 1.1 μM + | Undetermined | CHN | [34] |
| Antibacterial | ageloxime B (6)/sponge | Alkaloid/terpenoid e | S. aureus inhibition | 7.2–9.2 μg/mL * | Undetermined | CHN, USA | [35] |
| Antibacterial | Alternaria sp. anthraquinones (7–9)/fungus | Polyketide d | E. coli & V. parahemolyticus inhibition | 0.62–5 μM + | Undetermined | CHN | [36] |
| Antibacterial | antimycin B2 (10)/bacterium | Shikimate/Polyketide d | L. hongkongensis inhibition | 8 μg/mL + | Undetermined | CHN | [37] |
| Antibacterial | Aspergillus sp. (−)sydonol (11)/fungus | Terpenoid e | S. albus & M. tetragenus inhibition | 1.2–5 μg/mL + | Undetermined | CHN, NLD | [38] |
| Antibacterial | axistatins 1–3 (12–14)/sponge | Alkaloid/terpenoid e | C. neoformans & S. aureus inhibition | 1–4 μg/mL + | Undetermined | AUS, USA | [39] |
| Antibacterial | bromophycoic acid A & E (15,16)/alga | Terpenoid e | S. aureus & E. faecilis inhibition | 1.6 μg/mL + | Undetermined | FJI, USA | [40] |
| Antibacterial | cadeolides C–F (17–20)/tunicate | Shikimate h | S. aureus inhibition | 0.13–3 μg/mL + | Undetermined | S. KOR | [41] |
| Antibacterial | cadiolides E–I (21–23)/ascidian | Shikimate h | S. aureus & B. subtilis inhibition | 0.8–12 μg/mL + | Undetermined | S. KOR | [42] |
| Antibacterial | citreamicin θ A & B (24,25)/bacterium | Polyketide d | S. aureus inhibition | 0.25–1 μg/mL * | Undetermined | CHN, SAU | [43] |
| Antibacterial | communol A & F (26,27)/fungus | Polyketide d | E. coli inhibition | 4.1, 6.4 μg/mL + | Undetermined | CHN | [44] |
| Antibacterial | D. spiralis dolabellanes (28,29)/alga | Terpenoid e | S. aureus inhibition | 2–8 μg/mL + | Undetermined | GRC, ESP, UK | [45] |
| Antibacterial | enhygrolide A (30)/bacterium | Shikimate h | A. cristallopoietes inhibition | 4 μg/mL + | Undetermined | DEU | [46] |
| Antibacterial | eudistomin Y11 (31)/ascidian | Alkaloid f | B. subtilis & S. typhimurium inhibition | 3.12 μg/mL + | Undetermined | S. KOR | [47] |
| Antibacterial | fradimycin B (32)/bacterium | Polyketide d | S. aureus inhibition | 2.0 μg/mL + | Undetermined | CHN | [48] |
| Antibacterial | Haliclona diAPS (33–35)/sponge | Alkaloid f | M. luteus inhibition | 3.1 μg/mL + | Undetermined | S. KOR | [49] |
| Antibacterial | hyrtimomine D (36)/sponge | Alkaloid f | S. aureus inhibition | 4 μg/mL + | Undetermined | JPN | [50] |
| Antibacterial | ianthelliformisamine A (37)/sponge | Alkaloid f | P. aeruginosa inhibition | 6.8 μM | Undetermined | AUS | [51] |
| Antibacterial | kocurin (38)/bacterium | Peptide f | MR S. aureus inhibition | 0.25 μg/mL + | Undetermined | ESP, USA | [52] |
| Antibacterial | lamellarin O (39)/sponge | Alkaloid f | B. subtilis inhibition | 2.5 μM | Undetermined | AUS | [53] |
| Antibacterial | Laurencia sesquiterpenes (40–42)/alga | Terpenoid e | E. coli & S. aureus inhibition | 5–7 μg/disk ++ | Undetermined | CHN, USA | [54] |
| Antibacterial | lobophorin H (43)/bacterium | Terpenoid glycoside | B. subtilis inhibition | 1.57 μg/mL + | Undetermined | CHN | [55] |
| Antibacterial | marthiapeptide A (44)/bacterium | Peptide f | M. luteus & B. thuringiensis inhibition | 2.0 μg/mL * | Undetermined | CHN | [56] |
| Antibacterial | napyradiomycin A1 & B3 (45,46)/bacterium | Terpenoid/polyketide d | S. aureus inhibition | 0.5–2 μg/mL + | Undetermined | CHN | [57,58] |
| Antibacterial | Nigrospora sp. anthraquinones (47,48)/fungus | Polyketide d | E. coli & S. aureus inhibition | 0.6–0.7 μM + | Undetermined | CHN | [59] |
| Antibacterial | ohmyungsamycin A (49)/bacterium | Peptide f | B. subtilis inhibition | 4.28 μM + | Undetermined | S. KOR | [60] |
| Antibacterial | penicifuran A (50)/fungus | Shikimate h | S. albus inhibition | 3.1 μM + | Undetermined | CHN | [61] |
| Antifungal | crambescidin-816 (51)/sponge | Alkaloid f | S. cerevisiae growth inhibition | 1 μM + | G2/M cell cycle arrest and apoptosis | ESP, FRA | [62] |
