First Report on Chitin in a Non-Verongiid Marine Demosponge: The Mycale euplectellioides Case
Abstract
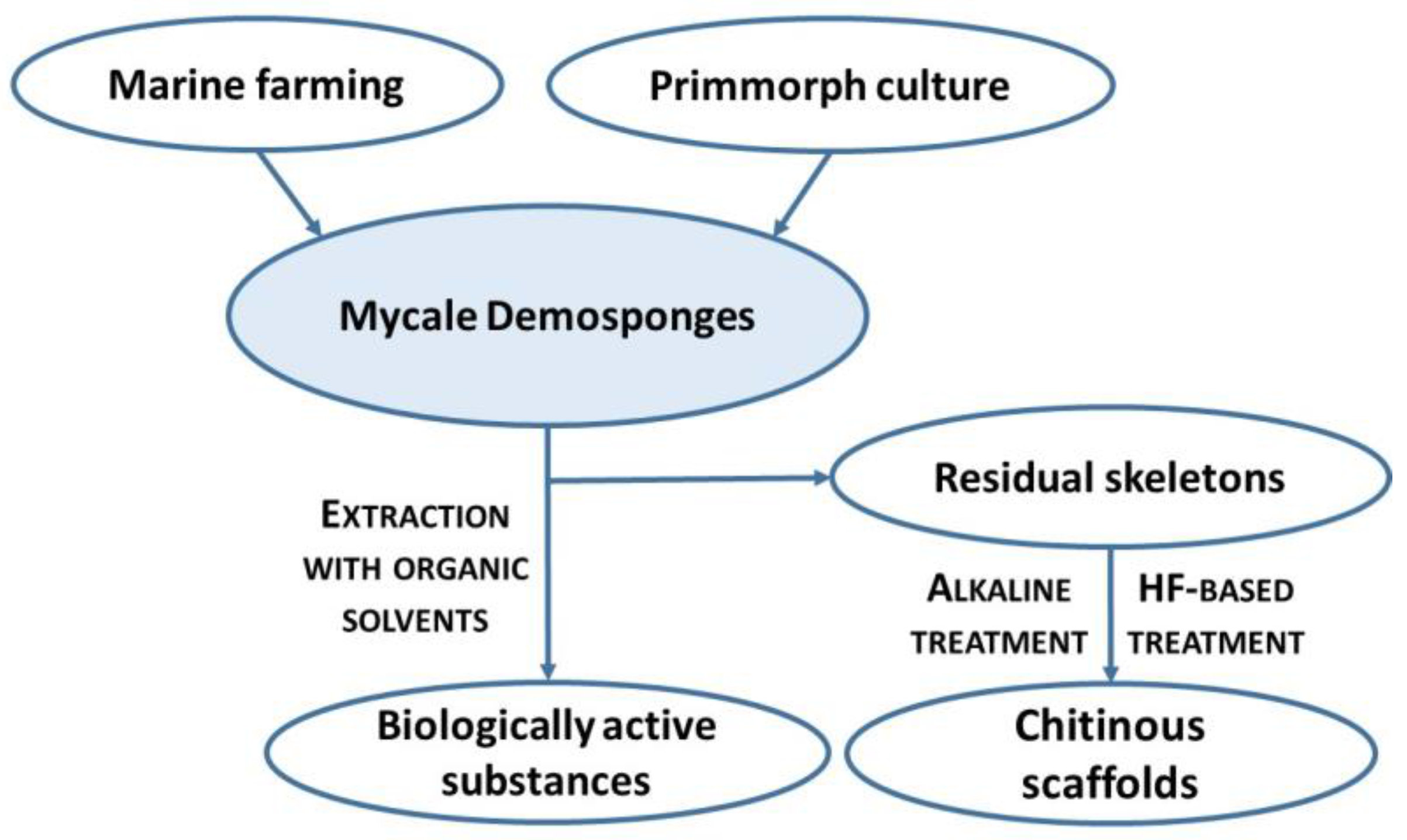
:1. Introduction
2. Results
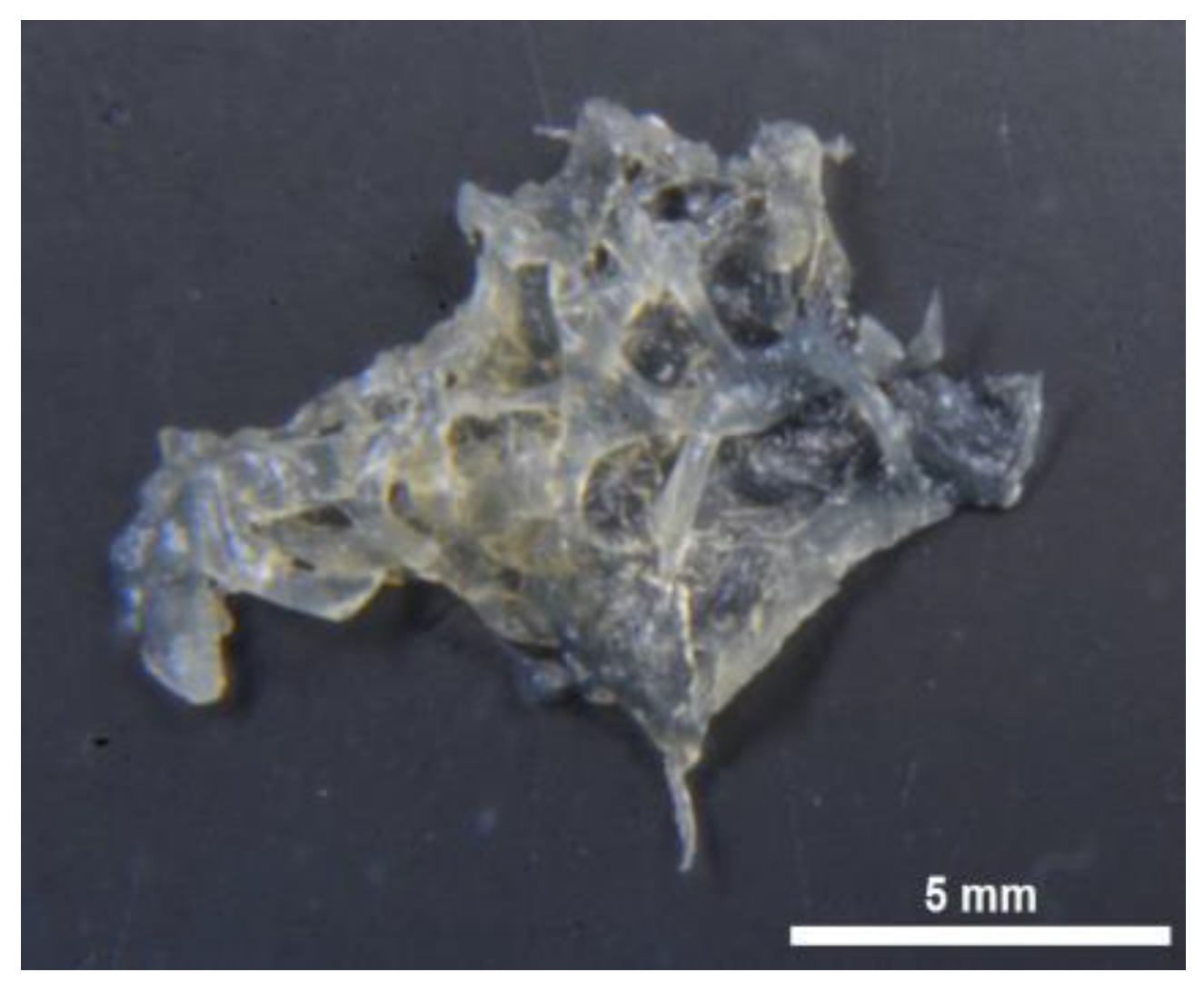
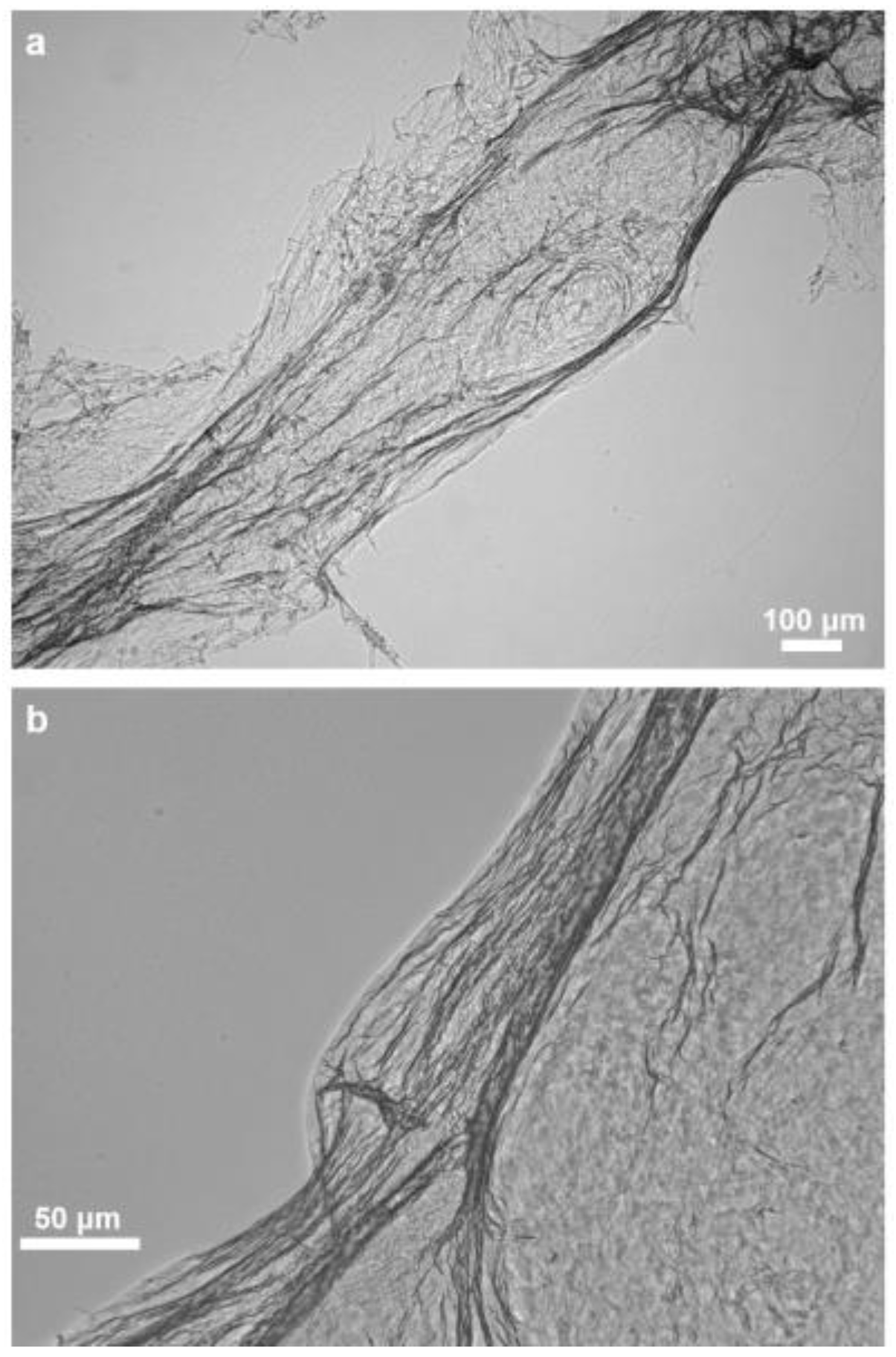
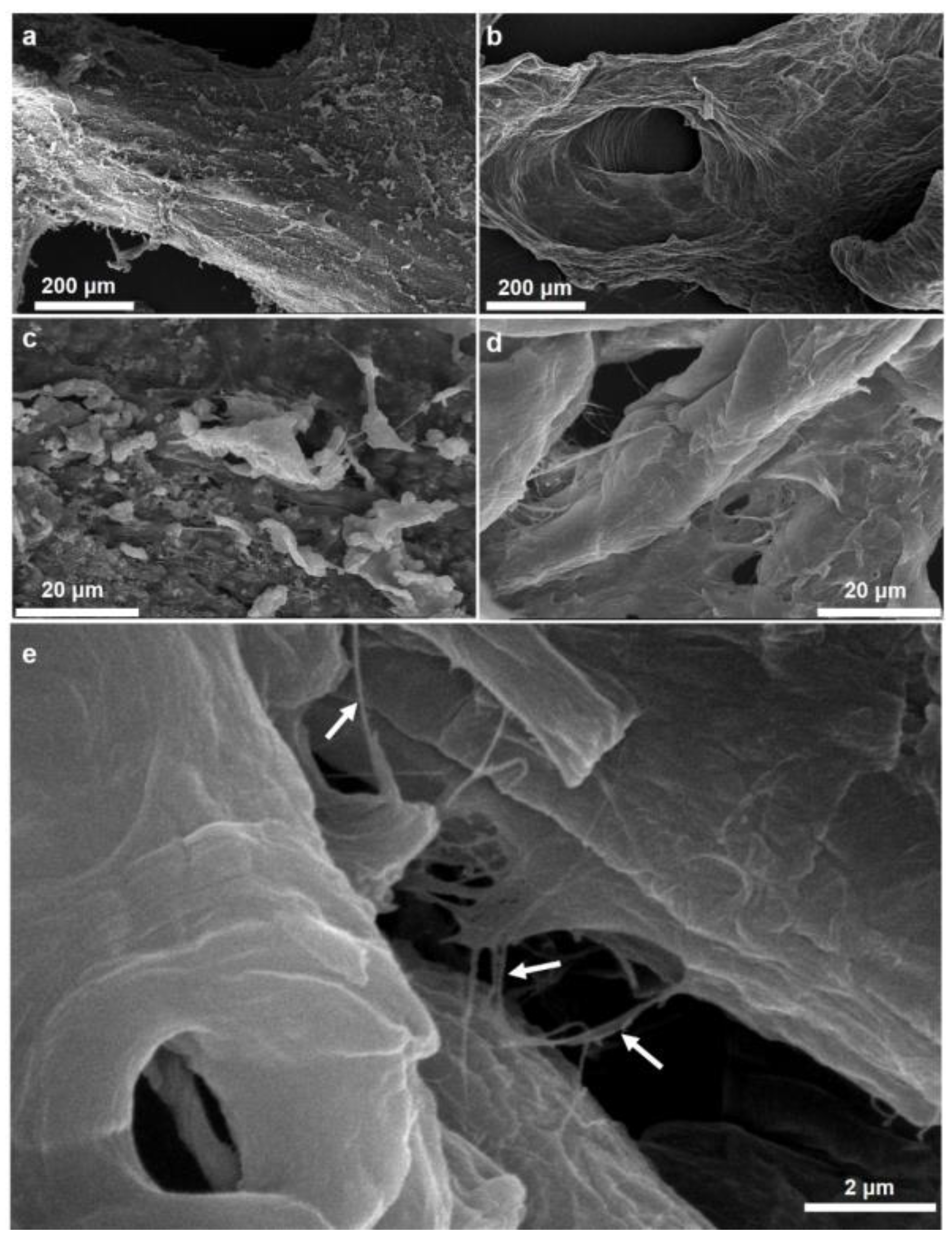
2.1. Morphology and Structural Peculiarities of Organic Scaffold Isolated from M. euplectellioides Skeleton
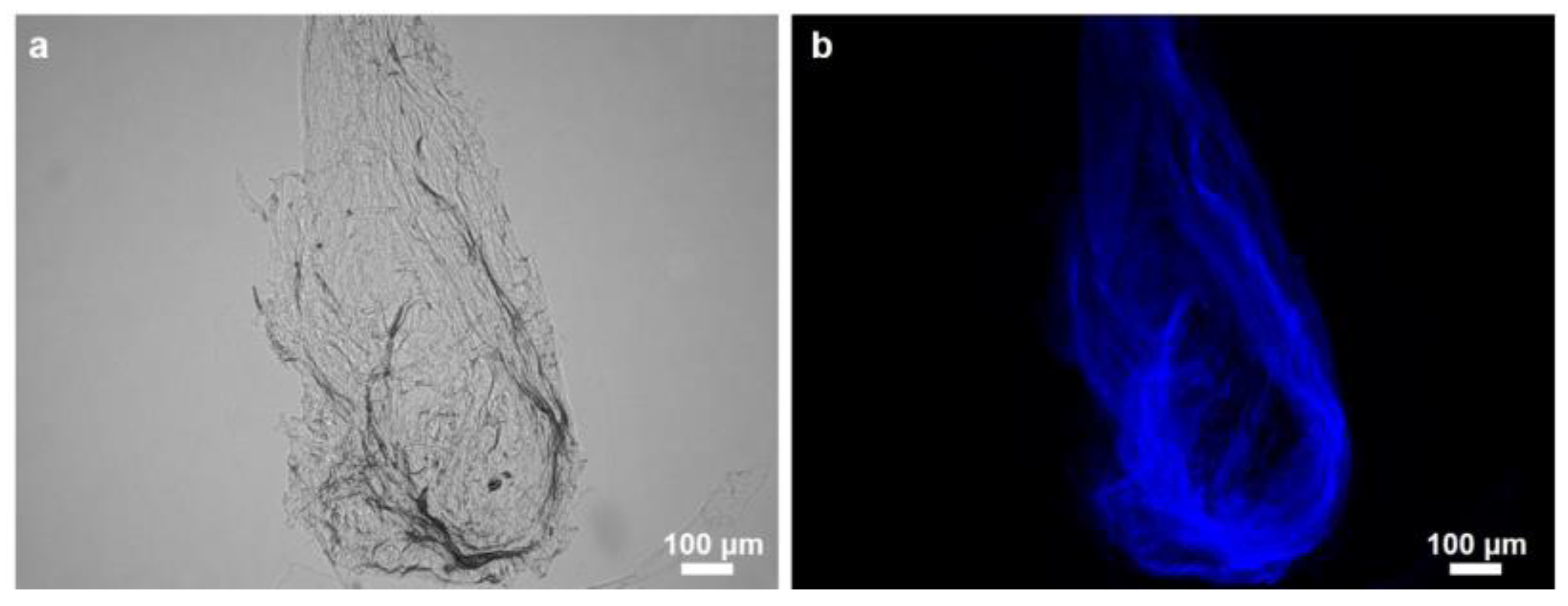
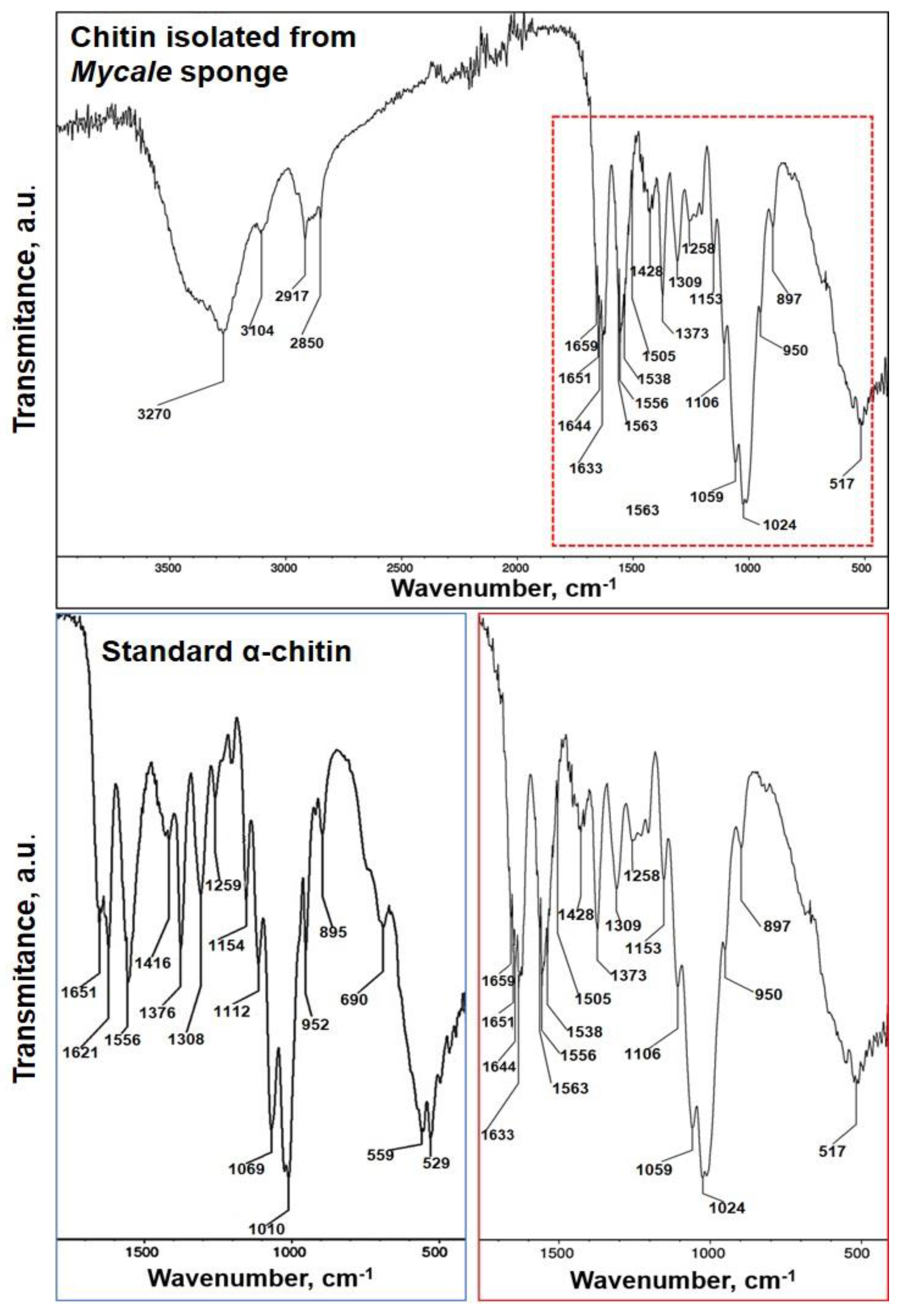
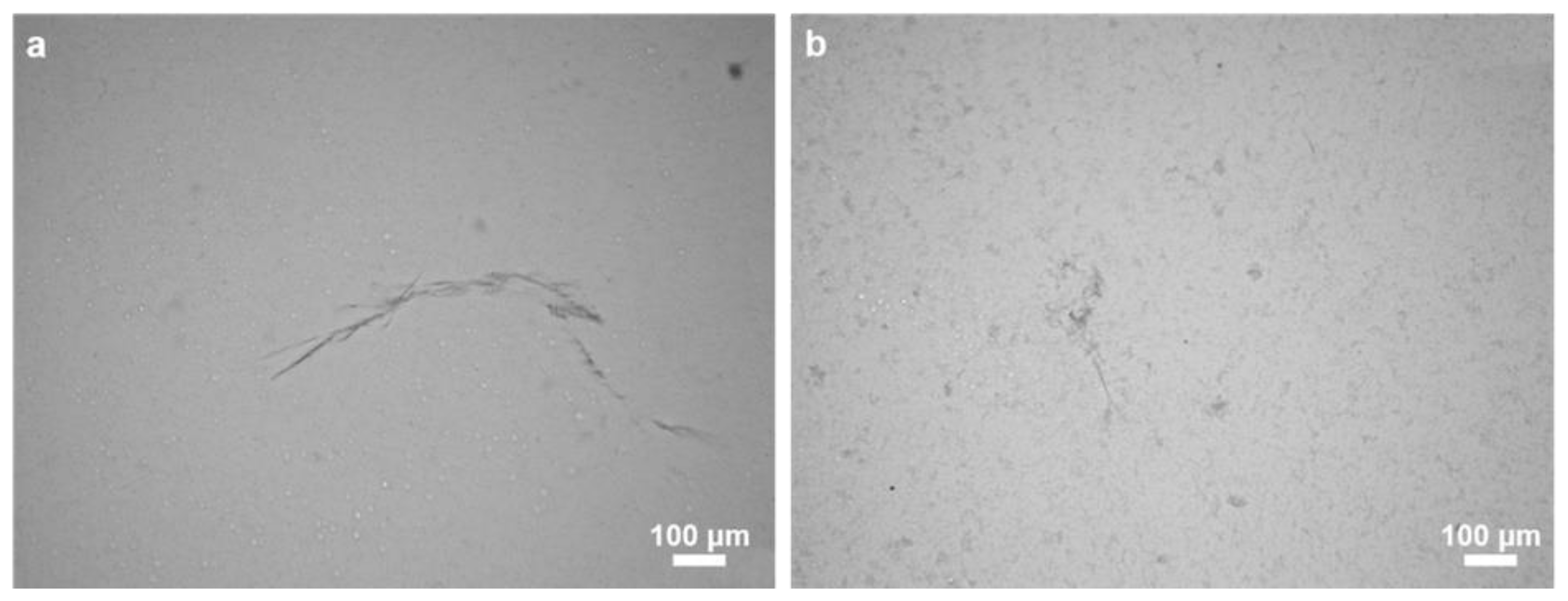
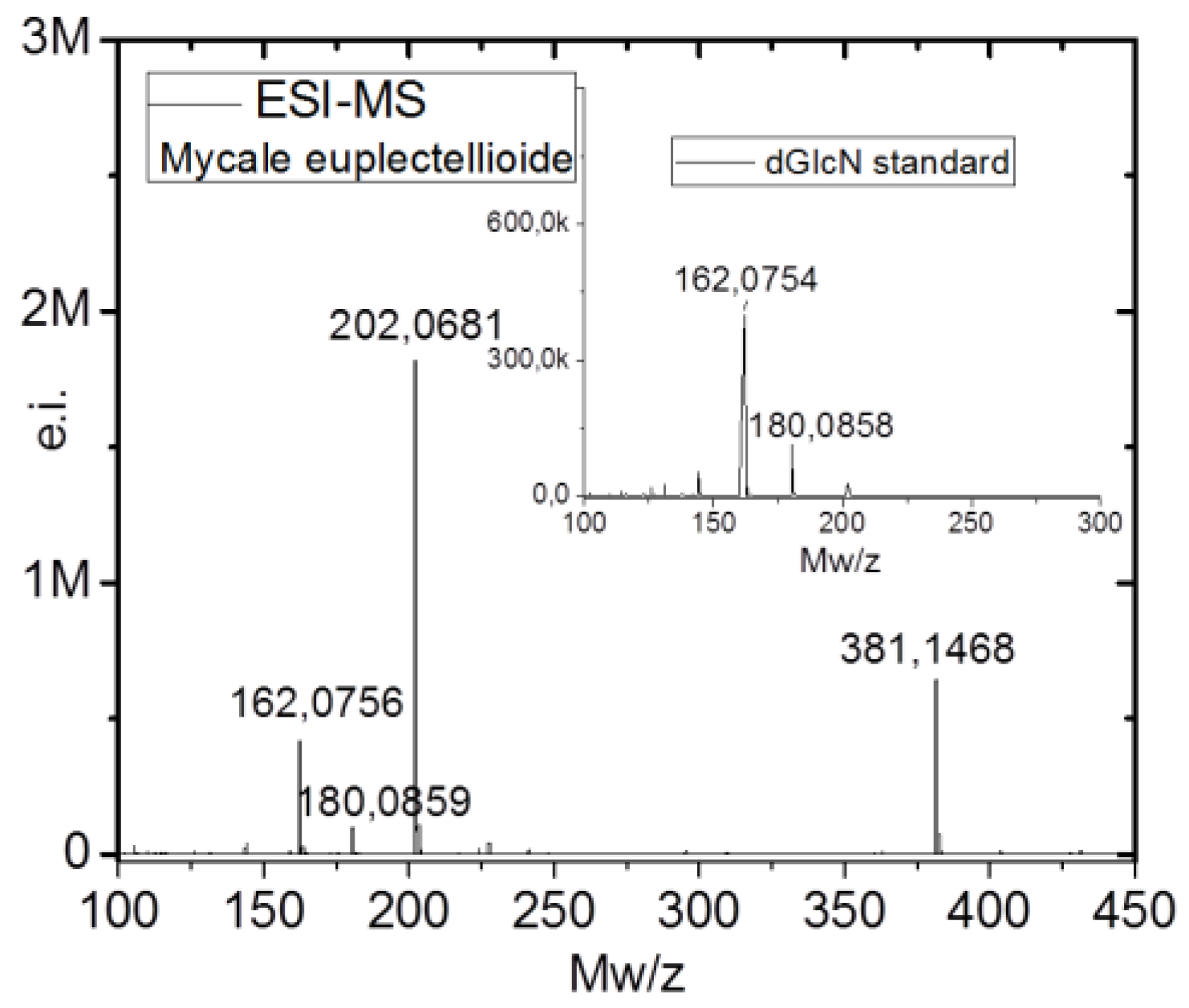
2.2. Identification of Chitin
3. Discussion
4. Materials and Methods
4.1. Collection of the Samples
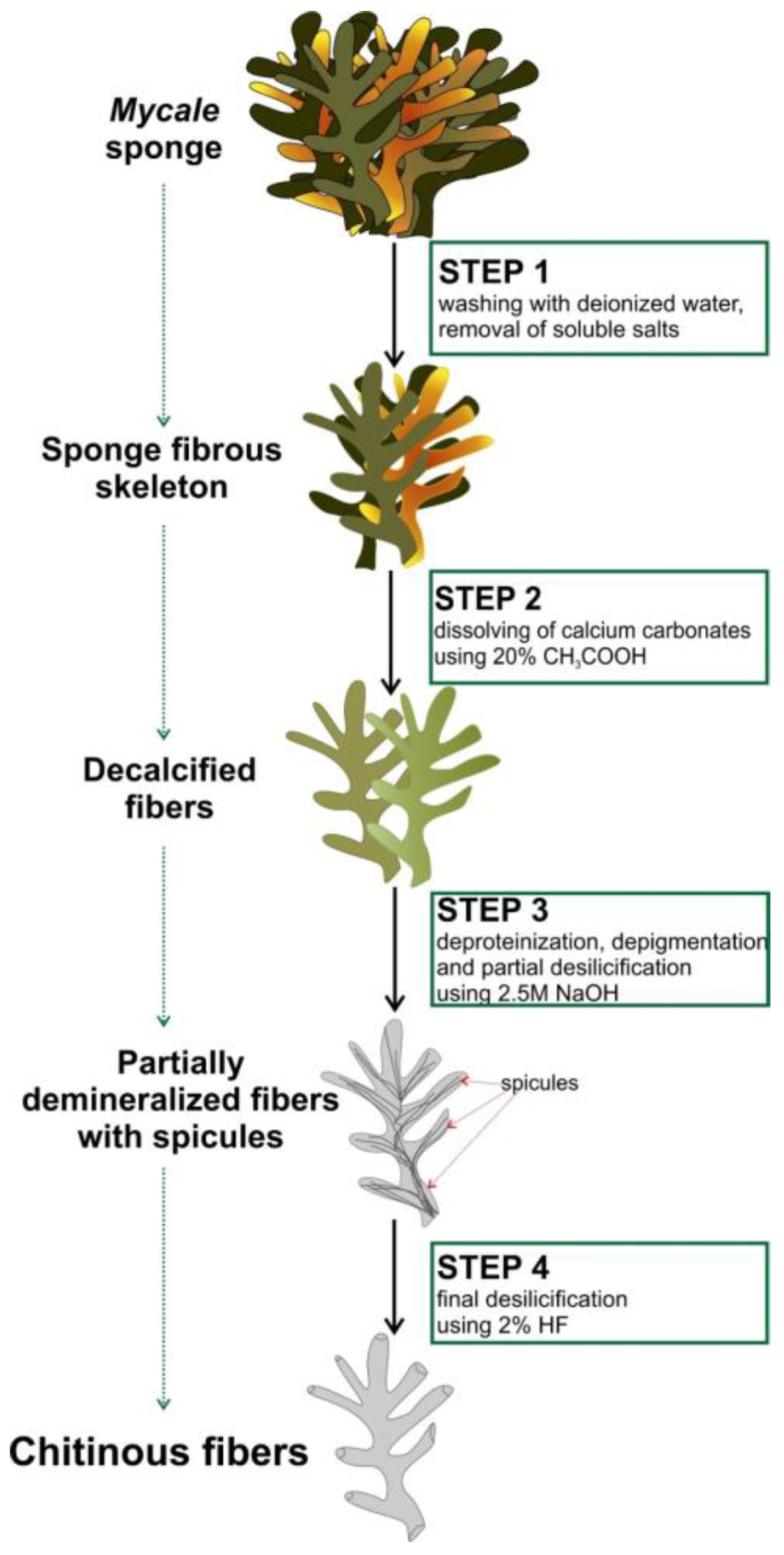
4.2. Isolation of Chitin from M. euplectellioides
4.3. Light and Fluorescent Microscopy Analysis and Imaging
4.4. Calcofluor White Staining Test
4.5. Scanning Electron Microscopy Analysis
4.6. Chitinase Digestion Test
4.7. Estimation of N-acetyl-d-glucosamine (NAG) Contents and Electrospray Ionization Mass Spectrometry
4.8. FTIR Spectroscopy
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Merzendorfer, H. The cellular basis of chitin synthesis in fungi and insects: Common principles and differences. Eur. J. Cell Biol. 2011, 90, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, S.; Tsujii, K.; Horikoshi, K. In situ microscopic observation of chitin and fungal cells with chitinous cell walls in hydrothermal conditions. Sci. Rep. 2015, 5, 11907. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.R.; Latge, J.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5, 1–25. [Google Scholar]
- Brunner, E.; Ehrlich, H.; Schupp, P.; Hedrich, R.; Hunoldt, S.; Kammer, M.; Machill, S.; Paasch, S.; Bazhenov, V.V.; Kurek, D.V.; et al. Chitin-based scaffolds are an integral part of the skeleton of the marine demosponge Ianthella basta. J. Struct. Biol. 2009, 168, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Maldonado, M.; Spindler, K.; Eckert, C.; Hanke, T.; Born, R.; Simon, P.; Heinemann, S.; Worch, H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (Demospongia: Porifera). J. Exp. Zool. Part B 2007, 356, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Krautter, M.; Hanke, T.; Simon, P.; Knieb, C.; Heinemann, S.; Worch, H. First evidence of the presence of chitin in skeletons of marine sponges. Part II. Glass sponges (Hexactinellida: Porifera). J. Exp. Zool. Part B 2007, 308B, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Bol. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Steck, E.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part II: Biomimetic potential and applications. Int. J. Biol. Macromol. 2010, 47, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Brunner, E.; Richthammer, P.; Ehrlich, H.; Paasch, S.; Simon, P.; Ueberlein, S.; van Pée, K.-H. Chitin-based organic networks: An integral part of cell wall biosilica in the diatom Thalassiosira pseudonana. Angew. Chem. Int. Ed. 2009, 48, 9724–9727. [Google Scholar] [CrossRef] [PubMed]
- Bo, M.; Bavestrello, G.; Kurek, D.; Paasch, S.; Brunner, E.; Born, R.; Galli, R.; Stelling, A.L.; Sivkov, V.N.; Petrova, O.V.; et al. Isolation and identification of chitin in the black coral Parantipathes larix (Anthozoa: Cnidaria). Int. J. Bol. Macromol. 2012, 51, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Connors, M.J.; Ehrlich, H.; Hog, M.; Godeffroy, C.; Araya, S.; Kallai, I.; Gazit, D.; Boyce, M.; Ortiz, C. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. 2012, 177, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Cuong, H.N.; Minh, N.C.; Van Hoa, N.; Trung, T.S. Preparation and characterization of high purity β-chitin from squid pens (Loligo chenisis). Int. J. Biol. Macromol. 2016, 93, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Guggolz, T.; Henne, S.; Politi, Y.; Schütz, R.; Mašić, A.; Müller, C.H.G.; Meißner, K. Histochemical evidence of β-chitin in parapodial glandular organs and tubes of Spiophanes (Annelida, Sedentaria: Spionidae), and first studies on selected Annelida. J. Morphol. 2015, 276, 1433–1447. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Chitin and collagen as universal and alternative templates in biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Wysokowski, M.; Petrenko, I.; Stelling, A.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan chitin as a versatile template for extreme biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef]
