Bioactivity Assessment of Indian Origin—Mangrove Actinobacteria against Candida albicans
Abstract
:1. Introduction
2. Results
2.1. Isolation and Identification of Mangrove Actinobacteria
2.2. Genetic Screening
2.3. Phylogenetic Analysis of the Actinobacteri Strains
2.4. Antifungal Activity Prospect Evaluations
2.5. Antibiotic Sensitivity Assessment of Actinobacteria Strains
2.6. GCMS Analysis and Screening of Bioactive Compounds
2.7. Pharmacological Property Predictions of Screened Compounds
3. Discussion
4. Materials and Methods
4.1. Isolation of Marine Mangrove Actinobacteria
4.2. Genomic DNA Extraction for Identification Through 16S rRNA Sequencing
4.3. Amplification of 16S rRNA Gene and Sequencing
4.4. PCR Screening for Biosynthetic Genes
4.5. Phylogenetic Analysis of the Strains
4.6. Evaluation of Antifungal Activity
4.7. Antibiotic Susceptibility Test
4.8. GCMS Results and Screening of Pharmacologically Important Bioactive Compounds
4.9. Pharmacological Property Predictions of Screened Compounds
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Bérdy, J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J. Antibiot. (Tokyo) 2012, 65, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Sanchez, S. Microbial drug discovery: 80 Years of progress. J. Antibiot. (Tokyo) 2009, 62, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Zotchev, S.B. Marine actinomycetes as an emerging resource for the drug development pipelines. J. Biotechnol. 2012, 158, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.H.; Chan, K.G.; Lee, L.H.; Goh, B.H. Streptomyces bacteria as potential probiotics in aquaculture. Front. Microbiol. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nikolouli, K.; Mossialos, D. Bioactive compounds synthesized by non-ribosomal peptide synthetases and type-I polyketide synthases discovered through genome-mining and metagenomics. Biotechnol. Lett. 2012, 34, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Weissman, K.J. Chapter 1 Introduction to Polyketide Biosynthesis. Methods Enzymol. 2009, 459, 3–16. [Google Scholar] [PubMed]
- Katz, L.; Donadio, S. Polyketide Synthesis: Prospects for Hybrid Antibiotics. Annu. Rev. Microbiol. 1993, 47, 875–912. [Google Scholar] [CrossRef] [PubMed]