| Antifungal | neothyonidioside (52)/sea cucumber | Terpenoid glycoside | S. cerevisiae inhibition | 1 μM + | Binding to plasma membrane sterols | NZL | [63] |
| Antifungal | ageloxime B (6)/sponge | Alkaloid/terpenoid | C. neoformans inhibition | 4.9 μg/mL * | Undetermined | CHN, USA | [35] |
| Antifungal | aurantoside K (53)/sponge | Polyketide/alkaloid glycoside | C. albicans inhibition | 1.95 μg/mL + | Undetermined | FJI | [64] |
| Antifungal | caulerprenylol B (54)/alga | Terpenoid e | C. glabrata & C. neoformans inhibition | 4.0 μg/mL + | Undetermined | CHN | [65] |
| Antifungal | didymellamide A (55)/fungus | Alkaloid f | C. albicans inhibition | 3.1 μg/mL + | Undetermined | JPN | [66] |
| Antifungal | hippolachnin A (56)/sponge | Polyketide d | T. rubrum, M. gypseum & C. neoformans inhibition | 0.41 μM + | Undetermined | CHN | [67] |
| Antifungal | holotoxins F & G (57,58)/sea cucumber | Terpenoid glycoside | C. albicans, Microsporum & Cryptococcus inhibition | 1.4–5.8 μM + | Undetermined | CHN, DEU | [68] |
| Antifungal | hyrtimomine D & E (36,59)/sponge | Alkaloid f | C. albicans & C. neoformans inhibition | 4–16 μg/mL + | Undetermined | JPN | [50] |
| Antifungal | nagelamide Z (60)/sponge | Alkaloid f | C. albicans inhibition | 0.25 μg/mL * | Undetermined | JPN | [69] |
| Antifungal | woodylide A (61)/sponge | Polyketide d | C. neoformans inhibition | 3.7 μg/mL * | Undetermined | CHN | [70] |
| Antiprotozoal | araplysillin I (62)/sponge | Alkaloid f | P. falciparum FcB1 & 3D7 strain inhibition | 4.5 μM | Undetermined | AUS, DEU, FJI, FRA | [71] |
| Antiprotozoal | ascidiathiazone A (63)/ascidian | Alkaloid f | P. falciparum K1 strain inhibition | 3.3 μM | Undetermined | NZL, CHE | [72] |
| Antiprotozoal | axidjiferosides A–C (64–66)/sponge | Glycosphingolipid | P. falciparum FcB1strain inhibition | 0.53 μM | Undetermined | FRA | [73] |
| Antiprotozoal | cytosporone E (67)/fungus | Polyketide d | P. falciparum inhibition | 13 μM ** | Undetermined | USA | [74] |
| Antiprotozoal | dicerandrol D (68)/fungus | Polyketide d | P. falciparum 3D7 strain inhibition | 0.6 μM | Undetermined | CHN, TWN, USA | [75] |
| Antiprotozoal | dihydroingenamine D (69)/sponge | Alkaloid f | P. falciparum D6 & W2 strain inhibition | 57–72 ng/mL | Undetermined | USA | [76] |
| Antiprotozoal | 19-hydroxypsammaplysin E (70)/sponge | Alkaloid f | P. falciparum 3D7strain inhibition | 6.4 μM | Undetermined | AUS, IDN | [77] |
| Antiprotozoal | kabiramide L (71)/sponge | Polyketide d | P. falciparum K1 strain inhibition | 2.6 μM | Undetermined | THAI, AUT | [78] |
| Antiprotozoal | meridianin C & G (72,73)/tunicate | Alkaloid f | P. falciparum D6 & W2 strain inhibition | 4.4–14.4 μM | Undetermined | IND | [79] |
| Antiprotozoal | orthidine F (74)/ascidian | Alkaloid f | P. falciparum K1 strain inhibition | 0.90 μM | Undetermined | CHE, NZL | [80] |
| Antiprotozoal | plakortide U (75)/sponge | Polyketide d | P. falciparum FcM29 strain inhibition | 0.8 μM | Undetermined | FRA, ITA | [81] |
| Antiprotozoal | thiaplakortone A (76)/sponge | Alkaloid f | P. falciparum 3D7 & Dd2 strain inhibition | 0.006–0.051 μM | Undetermined | AUS | [82] |
| Antiprotozoal | tsitikammamine C (77)/sponge | Alkaloid f | P. falciparum 3D7 & Dd2 strain inhibition | 13 & 18 nM | Undetermined | AUS | [83] |
| Antiprotozoal | urdamycinone E (78)/bacterium | Polyketide d | P. falciparum K1 strain inhibition | 0.05 μg/mL | Undetermined | THAI | [84] |
| Antiprotozoal | almiramide (79,80)/bacterium | Peptide f | T. brucei inhibition | 0.4–3.5 μM | Glycosome function inhibition | USA | [85] |
| Antiprotozoal | diazepinomicin (81)/bacterium | Alkaloid/terpenoid | T. brucei inhibition | 13.5 μM | Rhodesain inhibition | EGY, DEU | [86] |