- Wysokowski, M.; Bazhenov, V.V.; Tsurkan, M.V.; Galli, R.; Stelling, A.L.; Stöcker, H.; Kaiser, S.; Niederschlag, E.; Gärtner, G.; Behm, T.; et al. Isolation and identification of chitin in three-dimensional skeleton of Aplysina fistularis marine sponge. Int. J. Biol. Macromol. 2013, 62, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Kaluzhnaya, O.V.; Tsurkan, M.V.; Ereskovsky, A.; Tabachnick, K.R.; Ilan, M.; Stelling, A.; Galli, R.; Petrova, O.V.; Nekipelov, S.V.; et al. First report on chitinous holdfast in sponges (Porifera). Proc. R. Soc. B 2013, 280, 20130339. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Kaluzhnaya, O.V.; Brunner, E.; Tsurkan, M.V.; Ereskovsky, A.; Ilan, M.; Tabachnick, K.R.; Bazhenov, V.V.; Paasch, S.; Kammer, M.; et al. Identification and first insights into the structure and biosynthesis of chitin from the freshwater sponge Spongilla lacustris. J. Struct. Biol. 2013, 183, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Simon, P.; Carrillo-Cabrera, W.; Bazhenov, V.V.; Botting, J.P.; Ilan, M.; Ereskovsky, A.V.; Muricy, G.; Worch, H.; Mensch, A.; et al. Insights into chemistry of biological materials: Newly discovered silica-aragonite-chitin biocomposites in Demosponges. Chem. Mater. 2010, 22, 1462–1471. [Google Scholar] [CrossRef]
- Ehrlich, H.; Maldonado, M.; Parker, A.R.; Kulchin, Y.N.; Schilling, J.; Köhler, B.; Skrzypczak, U.; Simon, P.; Reiswig, H.M.; Tsurkan, M.V.; et al. Supercontinuum Generation in Naturally Occurring Glass Sponges Spicules. Adv. Opt. Mater. 2016, 4, 1608–1613. [Google Scholar] [CrossRef]
- Philibert, T.; Lee, B.H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef] [PubMed]
- Anastopoulos, I.; Bhatnagar, A.; Bikiaris, D.; Kyzas, G. Chitin Adsorbents for Toxic Metals: A Review. Int. J. Mol. Sci. 2017, 18, 114. [Google Scholar] [CrossRef] [PubMed]
- Bąk, M.; Gutlowska, O.; Wagner, E.; Gosk, J. The role of chitin and chitosan in peripheral nerve reconstruction. Polym. Med. 2017, 47, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Shitiz, K.; Singh, A. Chitin and chitosan: Biopolymers for wound management. Int. Wound J. 2017, 14, 1276–1289. [Google Scholar] [CrossRef] [PubMed]
