Tetrocarcin Q, a New Spirotetronate with a Unique Glycosyl Group from a Marine-Derived Actinomycete Micromonospora carbonacea LS276
Abstract
:1. Introduction
2. Results and Discussion
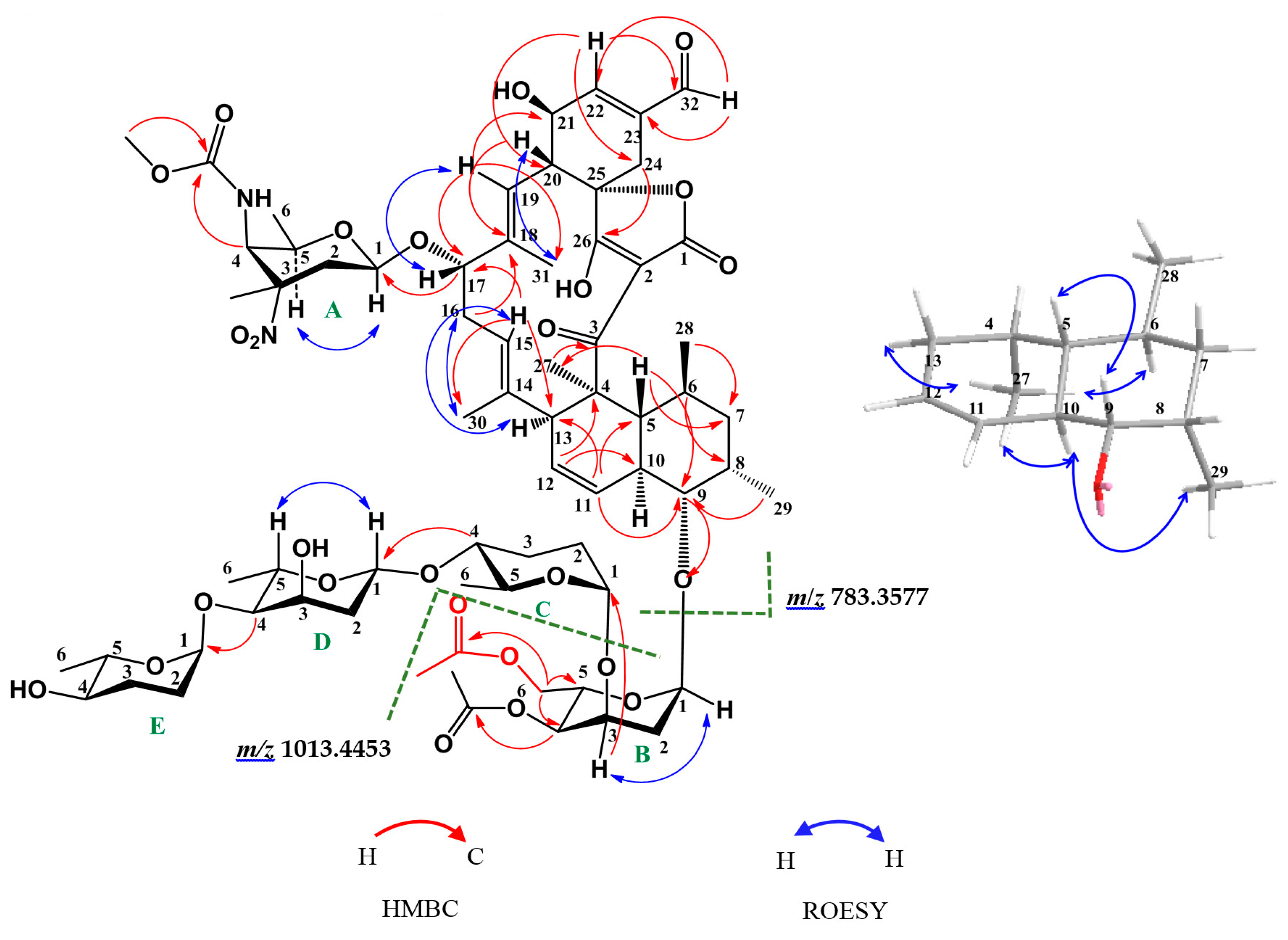
2.1. Structure Elucidation of Compounds
2.2. Biological Assays
3. Materials and Methods
3.1. General
3.2. Bacterial Material and Fermentation
3.3. Extraction and Isolation
3.4. Biological Assays
3.4.1. Antibacterial Activity
3.4.2. Antitumor Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lacoske, M.H.; Theodorakis, E.A. Spirotetronate polyketides as leads in drug discovery. J. Nat. Prod. 2015, 78, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, Y.; Huang, L.; Jia, X.; Zhang, Q.; Zhang, X.; Tang, G.; Liu, W. Cloning and characterization of the tetrocarcin A gene cluster from Micromonospora chalcea NRRL 11289 reveals a highly conserved strategy for tetronate biosynthesis in spirotetronate antibiotics. J. Bacteriol. 2008, 190, 6014–6025. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.Q.; Zhang, S.Y.; Wang, N.; Li, Z.L.; Hua, H.M.; Hu, J.C.; Wang, S.J. New spirotetronate antibiotics, lobophorins H and I, from a South China Sea-derived Streptomyces sp. 12A35. Mar. Drugs 2013, 11, 3891–3901. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Hu, Y.; Wang, Q.; Zhou, H.; Wang, Y.; Gan, M. Tetrocarcins N and O, glycosidic spirotetronates from a marine-derived Micromonospora sp. identified by PCR-based screening. RSC Adv. 2016, 6, 91773–91778. [Google Scholar] [CrossRef]
- Tamaoki, T.; Kasai, M.; Shirahata, K.; Ohkubo, S.; Morimoto, M.; Mineura, K.; Ishii, S.; Tomita, F. Tetrocarcins, novel antitumor antibiotics. II. Isolation, characterization and antitumor activity. J. Antibiot. 1980, 33, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Fukui, M.; Ohkubo, S.; Tamaoki, T.; Tomita, F. Tetrocarcins, new antitumor antibiotics. 3. Antitumor activity of tetrocarcin A. J. Antibiot. 1982, 35, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Ohtomo, M.; Yamazaki, K.; Ito, S.; Shimura, K.; Shimizu, S.; Minami, T.; Fujinaga, T.; Shimada, K. Effects of tetrocarcin-A on bovine theileriosis in Japan. Nihon Juigaku Zasshi Jpn. J. Vet. Sci. 1985, 47, 581–587. [Google Scholar] [CrossRef]
- Namikawa, K.; Sakuma, Y.; Sunaga, F.; Kanno, Y. Characteristics of tetrocarcin-A compared with other anti-piroplasmotic drugs. Nihon Juigaku Zasshi Jpn. J. Vet. Sci. 1988, 50, 605–612. [Google Scholar] [CrossRef]
- Furumai, T.; Takagi, K.; Igarashi, Y.; Saito, N.; Oki, T. Arisostatins A and B, new members of tetrocarcin class of antibiotics from Micromonospora sp. TP-A0316. I. Taxonomy, fermentation, isolation and biological properties. J. Antibiot. 2000, 53, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Miura, M.; Hara, M. Tetrocarcin A inhibits mitochondrial functions of Bcl-2 and suppresses its anti-apoptotic activity. Cancer Res. 2000, 60, 1229–1235. [Google Scholar] [PubMed]