| Antiprotozoal | (−)-elatol (82)/alga | Terpenoid e | T. cruzi inhibition | 1.5–3 μM * | Mitochondrial disfunction | BRA | [87] |
| Antiprotozoal | ascidiathiazone A (63)/ascidian | Alkaloid f | T. b. rhodesiense inhibition | 3.1 μM | Undetermined | NZL, CHE | [72] |
| Antiprotozoal | coibacin A (83)/bacterium | Polyketide d | L. donovani inhibition | 2.4 μM | Undetermined | USA, PAN | [88] |
| Antiprotozoal | cristaxenicin A (84)/gorgonian | Terpenoid e | T. congolense & L. amazonensis inhibition | 0.25 & 0.088 μM | Undetermined | JPN | [89] |
| Antiprotozoal | manadoperoxide B analogues (85,86)/sponge | Polyketide d | T. b. rhodesiense inhibition | 3–11 ng/mL | Undetermined | ITA, IDN, CHE, IRL | [90] |
| Antituberculosis | asperterpenoid A (87)/fungus | Terpenoid e | M. tuberculosis PTP inhibition | 2.2 μM | Undetermined | CHN | [91] |
| Antituberculosis | brevianamide S (88)/fungus | Alkaloid f | BCG inhibition | 6.25 μg/mL + | Undetermined | AUS, CHN | [92] |
| Antituberculosis | lobophorin G (89)/bacterium | Terpenoid e glycoside | BCG inhibition | 1.56 μg/mL + | Undetermined | CHN | [93] |
| Antituberculosis | neamphamide B (90)/sponge | Peptide f | M. bovis inhibition | 1.56 μg/mL + | Undetermined | JPN | [94] |
| Antituberculosis | S. flava diterpenes (91,92)/sponge | Terpenoid e | M. tuberculosis H37Rv inhibition | 15, 32 μg/mL + | Undetermined | USA | [95] |
| Antituberculosis | urdamycinone E (78)/bacterium | Polyketide d | M. tuberculosis H37Ra inhibition | 3.13 μg/mL + | Undetermined | THAI | [84] |
| Antiviral | halistanol sulfates (93,94)/sponge | Terpenoid f | Human Herpes simplex virus-1 inhibition | 0.5–12.2 μg/mL | Attachment & penetration inhibition | ARG, BRA | [96] |
| Antiviral | L. arboreum metabolites (95–97)/soft coral | Terpenoid/sphingolipid | HIV-1 protease inhibition | 4.8–7.2 μM * | Molecular docking & HIV-1 protease receptor | ZAF | [97] |
| Antiviral | manoalide (98)/sponge | Terpenoid e | Hepatitis C virus inhibition | 15–70 μM | RNA helicase and ATPase inhibition | JPN | [98] |
| Antiviral | N. aculeata metabolites (99,100)/alga | Polyketide d | Human rhinoviruses 2 & 3 inhibition | 2.5–7.1 μg/mL | Cytopathic effect inhibition | S. KOR | [99] |
| Antiviral | stachybotrin D (101)/fungus | Alkaloid/terpenoid | HIV-1 replication inhibition | 8.4 μM | Reverse transcriptase inhibition | CHN | [100] |
| Antiviral | streptoseolactone (102)/bacterium | Terpenoid f | Neuraminidase inhibition | 3.9 μM | Noncompetitive inhibition | CHN | [101] |
| Antiviral | asperterrestide A(103)/fungus | Peptide f | H3N2 influenza virus inhibition | 8.1 μM | Undetermined | CHN | [102] |
| Antiviral | Cladosporium sp. alkaloids (104,105)/fungus | Alkaloid f | H1N1 influenza virus inhibition | 82–85 μM | Undetermined | CHN | [103] |
| Antiviral | isorhodoptilometrin-1-methyl ether (106)/fungus | Polyketide d | Hepatitis C NS3/4A protease inhibition | >1 ng/mL * | Undetermined | EGY | [104] |
| Antiviral | massarilactone H (107)/fungus | Polyketide d | Influenza virus neuraminidase inhibition | 8.2 μM | Undetermined | CHN, MYS | [105] |
| Antiviral | pyronepolyene C-glucoside (108)/fungus | Polyketide d | H1N1 influenza virus inhibition | 91.5 μM | Undetermined | CHN | [106] |
| Antiviral | S. candidula sterol (109,110)/soft coral | Terpenoid/sphingolipid | H5N1 avian influenza virus inhibition | 1 ng/mL * | Undetermined | EGY | [107] |
| Antiviral | S. vulgare glycolipid (111)/alga | Glycolipid | Human herpes simplex virus-1 & 2 inhibition | <50 μg/mL | Undetermined | BRA | [108] |
| Anthelmintic | echinosides A & B (112,113)/sea cucumber | Terpenoid glycoside | S. mansoni worm lethality | 0.19, 0.27 μg/mL +++ | Undetermined | EGY | [109] |
| Drug Class | Compound/Organism a | Chemistry | Pharmacological Activity | IC50 b | MMOA c | Country d | References |
|---|---|---|---|---|---|---|---|