- Schleuter, D.; Günther, A.; Paasch, S.; Ehrlich, H.; Kljajić, Z.; Hanke, T.; Bernhard, G.; Brunner, E. Chitin-based renewable materials from marine sponges for uranium adsorption. Carbohydr. Polym. 2013, 92, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Sowmya, S.; Kumar, P.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Ehrlich, H.; Rigby, J.K.; Botting, J.P.; Tsurkan, M.; Werner, C.; Schwille, P.; Petrasek, Z.; Pisera, A.; Simon, P.; Sivkov, V.; et al. Discovery of 505-million-year old chitin in the basal demosponge Vauxia gracilenta. Sci. Rep. 2013, 3, 3497. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Biomimetic potential of chitin-based composite biomaterials of poriferan origin. In Biomimetic Biomaterials: Structure and Applications; Ruys, A.J., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 46–66. [Google Scholar]
- Wysokowski, M.; Motylenko, M.; Rafaja, D.; Koltsov, I.; Stöcker, H.; Szalaty, T.J.; Bazhenov, V.V.; Stelling, A.L.; Beyer, J.; Heitmann, J.; et al. Extreme biomimetic approach for synthesis of nanocrystalline chitin-(Ti,Zr)O2 multiphase composites. Mater. Chem. Phys. 2017, 188, 115–124. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Bazhenov, V.V.; Stawski, D.; Petrenko, I.; Ehrlich, A.; Behm, T.; Kljajic, Z.; Stelling, A.L.; Jesionowski, T.; et al. Poriferan chitin as a template for hydrothermal zirconia deposition. Front. Mater. Sci. 2013, 7, 248–260. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Beyer, J.; Makarova, A.; Stöcker, H.; Walter, J.; Galli, R.; Kaiser, S.; Vyalikh, D.; Bazhenov, V.V.; et al. Extreme biomimetic approach for developing novel chitin-GeO2 nanocomposites with photoluminescent properties. Nano Res. 2015, 8, 2288–2301. [Google Scholar] [CrossRef]
- Stepniak, I.; Galinski, M.; Nowacki, K.; Wysokowski, M.; Jakubowska, P.; Bazhenov, V.V.; Leisegang, T.; Ehrlich, H.; Jesionowski, T. A novel chitosan/sponge chitin origin material as a membrane for supercapacitors – preparation and characterization. RSC Adv. 2016, 6, 4007–4013. [Google Scholar] [CrossRef]