- Strieker, M.; Tanović, A.; Marahiel, M.A. Nonribosomal peptide synthetases: Structures and dynamics. Curr. Opin. Struct. Biol. 2010, 20, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Ayuso-Sacido, A.; Genilloud, O. New PCR primers for the screening of NRPS and PKS-I systems in actinomycetes: Detection and distribution of these biosynthetic gene sequences in major taxonomic groups. Microb. Ecol. 2005, 49, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Chen, Y.Y.; Ge, L.; Fang, T.T.; Meng, J.; Liu, Z.; Fang, X.Y.; Ni, S.; Lin, C.; Wu, Y.Y.; et al. PCR screening reveals considerable unexploited biosynthetic potential of ansamycins and a mysterious family of AHBA-containing natural products in actinomycetes. J. Appl. Microbiol. 2013, 115, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A.; Walsh, C.T. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: Logic machinery, and mechanisms. Chem. Rev. 2006, 106, 3468–3496. [Google Scholar] [CrossRef] [PubMed]
- Iii, J.E.C. Impact of 16S rRNA Gene Sequence Analysis for Identification of Bacteria on Clinical Microbiology and Infectious Diseases. Clin. Microbiol. Rev. 2004, 17, 840–862. [Google Scholar] [CrossRef]
- Azman, A.S.; Othman, I.; Velu, S.S.; Chan, K.G.; Lee, L.H. Mangrove rare actinobacteria: Taxonomy, natural compound, and discovery of bioactivity. Front. Microbiol. 2015, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Emerson, R.; Procópio, D.L.; Reis, I.; Kassawara, M.; Lúcio, J.; Azevedo, D.; Magali, J.; Araújo, D. Review article Antibiotics produced by Streptomyces. Br. J. Infect. Dis. 2012, 16, 466–471. [Google Scholar] [CrossRef]
- Keikha, N.; Mousavi, S.A.A.; Nakhaei, A.R.; Yadegari, M.H.; Bonjar, G.H.S.; Amiri, S. In vitro evaluation of enzymatic and antifungal activities of soil-actinomycetes isolates and their molecular identification by PCR. Jundishapur J. Microbiol. 2015, 8, 1–6. [Google Scholar] [CrossRef]
- Kamjam, M.; Sivalingam, P.; Deng, Z.; Hong, K. Deep Sea Actinomycetes and Their Secondary Metabolites. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.C.P.; Gleason, J.E.; Sanchez, H.; Schatzman, S.S.; Culbertson, M.; Johnson, C.J.; Mcnees, C.A.; Coelho, C.; Nett, E.; Andes, D.R.; et al. Candida albicans FRE8 encodes a member of the NADPH oxidase family that produces a burst of ROS during fungal morphogenesis. PLoS Pathog. 2017, 13, e1006763. [Google Scholar] [CrossRef] [PubMed]