| Antidiabetic | octaphlorethol A (114)/alga | Polyketide e | Increased glucose uptake in rat myoblast cells | 50 μM * | Glucose transporter 4 translocation | S. KOR | [120] |
| Anti-inflammatory | apo-9′-fucoxanthinone (115)/alga | Terpenoid f | Macrophage TNF-α, IL-6 & 12 expression inhibition | 5–14 μM | MAPK pathway inhibition | S. KOR | [121] |
| Anti-inflammatory | astaxanthin (116)/alga | Terpenoid f | Macrophage cytokine inhibition | 10 μM * | SHP-1 restoration | ITA | [122] |
| Anti-inflammatory | bengamide A & B (117,118)/sponge | Alkaloid g | Macrophage TNF-α & IL-6 inhibition | 0.5 μM * | IĸBα phosphorylation inhibition | USA | [123] |
| Anti-inflammatory | bis-N-norgliovictin (119)/fungus | Alkaloid g | Macrophage TNF-α, IL1-6, MCP-1 release inhibition in vitro | 0.5 μg/mL * | Inflammatory gene inhibition | CHN | [124] |
| Anti-inflammatory | 6,6′-bieckol (120)/alga | Polyketide e | Macrophage TNF-α & IL-6 expression inhibition | 25 μM * | Inhibition of NFκB | S. KOR, USA | [125] |
| Anti-inflammatory | coibacin B (121)/bacterium | Polyketide e | Macrophage NO inhibition | 5 μM | iNOS, TNF-α, IL-1, IL-6 transcription inhibition | USA, PAN | [88] |
| Anti-inflammatory | 11-epi-sinulariolide acetate (122)/soft coral | Terpenoid f | Macrophage COX-2 & IL-8 expression inhibition | 10 μM | Ca2+ signaling inhibition | TWN | [126] |
| Anti-inflammatory | honaucin A (123)/bacterium | Polyketide e | Macrophage NO inhibition | 4 μM | iNOS, TNF-α, IL-1, IL-6 transcription inhibition | USA, PAN | [127] |
| Anti-inflammatory | Hymeniacidon sp. amphilectanes (124,125)/sponge | Terpenoid f | Brain microglia TXB2 inhibition | 0.2 μM | SOX independent & COX dependent | USA | [128] |
| Anti-inflammatory | largazole (126)/bacterium | Peptide g | Modulation of human RA synovial fibroblasts in vitro | 5 μM * | Enhanced HDAC6 & ICAM-1 | USA | [129] |
| Anti-inflammatory | lemnalol (127)/soft coral | Terpenoid f | In vivo arthritis inhibition | 30 mg/kg* | iNOS, COX-2 and c-Fos expression inhibition | TWN | [130] |
| Anti-inflammatory | neoechinulin A (128)/fungus | Alkaloidg | Macrophage PGE2 and NO expression inhibition | 25–50 μM * | Inhibition of NFκB & MAPK | S. KOR; CHN | [131] |
| Anti-inflammatory | penstyrylpyrone (129)/fungus | Shikimate/polyketide | Macrophage NO, PGE2, IL1β inhibition | 9.3–13.5 μM | PTP1B inhibition | S. KOR | [132] |
| Anti-inflammatory | perthamide C (130)/sponge | Peptide g | Carrageenan-induced paw edema inhibition | ND | Induction of proteome changes | ITA | [133] |
| Anti-inflammatory | R-prostaglandins (131,132)/soft coral | Polyketide e | Topical inflammation inhibition | ND | PMN elastase inhibition | COL | [134] |
| Anti-inflammatory | sinularin (133)/soft coral | Terpenoid f | Carrageenan-induced spinal neuroinflammation inhibition | 0.1 μM * | iNOS & COX-2 inhibition | TWN | [135] |
| Anti-inflammatory | swinhosterol B (134)/sponge | Terpenoid f | Lymphocyte release of IL-10 | 10 μM * | Pregnane-X-receptor agonist | ITA, FRA | [136] |
| Anti-inflammatory | A. polyacanthus steroids (135,136)/starfish | Terpenoid f | Bone marrow-derived dendritic cells IL-6 and TNF-α inhibition | 1.8–7.0 μM | Undetermined | S. KOR, VNM | [137] |
| Anti-inflammatory | barettin (137)/sponge | Alkaloid g | Macrophage anti-inflammatory IL-10 release in vitro | 50 μg/mL | Undetermined | NOR | [138] |
| Anti-inflammatory | briarenolide F (138)/octocoral | Terpenoid f | Neutrophil superoxide inhibition | 3.82 μg/mL | Undetermined | TWN | [139] |
| Anti-inflammatory | Callyspongia sp. diketopiperazine (139)/sponge | Peptide g | Macrophage IL1β release inhibition in vitro | 5 μg/mL * | Undetermined | CHN | [140] |
| Anti-inflammatory | 6-epi-cladieunicellin F (140)/octocoral | Terpenoid f | Neutrophil superoxide and elastase inhibition | 10 μM * | Undetermined | TWN | [141] |