- Petrenko, I.; Bazhenov, V.V.; Galli, R.; Wysokowski, M.; Fromont, J.; Schupp, P.J.; Stelling, A.L.; Niederschlag, E.; Stöker, H.; Kutsova, V.Z.; et al. Chitin of poriferan origin and the bioelectrometallurgy of copper/copper oxide. Int. J. Biol. Macromol. 2017, 104, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Mutsenko, V.V.; Bazhenov, V.V.; Rogulska, O.; Tarusin, D.N.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; Meißner, H.; Lode, A.; et al. 3D chitinous scaffolds derived from cultivated marine demosponge Aplysina aerophoba for tissue engineering approaches based on human mesenchymal stromal cells. Int. J. Biol. Macromol. 2017, 104, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Mutsenko, V.V.; Gryshkov, O.; Lauterboeck, L.; Rogulska, O.; Tarusin, D.N.; Bazhenov, V.V.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; et al. Novel chitin scaffolds derived from marine sponge Ianthella basta for tissue engineering approaches based on human mesenchymal stromal cells: Biocompatibility and cryopreservation. Int. J. Biol. Macromol. 2017, 104, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.E. Notes on the arrangement of sponges, with the descriptions of some new genera. Proc. Zool. Soc. London 1867, 2, 492–558. [Google Scholar]
- Topsent, E. Révision des Mycale de l’Eéurope occidentale. Annales de l’Institut océanographique. Ann. l’Institut Océanographique 1924, 1, 77–118. [Google Scholar]
- Doumenc, D.; Levi, C. Anisochelae analysis and taxonomy of the genus Mycale Gray (Demospongiae). In Taxonomy of Porifera from the Northeast Atlantic and Mediterranean Sea; Vacelet, J., Boury-Esnault, N., Eds.; Springer-Verlag: Berlin, Germany, 1987; pp. 73–92. [Google Scholar]
- Bergquist, P.R.; Fromont, P.J. The Marine Fauna of New Zealand: Porifera, Demospongiae. Part 4 (Poecilosclerida); New Zealand Oceanographic Institute: Wellington, New Zealand, 1988. [Google Scholar]
- Reiswig, H.M.; Kaiser, H. Description of Mycale bamfieldense n. sp. (Porifera, Demospongiae, Poecilosclerida) from Vancouver Island, British Columbia. Can. J. Zool. 1989, 67, 674–677. [Google Scholar] [CrossRef]
- Carballo, J.L.; García-Gómez, J.C. The Northeastern Atlantic species Mycale micracanthoxea Buizer Van Soest, 1977 (Porifera, Poecilosclerida) in the Strait of Gibraltar (Southern Spain). Beaufortia 1994, 44, 11–16. [Google Scholar]
- Hajdu, E.; Desquevroux-Faundez, R. A synopsis of South American Mycale (Mycale) (Poecilosclerida, Demospongiae), with description of three new species and a cladistic analysis of Mycalidae. Rev. Suisse Zool. 1994, 101, 563–600. [Google Scholar] [CrossRef]