- Brothers, K.M.; Gratacap, R.L.; Barker, S.E.; Newman, Z.R.; Norum, A.; Wheeler, R.T. NADPH Oxidase-Driven Phagocyte Recruitment Controls Candida albicans Filamentous Growth and Prevents Mortality. PLoS Pathog. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.B.; Gulati, M.; Johnson, A.D.; Nobile, C.J. Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Meena, B.; Rajan, L.A.; Vinithkumar, N.V.; Kirubagaran, R. Novel marine actinobacteria from emerald Andaman & Nicobar Islands: A prospective source for industrial and pharmaceutical byproducts. BMC Microbiol. 2013, 13, 145. [Google Scholar] [CrossRef]
- Baskaran, R.; Vijayakumar, R.; Mohan, P.M. Enrichment method for the isolation of bioactive actinomycetes from mangrove sediments of Andaman Islands, India. Malays. J. Microbiol. 2011, 7, 26–32. [Google Scholar] [CrossRef]
- Das, A.; Bhattacharya, S.; Yegoup, A.; Mohammed, H. In vitro Antimicrobial Activity and Characterization of Mangrove Isolates of Streptomycetes Effective against Bacteria and Fungi of Nosocomial Origin. Braz. Arch. Biol. Technol. 2014, 57, 349–356. [Google Scholar] [CrossRef]
- Erdogan, A.; Rao, S.S.C. Small Intestinal Fungal Overgrowth. Curr. Gastroenterol. Rep. 2015, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Ferreira, I.C.F.R.; Barros, L.; Silva, S.; Henriques, M. Candidiasis: Predisposing Factors, Prevention, Diagnosis and Alternative Treatment. Mycopathologia 2014, 177, 223–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.H.; Díaz, J.A.; Lee, S.A.; Candida, N. Emerging opportunistic yeast infections Emerging yeasts. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef]
- Hadjithomas, M.; Chen, I.M.A.; Chu, K.; Ratner, A.; Palaniappan, K.; Szeto, E.; Huang, J.; Reddy, T.B.K.; Cimermančič, P.; Fischbach, M.A.; et al. IMG-ABC: A knowledge base to fuel discovery of biosynthetic gene clusters and novel secondary metabolites. MBio 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meklat, A.; Sabaou, N.; Zitouni, A.; Mathieu, F.; Lebrihi, A. Isolation, taxonomy, and antagonistic properties of halophilic actinomycetes in Saharan soils of Algeria. Appl. Environ. Microbiol. 2011, 77, 6710–6714. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Braga-Silva, L.A. Aspartic Protease Inhibitors as Potential Anti-Candida albicans Drugs: Impacts on Fungal Biology, Virulence and Pathogenesis. Curr. Med. Chem. 2011, 18, 2401–2419. [Google Scholar]