| Anti-inflammatory | crassarosteroside A (141)/soft coral | Terpenoid glycoside f | Macrophage iNOS protein inhibition | 10 μM * | Undetermined | TWN | [142] |
| Anti-inflammatory | cystodione A (142)/alga | Terpenoid f | Radical-scavenging and macrophage TNF-α inhibition in vitro | 8–22 μM * | Undetermined | ESP, MAR | [143] |
| Anti-inflammatory | densanins A & B (143,144)/sponge | Alkaloid g | Macrophage NO release inhibition | 1–2.1 μM | Undetermined | S. KOR | [144] |
| Anti-inflammatory | dissesterol (145)/soft coral | Terpenoid f | Bone marrow dendritic cells IL-12 release inhibition | 4 μM | Undetermined | S. KOR, VNM | [145] |
| Anti-inflammatory | echinohalimane A (146)/gorgonian | Terpenoid f | Neutrophil elastase inhibition | 0.38 μg/mL | Undetermined | TWN | [146] |
| Anti-inflammatory | eunicidiol (147)/gorgonian | Terpenoid f | PMA-induced mouse ear edema inhibition | 100 μg/ear | Undetermined | CAN | [147] |
| Anti-inflammatory | flexibilisolide C (148)/soft coral | Terpenoid f | Macrophage COX-2 & iNOS expression inhibition | 10 μM * | Undetermined | TWN | [148] |
| Anti-inflammatory | flexibilisquinone (149)/soft coral | Terpenoid f | Macrophage COX-2 & iNOS expression inhibition | 10–20 μM * | Undetermined | TWN | [149] |
| Anti-inflammatory | lobocrassin F (150)/soft coral | Terpenoid f | Neutrophil elastase release inhibition | 6.3 μM * | Undetermined | TWN | [150] |
| Anti-inflammatory | perthamide J (151)/sponge | Peptide g | Carrageenan-induced paw edema reduction | 0.3 mg/kg * | Undetermined | ITA, FRA | [151] |
| Anti-inflammatory | pseudoalteromone A (152)/bacterium | Terpenoid f | Neutrophil elastase inhibition | 10 μg/mL * | Undetermined | TWN | [152] |
| Anti-inflammatory | sarcocrassocolide M (153)/soft coral | Terpenoid f | Macrophage COX-2 & iNOS expression inhibition | 10 μM * | Undetermined | TWN | [153] |
| Anti-inflammatory | sclerosteroids K & M (154,155)/soft coral | Terpenoid f | Macrophage COX-2 & iNOS expression inhibition | 10 μM * | Undetermined | TWN | [154] |
| Anti-inflammatory | seco-briarellinone (156)/octocoral | Terpenoid f | Macrophage NO release inhibition | 4.7 μM | Undetermined | PAN | [155] |
| Anti-inflammatory | sinularioside (157)/soft coral | Glycolipid | Macrophage NO release inhibition | 30 μM * | Undetermined | ITA | [156] |
| Immune system | lobocrassin B (158)/soft coral | Terpenoid f | Dendritic cell activation inhibition | 39 μM * | NF-κB translocation and TNF-α release inhibition | TWN | [157] |
| Immune system | penicacid B(159)/fungus | Polyketide e | T lymphocyte proliferation inhibition | 0.23–20 μM | IMPDH inhibition | CHN | [158] |
| Nervous system | APETx2 peptide (160)/sea anemone | Peptide g | ASIC3 inhibition | 61 nM | N- and C- termini truncation decrease inhibition | AUS | [159] |
| Nervous system | asteropsin A (161)/sponge | Peptide g | Enhancement of neuronal Ca2+ influx | 14 nM | No binding with VGSC site 2 | S. KOR, USA | [160] |
| Nervous system | BcsTx peptides (162,163)/sea anemone | Peptide g | rKv1.1 inhibition | 0.02–80 nM | Potassium influx inhibition | BRA, BEL | [161] |
| Nervous system | C. consors peptide (164)/cone snail | Peptide g | Muscle relaxation induction | 0.15 μM | Nav1.4 & Nav1.2 channel inhibition | BEL, FRA, CHE, CHL, DEU, NLD, | [162] |
| Nervous system | C. magnificus conotoxin MfVIA(165)/cone snail | Peptide g | Neuronal Na+ current inhibition | 95 nM | Nav1.8 and Nav1.4 channel inhibition | AUS | [163] |
| Nervous system | C. regius conotoxin RegIIA (166)/cone snail | Peptide g | ACH-current inhibition | 33 nM | Α2β2 ACH receptor | AUS, DEU, USA | [164] |
| Nervous system | C. regularis peptide (167)/cone snail | Peptide g | Antinociceptive activity | 0.85 mg/kg * | Cav2.2 channel inhibition | MEX | [165] |