- Hajdu, E.; Zea, S.; Kielman, M.; Peixinho, S. Mycale escarlatei n. sp. and Mycale unguifera n. sp. (Demospongiae) from the tropical-western Atlantic. Beaufortia 1995, 45, 1–16. [Google Scholar]
- Carballo, J.L.; Hajdu, E. Micromorphology in Mycale taxonomy (Mycalidae, Poecilosclerida, Demospongiae), with the description of two new micracanthoxea-bearing species. Contrib. to Zool. 1998, 67, 187–195. [Google Scholar]
- Coles, S.L.; Bolick, H. Assessment of Invasiveness of the Orange Keyhole Sponge Mycale armata in Kaneohe Bay, Oahu, Hawaii. Final Report Year 1 Prepared for Hawaii Coral Reef Initiative; Bishop Museum: Honolulu, HI, USA, 2006. [Google Scholar]
- Coles, S.L.; Bolick, H. Invasive introduced sponge Mycale grandis overgrows reef corals in Kāne’ohe Bay, O’ahu, Hawai’i. Coral Reefs 2007, 26, 911. [Google Scholar] [CrossRef]
- Carballo, J.L.; Cruz-Barraza, J.A. A revision of the genus Mycale (Poecilosclerida: Mycalidae) from the Mexican Pacific Ocean. Contrib. Zool. 2010, 79, 165–194. [Google Scholar]
- Van Soest, R.W.M.; Beglinger, E.J.; De Voogd, N.J. Mycale species (Porifera: Poecilosclerida) of Northwest Africa and the Macaronesian Islands. Zool. Med. Leiden 2014, 88, 59–109. [Google Scholar]
- Riesgo, A.; Taboada, S.; Sánchez-Vila, L.; Solà, J.; Bertran, A.; Avila, C. Some like it fat: Comparative ultrastructure of the embryo in two demosponges of the genus Mycale (order poecilosclerida) from Antarctica and the Caribbean. PLoS ONE 2015, 10, e0118805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Soest, R.W.M.; Hajdu, E. Family Mycalidae Lundbeck, 1905. In Systema Porifera: A Guide to the Classification of Sponges; Hooper, J.N.A., Van Soest, R.W.M., Willenz, P., Eds.; Springer: Boston, MA, USA, 2002; pp. 669–690. [Google Scholar]
- Van Soest, R.W.M.; Boury-Esnault, N.; Hooper, J.N.A.; Rützler, K.; de Voogd, N.J.; Alvarez, B.; Hajdu, E.; Pisera, A.B.; Manconi, R.; Schönberg, C.; et al. World Porifera Database. Mycale grandis Gray, 1867. Available online: http://www.marinespecies.org/porifera/porifera.php?p=taxdetails&id=192533 (accessed on 20 February 2018).