- Potter, V.R.; Dubois, K.P. Studies on the mechanism of hydrogen transport in animal tissues: VI. Inhibitor studies with succinic dehydraganase. J. Gen. Physiol. 1943, 26, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Fair, R.J.; Tor, Y. Perspectives in Medicinal Chemistry Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef]
- Özcan, K.; Aksoy, S.Ç.; Kalkan, O.; Uzel, A.; Hames-Kocabas, E.E.; Bedir, E. Diversity and antibiotic-producing potential of cultivable marine-derived actinomycetes from coastal sediments of Turkey. J. Soils Sediments 2013, 13, 1493–1501. [Google Scholar] [CrossRef]
- Seong, C.N.; Choi, J.H.; Baik, K. An improved selective isolation of rare actinomycetes from forest soil. J. Microbiol. 2001, 39, 17–23. [Google Scholar]
- Chater, K.F.; Hopwood, D.A. The Biology of Actinomycetes. In The Biology of Actinomycetes; Academic Press: London, UK, 1984; pp. 229–286. ISBN 012289670X. [Google Scholar]
- Rainey, F.A.; Ward-Rainey, N.; Kroppenstedt, R.M.; Stackebrandt, E. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: Proposal of Nocardiopsaceae fam. nov. Int. J. Syst. Bacteriol. 1996, 46, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Metsä-Ketelä, M.; Halo, L.; Munukka, E.; Hakala, J.; Mäntsäla, P.; Ylihonko, K. Molecular evolution of aromatic polyketides and comparative sequence analysis of polyketide ketosynthase and 16S ribosomal DNA genes from various Streptomyces species. Appl. Environ. Microbiol. 2002, 68, 4472–4479. [Google Scholar] [CrossRef] [PubMed]
- Palomo, S.; González, I.; De La Cruz, M.; Martín, J.; Tormo, J.R.; Anderson, M.; Hill, R.T.; Vicente, F.; Reyes, F.; Genilloud, O. Sponge-derived Kocuria and Micrococcus spp. as sources of the new thiazolyl peptide antibiotic kocurin. Mar. Drugs 2013, 11, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J.S. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, I.; Muhana, A. Optimal production conditions, extraction, partial purification and characterization of inhibitory compound(s) produced by. Trends Biotechnol. 2008, 2, 402–420. [Google Scholar]