| Nervous system | convolutamydine A (168)/bryozoa | Alkaloid g | Antinociceptive activity | 1 mg/kg | Cholinergic, opioid and nitric oxide | BRA | [166] |
| Nervous system | H. crispa polypeptides (169)/sea anemone | Peptide g | Antinociceptive and analgesic activity in vivo | 0.01–0.1 mg/kg * | Inhibition of TRPV1 vanilloid 1 receptor | RUS | [167] |
| Nervous system | ianthellamide A (170)/sponge | Alkaloid g | Increased kynurenic acid in vivo | 200 mg/kg * | Kynurenine 3- hydroxylase inhibition | AUS | [168] |
| Nervous system | leucettamine B (171)/sponge | Alkaloid g | Reduction of neurodegeneration in brain slices by analog leucettine L41 | 0.6–4.1 μM | Dual tyrosine phosphorylation kinase inhibition | FRA, UK, USA | [169] |
| Nervous system | pulchranin A (172)/sponge | Alkaloid g | TRPV1 receptor inhibition | 41.2 μM | Ca2+ response inhibition | RUS, S. KOR | [170] |
| Nervous system | serinolamide B (173)/bacterium | Alkaloid g | CB1 & CB2 binding | ** | cAMP accumulation inhibition | USA | [171] |
| Nervous system | arigsugacin I (174)/fungus | Terpenoid f | acetylcholinesterase inhibition | 0.64 μM | Undetermined | CHN | [172] |
| Nervous system | asperterpenol A (175)/fungus | Terpenoid f | acetylcholinesterase inhibition | 2.3 μM | Undetermined | CHN | [173] |
| Nervous system | cymatherelactone (176)/alga | Polyketide e | voltage-gated sodium channel inhibition | 16 μM | Undetermined | USA | [174] |
| Nervous system | dictyodendrin H (177)/sponge | Alkaloid g | BACE inhibition | 1 μM | Undetermined | AUS | [175] |
| Nervous system | geranylphenazinediol (178)/bacterium | Alkaloid g | acetylcholinesterase inhibition | 2.62 μM | Undetermined | DEU | [176] |
| Nervous system | halomadurones C & D (179,180)/bacteria | Terpenoid e | Nrf2-ARE activation | 3.7 μM * | Undetermined | USA | [177] |
| Nervous system | lamellarin O (39)/sponge | Alkaloid g | BACE inhibition | <10 μM | Undetermined | AUS | [53] |
| Nervous system | Psammocinia sp. ircinianin lactams (181,182)/sponge | Terpenoid f | A3 GlyR potentiation | 8.5 μM | Undetermined | AUS, DEU | [178] |
| Nervous system | starfish polar steroids (183–188)/starfish | Terpenoid f | Neuritogenic and neuroprotective | 1–100 nM | Undetermined | RUS | [179] |
| Compound/Organism a | Chemistry | Pharmacological Activity | IC50 b | MMOA c | Country d | References |
|---|---|---|---|---|---|---|
| astaxanthin (189)/alga | Terpenoid f | Human sperm capacitation | 2 μM * | Increased tyrosine phosphorylation | ITA | [182] |
| astaxanthin (189)/alga | Terpenoid f | Apoptosis reduction in retinal ganglion cells | 2 μM | H2O2 inhibition | CHN | [242] |
| biselyngbyaside (190)/bacterium | Polyketide e | Osteoclast apoptosis induction | 30 nM * | c-Fos and NFATc1 inhibition | JPN | [183] |
| Callyspongia sp. bisacetylenic alcohol (191)/sponge | Polyketide e | Lymphatic endothelial cell proliferation inhibition | 0.11 μM | Cell cycle arrest | JPN, NLD | [184] |
| conicasterol E (192)/sponge | Terpenoid f | Bile acid detoxification | 10 μM * | Farnesoid and pregnane receptor activity modulation | ITA, PYF | [185] |
| 6′′-debromohamacanthin A (193)/sponge | Alkaloid g | Angiogenesis inhibition | 14.8 μM | PI3K/AKT/mTOR signaling inhibition | CAN, S. KOR | [186] |
| dieckol (194)/alga | Polyketide e | Inhibition of melanin synthesis | >120 µM * | Cellular tyrosinase inhibition | S. KOR | [187] |
| fructigenine A (195)/fungus | Alkaloid g | PTP1B inhibition | 10.7 μM | Noncompetitive inhibition | S. KOR | [188] |
| geoditin A (196)/sponge | Terpenoid f | Melanogenesis inhibition | 1 μg/mL | cAMP-dependent signaling inhibition | CHN, USA | [189] |
| gorgosterol (197)/soft coral | Terpenoid f | FXR transactivation antagonism | 10 μM | Inhibition of OSTα & BSEP genes | ITA | [190] |
| gracilioether B (198)/sponge | Polyketide e | PPARγ binding | 5 μM * | Cys285 covalent binding | FRA, ITA | [191] |