- Vincente, J.; Silbiger, N.J.; Beckley, B.A.; Raczkowski, C.W.; Hill, R.T. Impact of high pCO2 and warmer temperatures on the process of silica biomineralization in the sponge Mycale grandis. ICES J. Mar. Sci. 2016, 73, 704–714. [Google Scholar] [CrossRef]
- Qiu, F.; Ding, S.; Ou, H.; Wang, D.; Chen, J.; Miyamoto, M.M. Transcriptome changes during the life cycle of the red sponge, Mycale phyllophila (Porifera, Demospongiae, Poecilosclerida). Genes 2015, 6, 1023–1052. [Google Scholar] [CrossRef] [PubMed]
- Corriero, G.; Scalera Liaci, L.; Nonnis Marzano, C.; Gaino, E. Reproductive strategies of Mycale contarenii (Porifera: Demospongiae). Mar. Biol. 1998, 131, 319–327. [Google Scholar] [CrossRef]
- Custódio, M.R.; Hajdu, E. In vivo study of microsclere formation in sponges of the genus Mycale (Demospongiae, Poecilosclerida). Zoomorphology 2002, 121, 203–211. [Google Scholar] [CrossRef]
- Mohamed, N.M.; Enticknap, J.J.; Lohr, J.E.; McIntosh, S.M.; Hill, R.T. Changes in bacterial communities of the marine sponge Mycale laxissima on transfer into aquaculture. Appl. Environ. Microbiol. 2008, 74, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Northcote, P.T.; Webb, V.L.; Mackey, S.; Handley, S.J. Aquaculture trials for the production of biologically active metabolites in the New Zealand sponge Mycale hentscheli (Demospongiae: Poecilosclerida). Aquaculture 2005, 250, 256–269. [Google Scholar] [CrossRef]
- Page, M.J.; Handley, S.J.; Northcote, P.T.; Cairney, D.; Willan, R.C. Successes and pitfalls of the aquaculture of the sponge Mycale hentscheli. Aquaculture 2011, 312, 52–61. [Google Scholar] [CrossRef]
- Huang, D.; Ou, H.; Wang, D.; Chen, J.; Ding, S. Sexual reproduction of the potentially cultivable sponge Mycale phyllophila (Porifera, Demospongiae). J. Mar. Biol. Assoc. U. K. 2016, 96, 1073–1081. [Google Scholar] [CrossRef]
- Row, R.W.H. Reports on the Marine Biology of the Sudanese Red Sea, from Collections made by Cyril Crossland, M.A., B.Sc., F.Z.S. XIX. Report on the Sponges collected by Mr. Cyril Crossland in 1904-5. Part II. Non-Calcarea. J. Linn. Soc. Zool. 1911, 31, 35–41. [Google Scholar] [CrossRef]
- Van Soest, R.W.M.; Boury-Esnault, N.; Hooper, J.N.A.; Rützler, K.; de Voogd, N.J.; Alvarez, B.; Hajdu, E.; Pisera, A.B.; Manconi, R.; Schönberg, C.; et al. World Porifera Database. Mycale euplectellioides (Row, 1911). Available online: http://www.marinespecies.org/porifera/porifera.php?p=taxdetails&id=194743 (accessed on 20 February 2018).
- Wysokowski, M.; Behm, T.; Born, R.; Bazhenov, V.V.; Meiβner, H.; Richter, G.; Szwarc-Rzepka, K.; Makarova, A.; Vyalikh, D.; Schupp, P.; et al. Preparation of chitin-silica composites by in vitro silicification of two-dimensional Ianthella basta demosponge chitinous scaffolds under modified Stöber conditions. Mater. Sci. Eng. C 2013, 33, 3935–3941. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Bazhenov, V.V.; Debitus, C.; de Voogd, N.; Galli, R.; Tsurkan, M.V.; Wysokowski, M.; Meissner, H.; Bulut, E.; Kaya, M.; et al. Isolation and identification of chitin from heavy mineralized skeleton of Suberea clavata (Verongida: Demospongiae: Porifera) marine demosponge. Int. J. Biol. Macromol. 2017, 104, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [PubMed]
- Lavall, R.L.; Assis, O.B.G.; Campana-Filho, S.P. Beta-chitin from the pens of Loligo sp.: Extraction and characterization. Bioresour. Technol. 2007, 98, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xu, S.; Yang, K.; He, H. Study on chemical constituents from Mycale parishi. Zhong Yao Cai 2003, 26, 715–718. [Google Scholar] [PubMed]
- Wang, R.P.; Lin, H.W.; Li, L.Z.; Gao, P.Y.; Xu, Y.; Song, S.J. Monoindole alkaloids from a marine sponge Mycale fibrexilis. Biochem. Syst. Ecol. 2012, 43, 210–213. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, X.; Guo, X.; Yang, B.; Yang, X.W.; Liu, Y. Chemical constituents of the sponge Mycale species from South China Sea. Rec. Nat. Prod. 2013, 7, 119–123. [Google Scholar]
- Northcote, P.T.; Blunt, J.W.; Munro, M.H.G. Pateamine: A potent cytotoxin from the New Zealand Marine sponge, Mycale sp. Tetrahedron Lett. 1991, 32, 6411–6414. [Google Scholar] [CrossRef]
- Matsunaga, S.; Sugawara, T.; Fusetani, N. New mycalolides from the marine sponge Mycale magellanica and their interconversion. J. Nat. Prod. 1998, 61, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; Blunt, J.W.; Munro, M.H.G.; Perry, N.B. Chemistry of the mycalamides, antiviral and antitumour compounds from a marine sponge. Part 5. Acid-catalysed hydrolysis and acetal exchange, double bond additions and oxidation reactions. J. Chem. Soc. Perkin Trans. 1 1995. [Google Scholar] [CrossRef]
- Thompson, A.M.; Blunt, J.W.; Murray, H.G.; Munro, S.M.H.G.; Perry, N.B.; Pannell, L.K. Chemistry of the Mycalamides, antiviral and antitumour compounds from a marine sponge. Part 3. Acyl, Alkyl and Silyl derivatives. J. Chem. Soc. Perikin. Trans. 1992, 1, 1335–1342. [Google Scholar] [CrossRef]
- West, L.M.; Northcote, P.T.; Hood, K.A.; Miller, J.H.; Page, M.J. Mycalamide D, a new cytotoxic amide from the New Zealand marine sponge Mycale species. J. Nat. Prod. 2000, 63, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Hood, K.A.; West, L.M.; Northcote, P.T.; Berridge, M.V.; Miller, J.H. Induction of apoptosis by the marine sponge (Mycale) metabolites, mycalamide A and pateamine. Apoptosis 2001, 6, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Fedorov, S.N.; Kalinovsky, A.I.; Shubina, L.K.; Bokemeyer, C.; Stonik, V.A.; Honecker, F. Mycalamide A shows cytotoxic properties and prevents EGF-induced neoplastic transformation through inhibition of nuclear factors. Mar. Drugs 2012, 10, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.B.; Blunt, J.W.; Munro, M.H.G.; Thompson, A.M. Antiviral and Antitumor Agents from a New Zealand Sponge, Mycale sp. 2. Structures and Solution Conformations of Mycalamides A and B. J. Org. Chem. 1990, 55, 223–227. [Google Scholar] [CrossRef]
- Perry, N.B.; Blunt, J.W.; Munro, M.H.G. Mycalamide A, an antiviral compound from a New Zeland sponge of the genus Mycale. J. Am. Chem. Soc. 1988, 110, 4850–4851. [Google Scholar] [CrossRef]