- Undabarrena, A.; Beltrametti, F.; Claverías, F.P.; González, M.; Moore, E.R.B.; Seeger, M.; Cámara, B. Exploring the diversity and antimicrobial potential of marine actinobacteria from the Comau Fjord in Northern Patagonia, Chile. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Jarrahpour, A.; Fathi, J.; Mimouni, M.; Parvez, A. Petra, Osiris and Molinspiration (POM) together as a successful support in drug design: Antibacterial activity and biopharmaceutical characterization of some azo Schiff bases. Med. Chem. Res. 2012, 21, 1984–1990. [Google Scholar] [CrossRef]
- Kumaresanetal, S.; Senthilkumar, V.; Stephen, A.; Balakumar, J.S. GC-MS analysis and PASS-assisted prediction of biological activity spectra of extract of Phomopsis sp. isolated from Andrographis paniculata. World J. Pharm. Res. 2015, 4, 1035–1053. [Google Scholar]
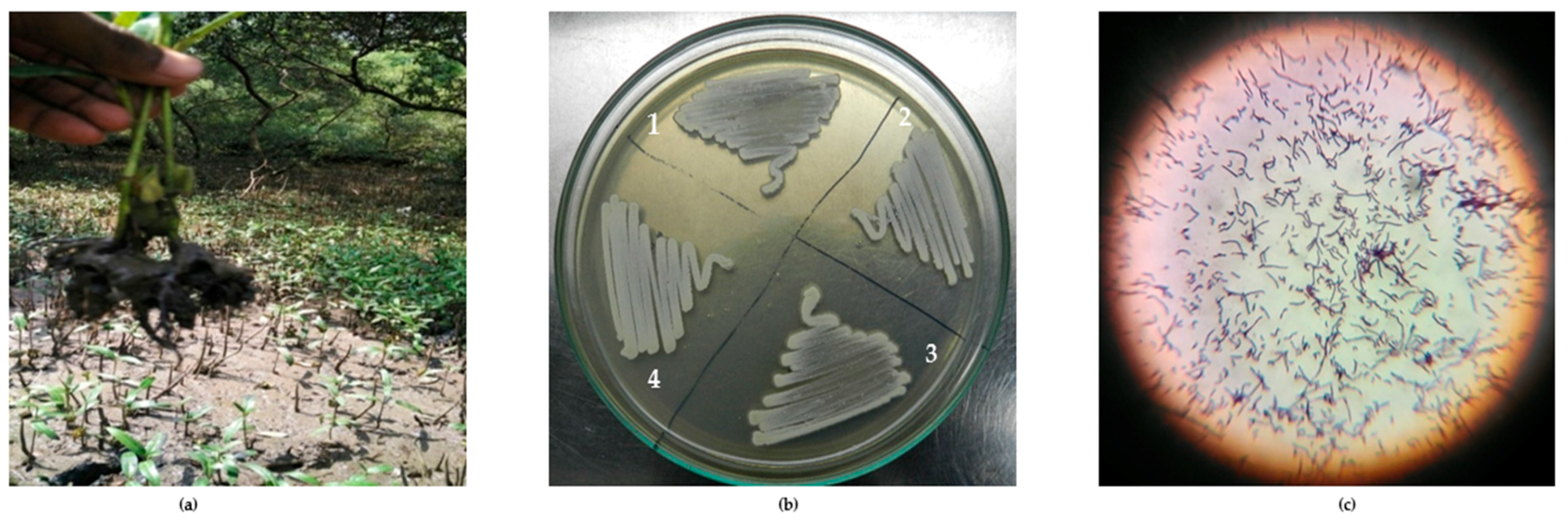
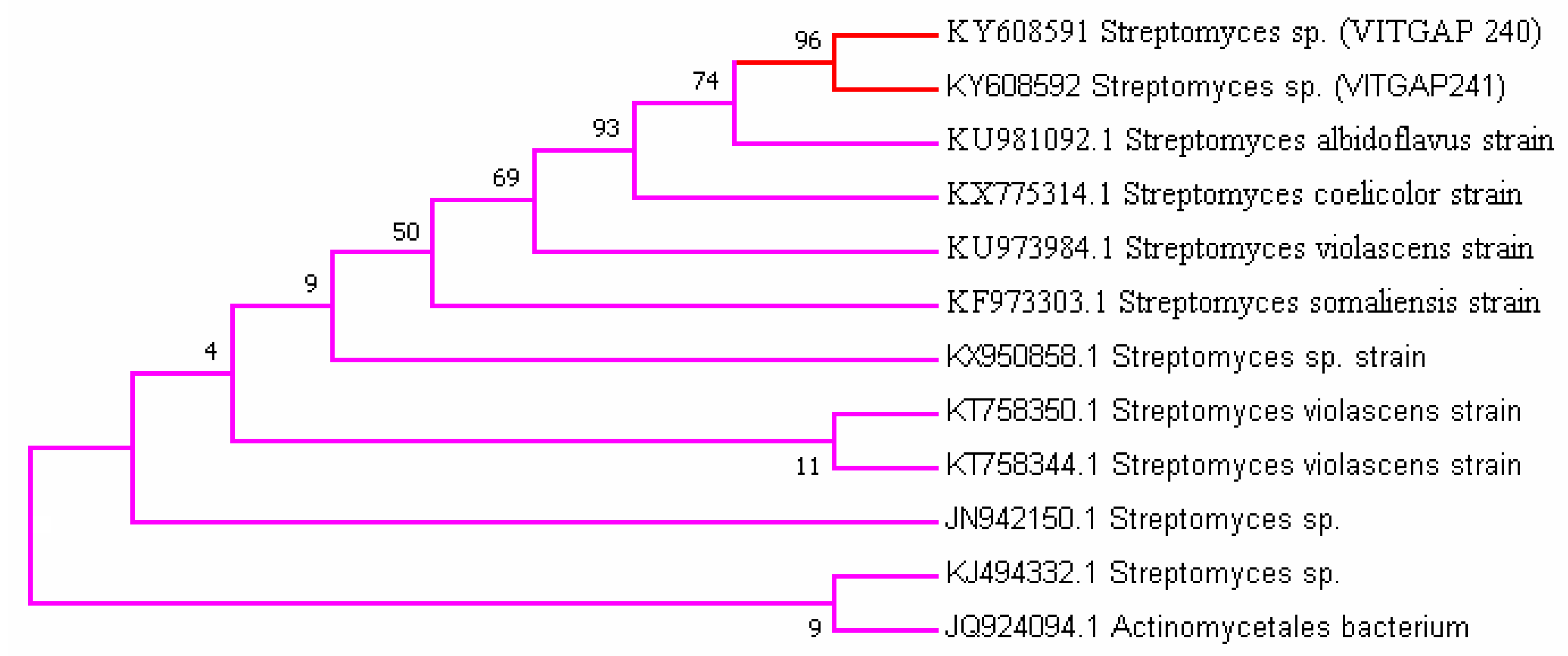
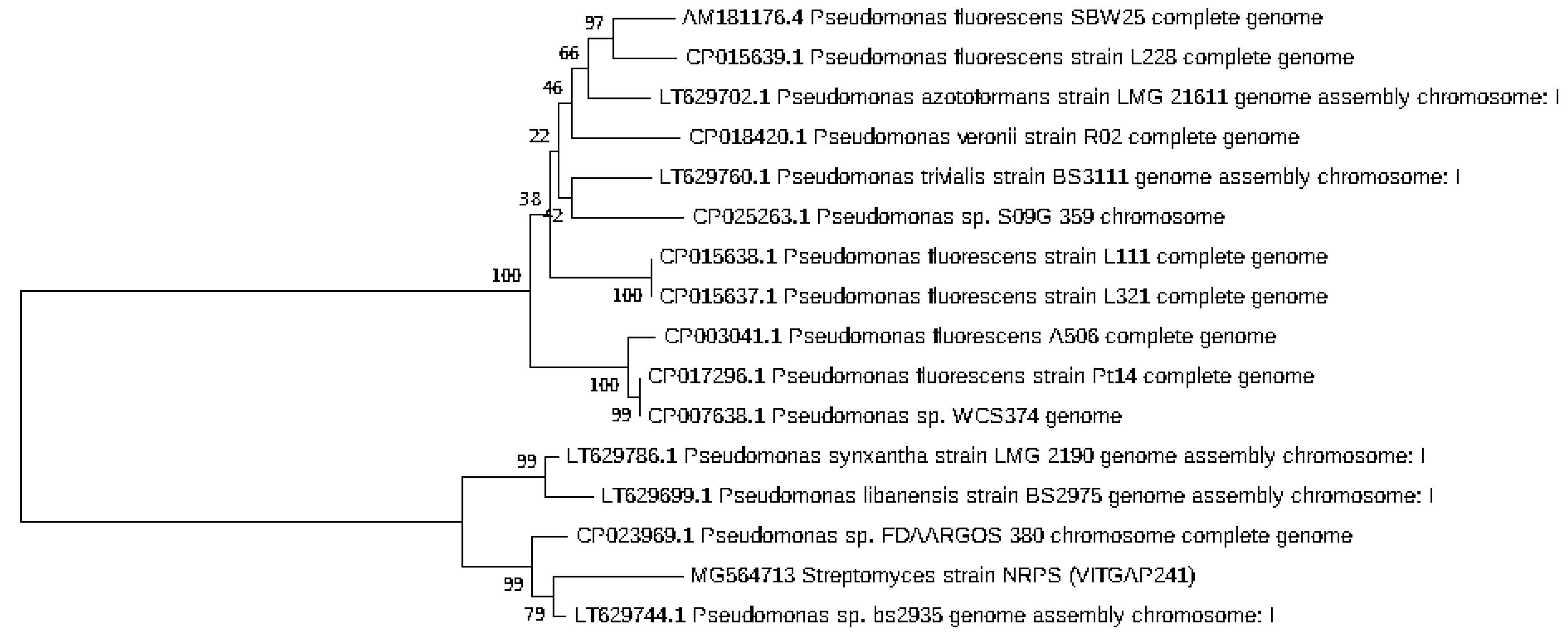
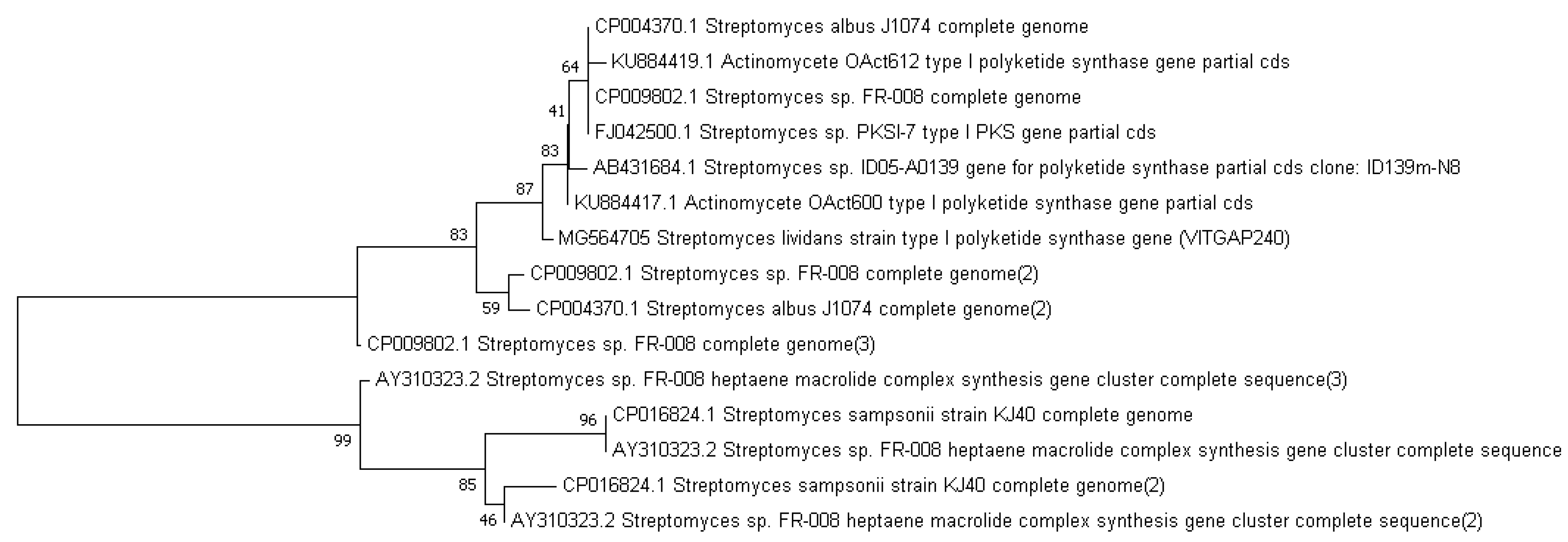

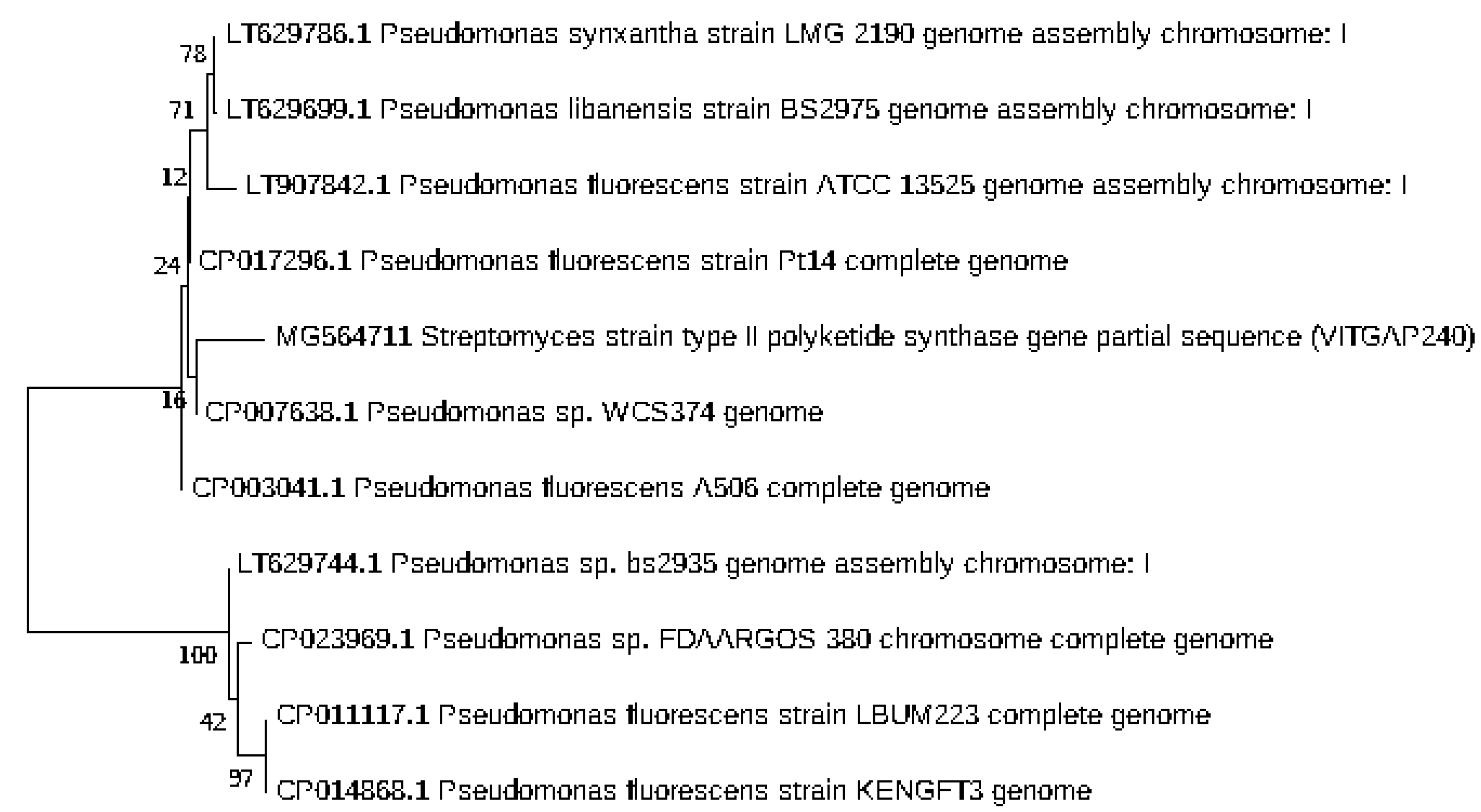
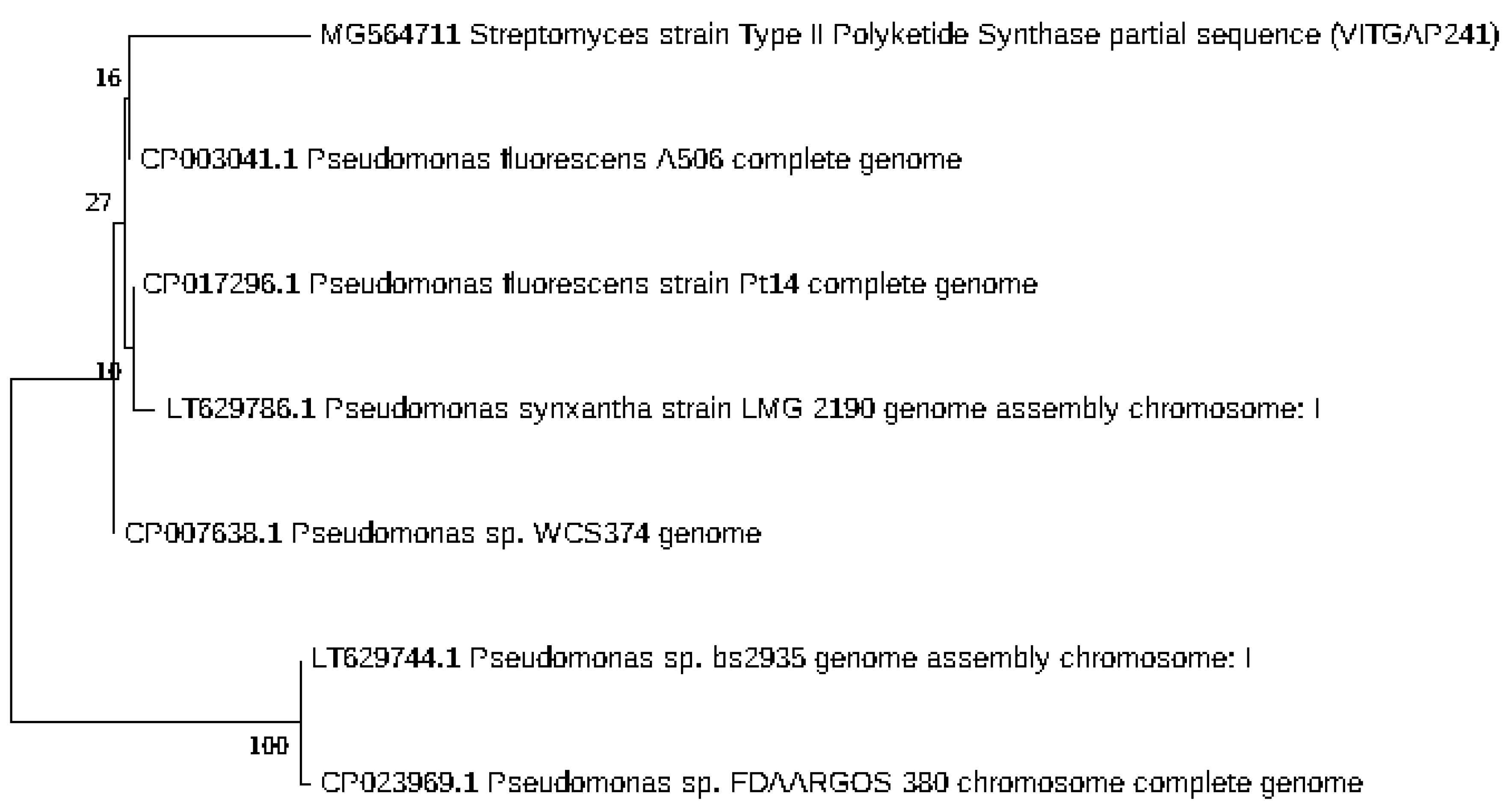
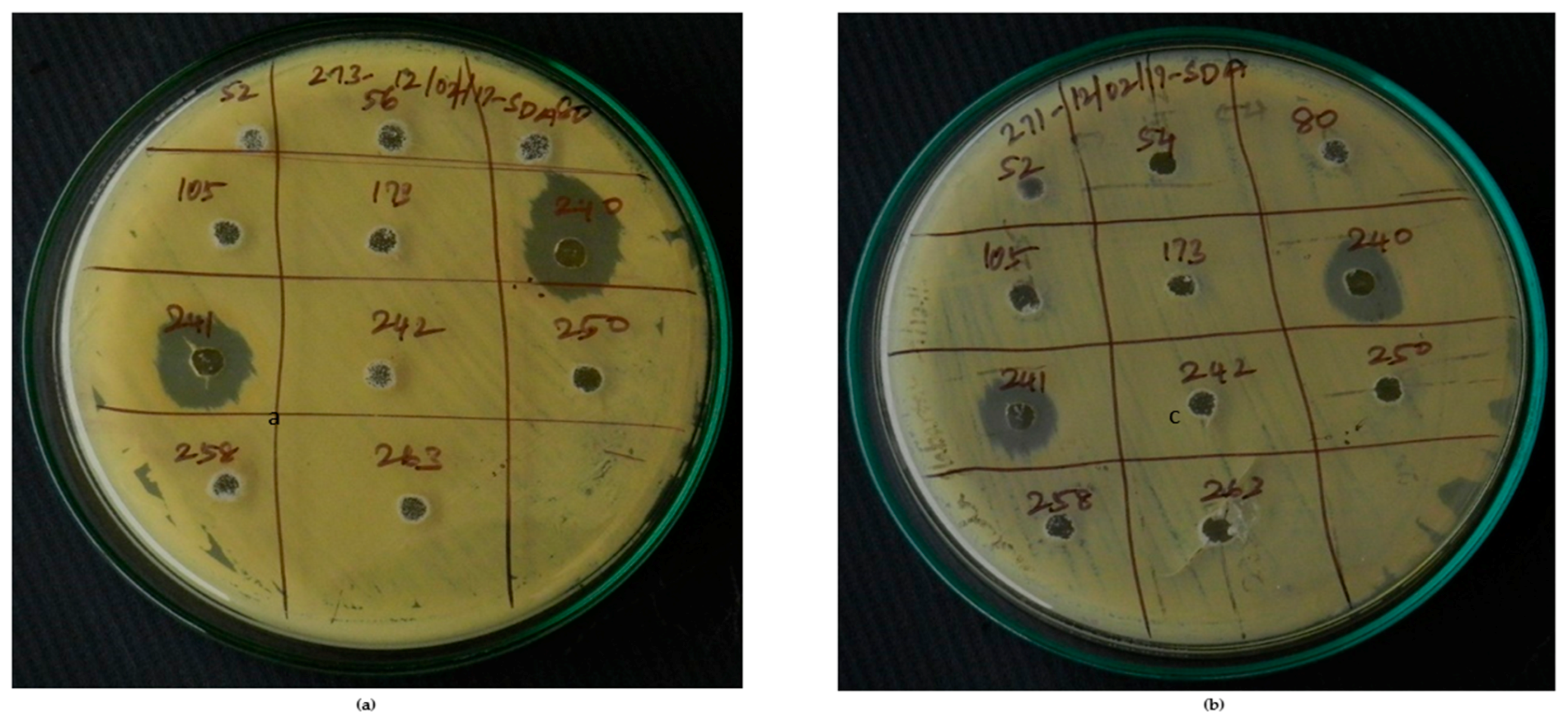
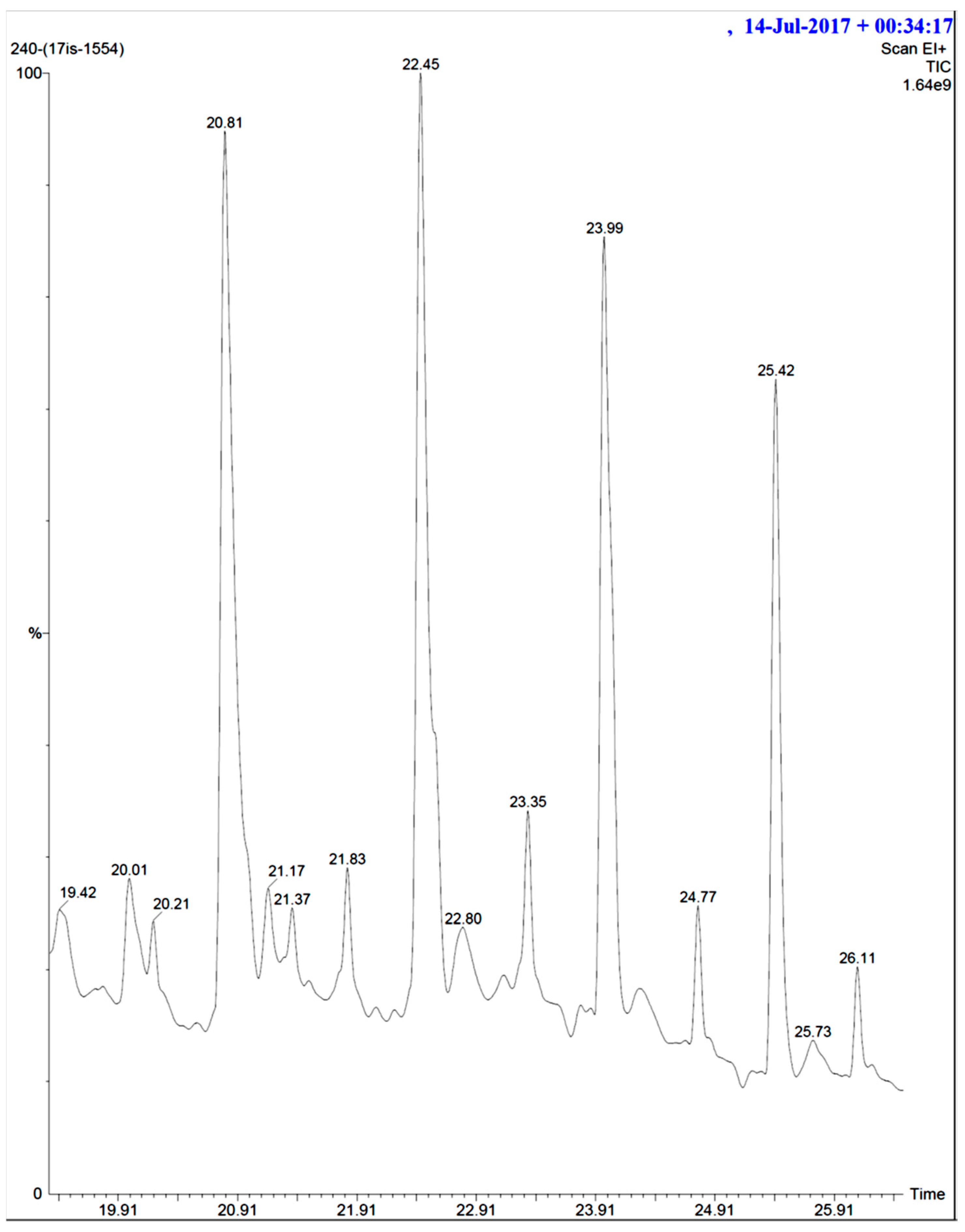

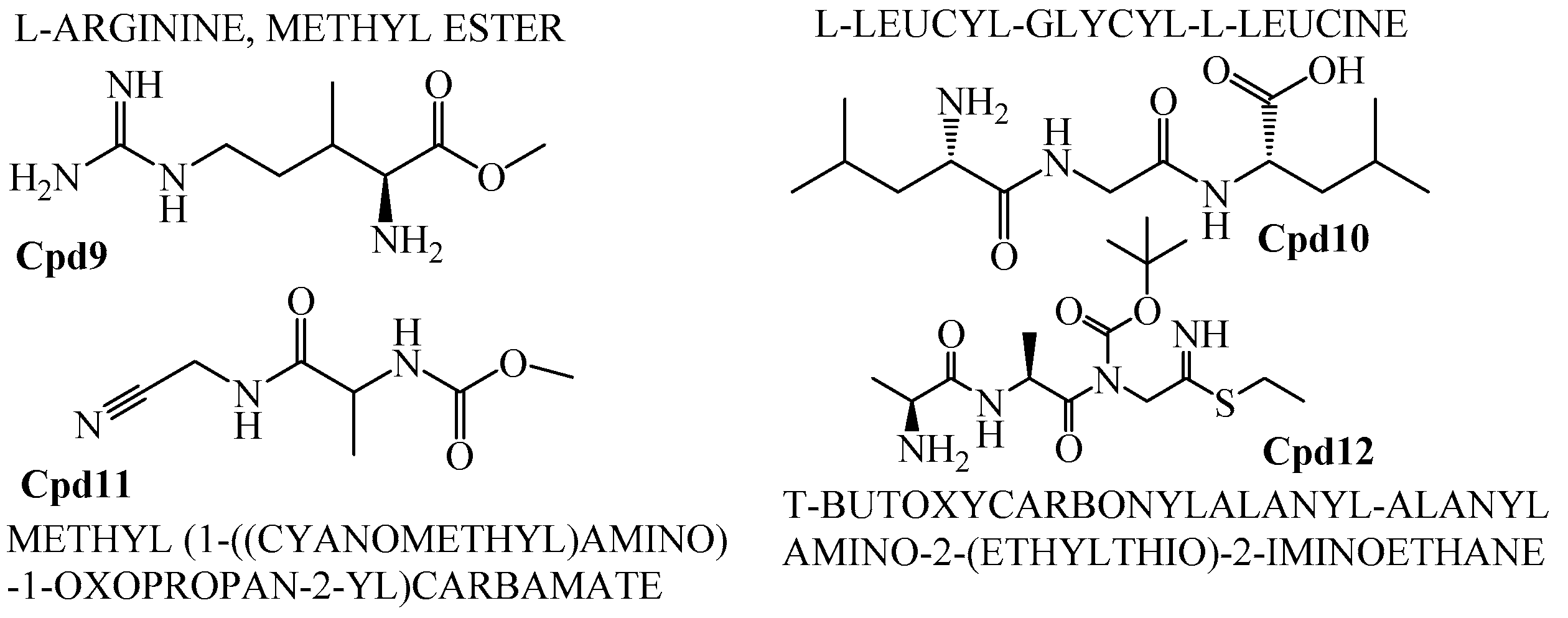
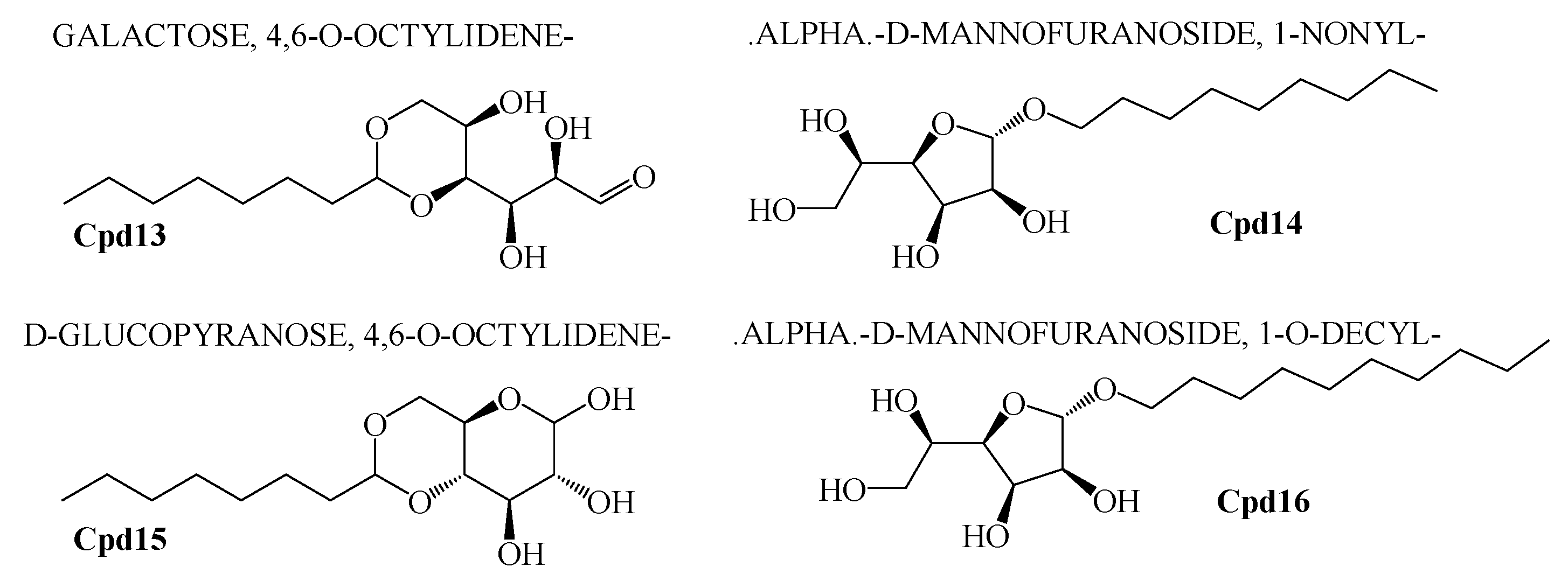
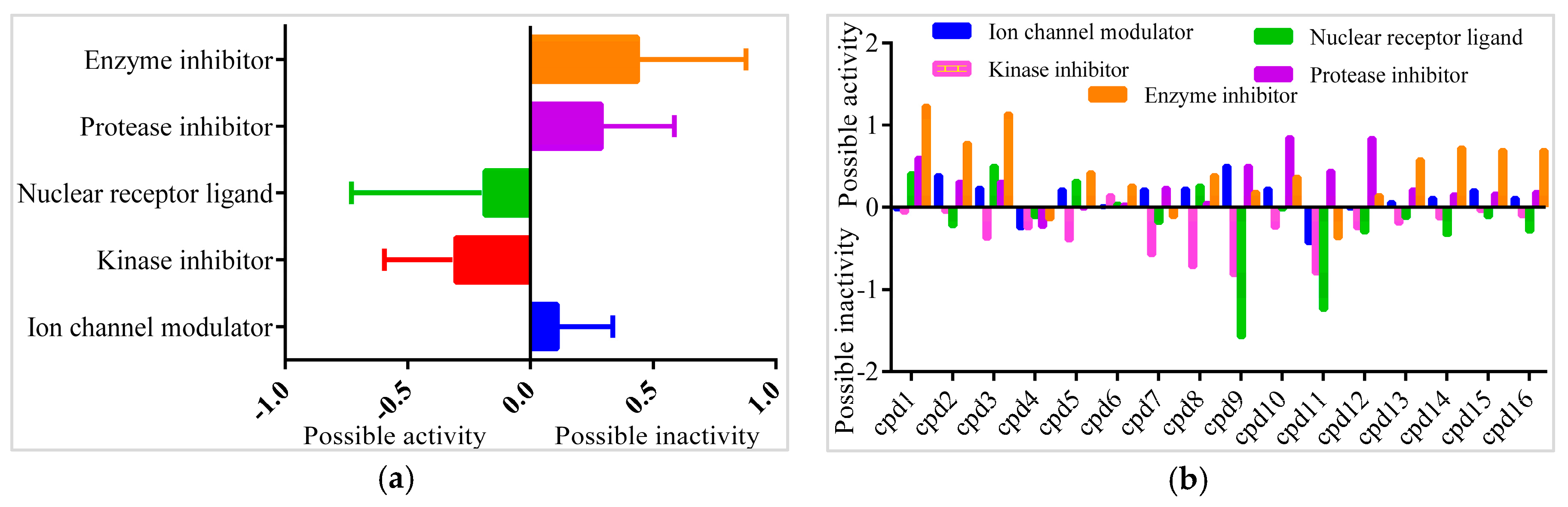
| Sequence-ID | Genbank Accession Numbers | Organism | Sampling Location | Latitude and Longitude |
|---|---|---|---|---|
| VITGAP080 | KY608546 | Streptomyces rochei | Muthupet, Thiruvarur, Tamil Nadu | 10.39 N, 79.49 E |
| VITGAP095 | KY608547 | Streptomyces variabilis | Muthupet, Thiruvarur, Tamil Nadu | 10.39 N, 79.49 E |
| VITGAP103 | KY608548 | Streptomyces sp. | Muthupet, Thiruvarur, Tamil Nadu | 10.39 N, 79.49 E |
| VITGAP104 | KY608549 | Streptomyces sp. | Muthupet, Thiruvarur, Tamil Nadu | 10.39 N, 79.49 E |
| VITGAP105 | KY608550 | Streptomyces sp. | Muthupet, Thiruvarur, Tamil Nadu | 10.39 N, 79.49 E |
| VITGAP229 | KY608585 | Streptomyces sp. | Port Mout, Andaman and Nicobar Islands | 11.40 N, 92.41 E |
| VITGAP231 | KY608586 | Streptomyces sp. | Port Mout, Andaman and Nicobar Islands | 11.40 N, 92.41 E |
| VITGAP232 | KY608587 | Streptomyces sp. | Port Mout, Andaman and Nicobar Islands | 11.40 N, 92.41 E |
| VITGAP233 | KY608588 | Streptomyces clavuligerus | Port Mout, Andaman and Nicobar Islands | 11.40 N, 92.41 E |
| VITGAP235 | KY608589 | Streptomyces sp. | Port Mout, Andaman and Nicobar Islands | 11.40 N, 92.41 E |
| VITGAP238 | KY608590 | Streptomyces sp. | Port Mout, Andaman and Nicobar Islands | 11.40 N, 92.41 E |
| VITGAP240 | KY608591 | Streptomyces lividans | Corbyn, Andaman and Nicobar Islands | 11.38 N, 92.44 E |