| gracilioether K (199)/sponge | Polyketide e | PXR agonistic activity | 10 μM * | Binding to LBD by molecular docking | ITA | [192] |
| herdmanine K (200)/ascidian | Alkaloid g | PPAR-γ agonist | 1 μg/mL * | mRNAexpression of target genes | S. KOR | [193] |
| hyrtioreticulin A (201)/sponge | Alkaloid g | Ubiquitin-activating enzyme inhibition | 2.4 μM | Putative ubiquitin-adenylate intermediate inhibition | IDN, JPN, NLD | [194] |
| InhVJ protease inhibitor (202)/sea anemone | Peptide g | Trypsin and α-chymotrysin inhibition | ** | Glu45 involved in InhVJ-trypsin complex | BEL, RUS | [195] |
| jaspamide (203)/sponge | Peptide g | Decreased cardiomyocyte activity and function | 1–19 μM * | Kv1.5 channel inhibition | USA | [196] |
| latonduine A (204)/sponge | Alkaloid g | F508del-CTFR correction | 1 μM * | PARP-3 inhibition | CAN | [197] |
| leucettine L41 (205)/sponge | Alkaloid g | DYR and CL tyrosine kinase inhibition | 21–77 nM | Primary and secondary targets identified | FRA | [169] |
| manzamine A (206)/sponge | Alkaloid g | Cholesterol esters inhibition | 4.1 μM | ACAT inhibition | JPN | [198] |
| nahuoic acid A (207)/bacterium | Polyketide e | SETDH inhibition | 6.5 μM | Competitive inhibition | PNG, CAN | [199] |
| namalide (208)/sponge | Peptide g | Carbopeptidase A inhibition | 0.25 μM | d-Lys presence required for activity | ITA, USA | [200] |
| ningalins C & D (209,210)/ascidian | Alkaloid g | CK1δ and GSK3β inhibition | 0.2 μM | Binding to ATP binding site | AUS | [201] |
| octaphlorethol A (114)/alga | Polyketide e | Glucose tansporter 4 increase | 10 μM * | AKT and AMPK activation | S. KOR | [120] |
| petrosaspongiolide M (211)/sponge | Terpenoid f | Proteasome inhibition | 0.085–1.05 μM | Pro-apoptotic bax induction | ITA | [202] |
| petrosiol A (212)/sponge | Polyketide e | PDGF-induced DNA synthesis inhibition | 0.73 μM | PDGF receptor-β signaling inhibition | JPN | [203] |
| phidianidine A (213)/mollusc | Alkaloid g | CXCR4 ligand antagonist | <50 μM | CXCL12-dependent DNA synthesis inhibition | ITA | [204] |
| Poly-APS (214)/sponge | Polyketide e | Thoracic aorta contraction inhibition in vitro | <10 μM * | Concentration-dependent LDH release | SVN | [205] |
| Pseudoceratina sp. Dibromotyrosine (215)/sponge | Alkaloid g | Apoptosis induction | 5 μg/mL | Mitochondrial disfunction | EGY, TWN | [206] |
| pseudopterosin A (216)/soft coral | Terpenoid f | Increased HUVEC proliferation | 13 nM | Enhancement potency by HPβCD | USA | [207] |
| sargachromanol G (217)/alga | Terpenoid f | Osteoclastogenesis inhibition | 20 Μm * | NF-ĸB phosphorylation of MAPK kinases inhibition | S. KOR | [208] |
| S. graminifolium polysaccharide (218)/alga | Polysaccharide h | Improved mitochondrial disfunction and oxidative stress | 25 mg/kg *** | Increased activity of antioxidant enzymes | CHN | [209] |
| S. patens phloroglucinol (219)/alga | Polyketide e | α-amylase inhibition | 3.2 μg/mL | Competitive α-amylase inhibitor | JPN | [210] |
| S. xiamenensis benzopyran (220)/bacterium | Mixed biogenesis | Fibrosis inhibition | 30 μg/mL * | Anti-proliferation, anti-contractile and anti-adhesion activity | CHN | [211] |
| theonellasterol (221)/sponge | Terpenoid f | Farnesoid receptor transactivation inhibition | 50 μM * | SAR showed OH at C-4 and oxidation at C-3 required | ITA, JPN | [212] |
| toluquinol (222)/fungus | Shikimate | Angiogenesis inhibition in vitro and in vivo | 2.5 μM * | Cell cycle arrest induction | ESP | [213] |
| U. lactuca fatty acid (223)/alga | Polyketide e | ARE activator | 10 μg/mL * | Nrf2 transcription factor activation | USA | [214] |
| alotaketal C (224)/sponge | Terpenoid f | cAMP signaling activation | 6.5 μM | Undetermined | CAN | [215] |
| aspergentisyl A(225)/fungus | Polyketide e | DPPH radical-scavenging | 9.3 μM | Undetermined | CHN | [216] |