- Gürel, G.; Blaha, G.; Steitz, T.A.; Moore, P.B. Structures of triacetyloleandomycin and mycalamide A bind to the large ribosomal subunit of Haloarcula marismortui. Antimicrob. Agents Chemother. 2009, 53, 5010–5014. [Google Scholar] [CrossRef] [PubMed]
- Phuwapraisirisan, P.; Matsunaga, S.; Van Soest, R.W.M.; Fusetani, N. Isolation of a new mycalolide from the marine sponge Mycale izuensis. J. Nat. Prod. 2002, 65, 942–943. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Yoshida, S.; Matsunaga, S.; Shindoh, N.; Terada, Y.; Nagai, K.; Yamashita, J.K.; Ganesan, A.; Van Soest, R.W.M.; Fusetani, N. Azumamides A–E: Histone deacetylase inhibitory cyclic tetrapeptides from the marine sponge Mycale izuensis. Angew. Chemie Int. Ed. 2006, 45, 7553–7557. [Google Scholar] [CrossRef] [PubMed]
- Maulucci, N.; Chini, M.G.; Di Micco, S.; Izzo, I.; Cafaro, E.; Russo, A.; Gallinari, P.; Paolini, C.; Nardi, M.C.; Casapullo, A.; et al. Molecular insights into azumamide E histone deacetylases inhibitory activity. J. Am. Chem. Soc. 2007, 129, 3007–3012. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Koimaru, K.; Ohta, T. Secomycalolide A: A new proteasome inhibitor isolated from a marine sponge of the genus Mycale. Mar. Drugs 2005, 3, 29–35. [Google Scholar] [CrossRef]
- Hood, K.A.; West, L.M.; Rouwé, B.; Northcote, P.T.; Berridge, M.V.; Wakefield, S.J.; Miller, J.H. Peloruside A, a novel antimitotic agent with paclitaxel-like microtubule-stabilizing activity. Cancer Res. 2002, 62, 3356–3360. [Google Scholar] [PubMed]
- Wilmes, A.; Bargh, K.; Kelly, C.; Northcote, P.T.; Miller, J.H. Peloruside A synergizes with other microtubule stabilizing agents in cultured cancer cell lines. Mol. Pharm. 2007, 4, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.H.; Singh, A.J.; Northcote, P.T. Microtubule-stabilizing drugs from marine sponges: Focus on peloruside A and zampanolide. Mar. Drugs 2010, 8, 1059–1079. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.H.; Rouwé, B.; Gaitanos, T.N.; Hood, K.A.; Crume, K.P.; Bäckström, B.T.; La Flamme, A.C.; Berridge, M.V.; Northcote, P.T. Peloruside A enhances apoptosis in H-ras-transformed cells and is cytotoxic to proliferating T cells. Apoptosis 2004, 9, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Kanakkanthara, A. Peloruside A: A lead non-taxoid-site microtubule-stabilizing agent with potential activity against cancer, neurodegeneration, and autoimmune disease. Nat. Prod. Rep. 2016, 33, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.J.; Xu, C.X.; Xu, X.; West, L.M.; Wilmes, A.; Chan, A.; Hamel, E.; Miller, J.H.; Northcote, P.T.; Ghosh, A.K. Peloruside B, a potent antitumor macrolide from the New Zealand marine sponge Mycale hentscheli: Isolation, structure, total synthesis, and bioactivity. J. Org. Chem. 2010, 75, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.C.; Liu, Y.; Morgan, J.B.; Jekabsons, M.B.; Zhou, Y.D.; Nagle, D.G. Lipophilic 2,5-disubstituted pyrroles from the marine sponge Mycale sp. inhibit mitochondrial respiration and HIF-1 activation. J. Nat. Prod. 2009, 72, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.G.; Dhananjaya, N. Chemical investigation of Mycale mytilorum and a study on toxicity and antidiabetic activity of 5-octadecylpyrrole-2-carboxaldehyde. Bioorganic Med. Chem. 2000, 8, 27–36. [Google Scholar] [CrossRef]
- Xue, D.Q.; Liu, H.L.; Chen, S.H.; Mollo, E.; Gavagnin, M.; Li, J.; Li, X.W.; Guo, Y.W. 5-Alkylpyrrole-2-carboxaldehyde derivatives from the Chinese sponge Mycale lissochela and their PTP1B inhibitory activities. Chinese Chem. Lett. 2017, 28, 1190–1193. [Google Scholar] [CrossRef]
- Carballeira, N.M.; Negron, V.; Reyes, E.D. Novel Monounsaturated Fatty Acids From the Sponges Amphimedon compressa and Mycale laevis. J. Nat. Prod. 1992, 55, 333–339. [Google Scholar] [CrossRef]
- Valle, H.A.Z.; Santafé, G.G.P. Free Sterols from the Marine Sponge Mycale laevis. Vitae 2009, 16, 103–109. [Google Scholar]
- Rochfort, S.J.; Gable, R.W.; Capon, R.J. Mycalone: A new steroidal lactone from a southern Australian marine sponge, Mycale sp. Aust. J. Chem. 1996, 49, 715–718. [Google Scholar]
- Antonov, A.S.; Afiyatullov, S.S.; Kalinovsky, A.I.; Ponomarenko, L.P.; Dmitrenok, P.S.; Aminin, D.L.; Agafonova, I.G.; Stonik, V.A. Mycalosides B–I, eight new spermostatic steroid oligoglycosides from the sponge Mycale laxissima. J. Nat. Prod. 2003, 66, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Abd-Elrazek, A.E.E.; Hassanean, H.A.; Alahdal, A.M.; Almohammadi, A.; Youssef, D.T.A. New fatty acids from the Red Sea sponge Mycale euplectellioides. Nat. Prod. Res. 2014, 28, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, R.; Elgawish, M.S.; Mira, A.; Ibrahim, A.K.; Ahmed, S.A.; Shimizu, K.; Yamada, K. Anti-choline esterase activity of ceramides from the Red Sea marine sponge Mycale euplectellioides. RSC Adv. 2016, 6, 20422–20430. [Google Scholar] [CrossRef]
- Ehrlich, H.; Brunner, E.; Richter, W.; Ilan, M.; Schupp, P. Two or Three-Dimensional Cleaned Chitin Skeleton of Dictyoceratid Sponges. Method for the Production and Use Thereof. WO2011023531A3, 23 June 2011. [Google Scholar]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żółtowska-Aksamitowska, S.; Shaala, L.A.; Youssef, D.T.A.; Elhady, S.S.; Tsurkan, M.V.; Petrenko, I.; Wysokowski, M.; Tabachnick, K.; Meissner, H.; Ivanenko, V.N.; et al. First Report on Chitin in a Non-Verongiid Marine Demosponge: The Mycale euplectellioides Case. Mar. Drugs 2018, 16, 68. https://doi.org/10.3390/md16020068
Żółtowska-Aksamitowska S, Shaala LA, Youssef DTA, Elhady SS, Tsurkan MV, Petrenko I, Wysokowski M, Tabachnick K, Meissner H, Ivanenko VN, et al. First Report on Chitin in a Non-Verongiid Marine Demosponge: The Mycale euplectellioides Case. Marine Drugs. 2018; 16(2):68. https://doi.org/10.3390/md16020068
Chicago/Turabian StyleŻółtowska-Aksamitowska, Sonia, Lamiaa A. Shaala, Diaa T. A. Youssef, Sameh S. Elhady, Mikhail V. Tsurkan, Iaroslav Petrenko, Marcin Wysokowski, Konstantin Tabachnick, Heike Meissner, Viatcheslav N. Ivanenko, and et al. 2018. "First Report on Chitin in a Non-Verongiid Marine Demosponge: The Mycale euplectellioides Case" Marine Drugs 16, no. 2: 68. https://doi.org/10.3390/md16020068