| VITGAP241 | KY608592 | Streptomyces albidoflavus | Corbyn, Andaman and Nicobar Islands | 11.38 N, 92.44 E |
| VITGAP242 | KY608593 | Streptomyces sp. | Corbyn, Andaman and Nicobar Islands | 11.38 N, 92.44 E |
| VITGAP244 | KY608594 | Rhodococcus sp. | Corbyn, Andaman and Nicobar Islands | 11.38 N, 92.44 E |
| VITGAP245 | KY608595 | Streptomyces violascens | Corbyn, Andaman and Nicobar Islands | 11.38 N, 92.44 E |
| VITGAP246 | KY608596 | Corynebacterineae bacterium | Corbyn, Andaman and Nicobar Islands | 11.38 N, 92.44 E |
| VITGAP247 | KY608597 | Streptomyces sp. | Sippighat, Andaman and Nicobar Islands | 11.36 N, 92.41 E |
| VITGAP248 | KY608598 | Streptomyces sp. | Sippighat, Andaman and Nicobar Islands | 11.36 N, 92.41 E |
| VITGAP250 | KY608599 | Streptomyces sp. | Sippighat, Andaman and Nicobar Islands | 11.36 N, 92.41 E |
| VITGAP253 | KY608600 | Streptomyces sp. | Wandoor Jetty, Andaman and Nicobar Islands | 11.35 N, 92.37 E |
| VITGAP255 | KY608601 | Actinomycetales bacterium | Burmanalla, Andaman and Nicobar Islands | 11.33 N, 92.43 E |
| VITGAP256 | KY608602 | Streptomyces sp. | Burmanalla, Andaman and Nicobar Islands | 11.33 N, 92.43 E |
| VITGAP257 | KY608603 | Streptomyces sp. | Burmanalla, Andaman and Nicobar Islands | 11.33 N, 92.43 E |
| VITGAP258 | KY608604 | Streptomyces sp. | Burmanalla, Andaman and Nicobar Islands | 11.33 N, 92.43 E |
| VITGAP259 | KY608605 | Actinomycetales bacterium | Burmanalla, Andaman and Nicobar Islands | 11.33 N, 92.43 E |
| VITGAP261 | KY608606 | Streptomyces sp. | Burmanalla, Andaman and Nicobar Islands | 11.33 N, 92.43 E |
| VITGAP263 | KY608607 | Streptomyces chumphonensis | Burmanalla, Andaman and Nicobar Islands | 11.33 N, 92.43 E |
| VITGAP270 | KY608608 | Streptomyces sp. | MundaPahad, Andaman and Nicobar Islands | 11.29 N, 92.42 E |
| VITGAP271 | KY608609 | Streptomyces sp. | Kalapahad, Andaman and Nicobar Islands | 11.36 N, 92.40 E |
| Isolate No. | Genbank Accession No. of the Isolates | Sampling Location of the Isolates | Closest Organism | Genebank No. | Similarity Percentage |
|---|---|---|---|---|---|
| VITGAP080 | KY608546 | Muthupet, Thiruvarur, Tamil Nadu | Streptomyces rochei strain | KP823705 | 96% |
| VITGAP095 | KY608547 | Muthupet, Thiruvarur, Tamil Nadu | Streptomyces variabilis | KU981101 | 95% |