| A. terreus butyrolactone (226)/fungus | Shikimate | β-glucuronidase inhibition | 6.2 μM | Undetermined | LKA, PAK, USA | [217] |
| caulerpine (227)/alga | Alkaloid g | Spasmolytic effect on guinea pig ileum | 0.05–0.13 μM | Undetermined | BRA | [218] |
| conicasterol F (228)/sponge | Terpenoid f | FXR antagonism | 10 μM * | Undetermined | GBR, ITA | [219] |
| D. avara sesquiterpene (229)/sponge | Terpenoid f | FAK, IGF1 & ERBB2 kinase inhibition | 1 μg/mL * | Undetermined | DEU, GBR, EGY, SAU | [220] |
| D. gigantea sterols (230,231)/soft coral | Terpenoid f | Farnesoid receptor transactivation inhibition | 14–15 μM | Undetermined | S. KOR | [221] |
| dysidavarone A (232)/sponge | Terpenoid f | PTP1B inhibition | 9.98 μM | Undetermined | CHN | [222] |
| galvaquinone B (233)/bacterium | Polyketide e | Epigenetic activity | 1.0 μM | Undetermined | USA | [223] |
| halicloic acids A & B (234,235)/sponge | Terpenoid f | IDO1 inhibition | 10 & 11 μM | Undetermined | CAN, NLD | [224] |
| isochromophilone XI (236)/fungus | Polyketide e | PD4 inhibition | 8.3 μM | Undetermined | DEU | [225] |
| leucettamols A & B (237,238)/sponge | Terpenoid f | TRPA1 and TRPM8 channel inhibition | 4.7–6.4 μM | Undetermined | ITA | [226] |
| manadosterol A (239)/sponge | Terpenoid f | Ubiquitin E2 enzyme UBc13-Uev1A complex inhibition | 90 nM | Undetermined | IDN. JPN, NLD | [227] |
| marilines A1 & A2 (240,241)/fungus | Mixed biogenesis | HLE inhibition | 0.86 μM | Undetermined | DEU, GRC, PAN | [228] |
| methyl sarcotroate B (242)/soft coral | Terpenoid f | PTP1B inhibition | 6.97 μM | Undetermined | CHN | [229] |
| P. citrinum sorbicillinoid (243)/fungus | Polyketide e | Antioxidant | 30 μM | Undetermined | JPN | [230] |
| phosphoiodyn A (244)/sponge | Polyketide e | hPPARδ inhibition | 23.7 nM | Undetermined | AUS, S. KOR | [231] |
| purpuroines A & D (245,246)/sponge | Alkaloid g | LCK kinase inhibition | 0.94, 2.35 μg/mL | Undetermined | DEU, CHN | [232] |
| santacruzamate A (247)/bacterium | Alkaloid g | HDAC2 inhibition | 0.110 nM | Undetermined | PAN, USA | [233] |
| sarcophytonolide N (248)/soft coral | Terpenoid f | PTP1B inhibition | 5.9 μM | Undetermined | CHN, ITA | [234] |
| sargassumol (249)/alga | Polyketide e | Antioxidant | 47 μM | Undetermined | S. KOR | [235] |
| sesquibastadin 1 (250)/sponge | Alkaloid g | Protein kinases inhibition | 0.1–6.5 μM | Undetermined | CHN, DEU | [236] |
| S. glaucum cembranoids (251–253)/soft coral | Terpenoid f | Cytochrome P450 1A inhibition | 12.7–3.7 nM * | Undetermined | EGY, SAU, USA | [237] |
| symplocin A (254)/bacterium | Peptide g | Cathepsin E inhibition | 0.3 nM | Undetermined | USA | [238] |
| tsitsikammamine A derivative (255)/sponge | Alkaloid g | IDO1 inhibition | 0.9 μM | Undetermined | BEL, FRA | [239] |
| V. lanosa bromophenol (256)/alga | Terpenoid f | Biochemical & cellular antioxidant activity | 30 μg/mL | Undetermined | NOR | [240] |
| X. testudinaria fatty acid (257)/sponge | Polyketide e | Adipogenesis stimulation | 2 μM | Undetermined | JPN | [241] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayer, A.M.S.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine Pharmacology in 2012–2013: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar. Drugs 2017, 15, 273. https://doi.org/10.3390/md15090273
Mayer AMS, Rodríguez AD, Taglialatela-Scafati O, Fusetani N. Marine Pharmacology in 2012–2013: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Marine Drugs. 2017; 15(9):273. https://doi.org/10.3390/md15090273
Chicago/Turabian StyleMayer, Alejandro M. S., Abimael D. Rodríguez, Orazio Taglialatela-Scafati, and Nobuhiro Fusetani. 2017. "Marine Pharmacology in 2012–2013: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action" Marine Drugs 15, no. 9: 273. https://doi.org/10.3390/md15090273