| VITGAP 103 | KY608548 | Muthupet, Thiruvarur, Tamil Nadu | Streptomyces sp. | CP013142 | 95% |
| VITGAP 105 | KY608550 | Muthupet, Thiruvarur, Tamil Nadu | Streptomyces sp. | JQ009379 | 96% |
| VITGAP 235 | KY608589 | Port Mout, Andaman and Nicobar Islands | Streptomyces sp. | KX279534 | 83% |
| VITGAP 240 | KY608591 | Corbyn, Andaman and Nicobar Islands | Streptomyces violascens | KU973980 | 91% |
| VITGAP 253 | KY608600 | Wandoor Jetty, Andaman and Nicobar Islands | Streptomyces sp. | KU884356 | 94% |
| VITGAP 255 | KY608601 | Burmanalla, Andaman and Nicobar Islands | Actinomycetales bacterium | EU368818 | 88% |
| VITGAP 257 | KY608603 | Burmanalla, Andaman and Nicobar Islands | Streptomyces sp. | JF736620 | 97% |
| VITGAP 258 | KY608604 | Burmanalla, Andaman and Nicobar Islands | Streptomyces sp. | KR817750 | 87% |
| VITGAP 261 | KY608606 | Burmanalla, Andaman and Nicobar Islands | Streptomyces sp. | KX928494 | 92% |
| VITGAP 263 | KY608607 | Burmanalla, Andaman and Nicobar Islands | Streptomyces chumphonensis | NR_126175 | 94% |
| VITGAP 271 | KY608609 | Kalapahad, Andaman and Nicobar Islands | Streptomyces sp. | KT588654 | 92% |
| Strains | PKS (Type I) | PKS (Type II) | NRPS |
|---|---|---|---|
| VITGAP080 | + | + | + |
| VITGAP095 | + | ||
| VITGAP105 | + | ||
| VITGAP240 | + | + | |
| VITGAP241 | + | + | + |
| VITGAP242 | + | + | + |
| VITGAP244 | + | ||
| VITGAP248 | + | ||
| VITGAP250 | + | ||
| VITGAP253 | + | ||
| VITGAP255 | + | ||
| VITGAP257 | + | ||
| VITGAP258 | + | + |
| Sequence | Query Coverage | % of Identity | No of Hits |
|---|---|---|---|
| NRPS_VITGAP241 | 99% | >83% | 15 |
| Type I PKS VITGAP-240 | 99% | >89% | 15 |
| Type I PKS VITGAP-241 | 80% | >79% | 13 |
| Type II PKS VITGAP-240 | 56% | >70% | 10 |
| Type II PKS VITGAP-241 | 68% | >67% | 6 |
| Antibiotics | Strains with the Biosynthetic Genes | Strains without the Biosynthetic Genes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 80 | 95 | 240 | 241 | 242 | 105 | 248 | 250 | 253 | 255 | 258 | 103 | 105 | 261 | 263 | |
| Piperacillin | R | R | R | R | S | R | R | R | R | R | R | R | R | R | R |
| Co-Trimoxazole | R | S | S | S | S | S | S | S | R | R | S | R | R | R | R |
| Ofloxacin | S | S | S | R | S | R | S | R | R | S | R | R | S | S | R |
| Amikacin | S | S | R | S | S | R | S | R | R | R | R | R | R | S | R |
| Erthyromicin | R | S | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Cefuroxime | S | S | R | R | R | R | S | R | R | R | R | R | R | R | R |
| Tobramycin | R | S | S | R | R | R | R | R | R | R | R | R | R | R | R |
| Ampicillin | R | S | R | R | R | S | S | R | R | R | S | R | R | R | R |
| Tetracycline | R | S | S | S | S | R | R | S | R | R | S | R | R | S | R |
| Ceftriazone | S | S | S | R | S | R | S | R | R | R | S | R | R | R | S |
| Polmycin | S | S | R | R | R | R | S | R | R | R | R | R | R | R | R |
| Nitrofurantin | R | R | R | S | R | R | R | R | R | R | R | R | R | R | R |
| Rifampicin | S | S | R | S | S | R | S | R | R | R | R | R | R | S | R |
| Clindamycin | R | R | R | R | S | R | R | R | R | R | R | R | R | R | R |
| Netillin | S | S | S | S | S | R | S | R | R | R | S | R | S | S | R |
| Ceftrazidime | R | S | R | R | S | R | R | R | R | R | R | R | R | R | R |
| Novobiocin | S | S | S | S | S | R | S | R | R | S | S | R | S | R | R |
| Aztreonam | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Cephotaxine | S | S | S | S | S | R | S | S | R | R | S | R | R | S | R |
| Chloramphenicol | R | R | R | S | R | R | R | R | R | R | R | R | R | R | R |
| Streptomycin | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Penicillin | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Methicillin | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Jancomycin | R | R | R | S | R | R | R | R | R | R | R | R | R | R | R |
| Gentamycin | S | S | R | S | R | R | S | R | R | R | S | R | S | S | R |
| Vancomycin | R | R | S | S | R | R | R | R | R | R | R | R | R | R | R |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavan Kumar, J.G.S.; Gomathi, A.; Vasconcelos, V.; Gothandam, K.M. Bioactivity Assessment of Indian Origin—Mangrove Actinobacteria against Candida albicans. Mar. Drugs 2018, 16, 60. https://doi.org/10.3390/md16020060
Pavan Kumar JGS, Gomathi A, Vasconcelos V, Gothandam KM. Bioactivity Assessment of Indian Origin—Mangrove Actinobacteria against Candida albicans. Marine Drugs. 2018; 16(2):60. https://doi.org/10.3390/md16020060
Chicago/Turabian StylePavan Kumar, J. G. S., Ajitha Gomathi, Vitor Vasconcelos, and K. M. Gothandam. 2018. "Bioactivity Assessment of Indian Origin—Mangrove Actinobacteria against Candida albicans" Marine Drugs 16, no. 2: 60. https://doi.org/10.3390/md16020060