- Tamaoki, T.; Kasai, M.; Shirahata, K.; Tomita, F. Tetrocarcins E1, E2, F and F-1, new antibiotics. Fermentation, isolation and characterization. J. Antibiot. 1982, 35, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Shimotohno, K.W.; Endo, T.; Furihata, K. Antibiotic AC6H, a new component of tetrocarcin group antibiotics. J. Antibiot. 1993, 46, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Takagi, K.; Kan, Y.; Fujii, K.; Harada, K.; Furumai, T.; Oki, T. Arisostatins A and B, new members of tetrocarcin class of antibiotics from Micromonospora sp. TP-A0316. II. Structure determination. J. Antibiot. 2000, 53, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Gui, C.; Zhang, S.; Zhu, X.; Ding, W.; Huang, H.; Gu, Y.C.; Duan, Y.; Ju, J. Antimicrobial spirotetronate metabolites from marine-derived Micromonospora harpali SCSIO GJ089. J. Nat. Prod. 2017, 80, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Nakashima, T.; Uosaki, Y.; Hara, M.; Ikeda, S.; Kanda, Y. Synthesis of tetrocarcin derivatives with specific inhibitory activity towards Bcl-2 functions. Bioorg. Med. Chem. Lett. 2001, 11, 887–890. [Google Scholar] [CrossRef]
- Hara, T.; Omura-Minamisawa, M.; Chao, C.; Nakagami, Y.; Ito, M.; Inoue, T. Bcl-2 inhibitors potentiate the cytotoxic effects of radiation in Bcl-2 overexpressing radioresistant tumor cells. Int. J. Radiat. Oncol. 2005, 61, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Q.; Liu, W.C.; Zhu, P.; Yang, J.L.; Cheng, K.D. Phylogenetic diversity of bacteria associated with the marine sponge Gelliodes carnosa collected from the Hainan Island coastal waters of the South China Sea. Microb. Ecol. 2011, 62, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Kobinata, K.; Uramoto, M.; Mizuno, T.; Isono, K. A new antibiotic, antlermicin A. J. Antibiot. 1980, 33, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Q.; Xu, S.; Zuo, L.; You, X.; Hu, H.Y. Aminoglycoside-based novel probes for bacterial diagnostic and therapeutic applications. Chem. Commun. 2017, 53, 1366–1369. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar] [PubMed]
- Zhen, X.; Gong, T.; Liu, F.; Zhang, P.C.; Zhou, W.Q.; Li, Y.; Zhu, P. A new analogue of echinomycin and a new cyclic dipeptide from a marine-derived Streptomyces sp. LS298. Mar. Drugs 2015, 13, 6947–6961. [Google Scholar] [CrossRef] [PubMed]

| No. | δH Mult. (J in Hz) | δC | No. | δH Mult. (J in Hz) | δC |
|---|---|---|---|---|---|
| Spiroteronate Skeleton | |||||
| 1 | - | 166.7 | 17 | 4.28, brs | 78.0 |
| 2 | - | 100.9 | 18 | - | 141.6 |
| 3 | - | 206.4 | 19 | 5.21, d (10.2) | 118.3 |
| 4 | - | 51.3 | 20 | 3.06, t (9.6) | 45.0 |
| 5 | 2.07, m | 43.4 | 21 | 4.85, m | 69.2 |
| 6 | 1.37, m | 31.3 | 22 | 6.92, s | 149.6 |
| 7 | 1.46, m; 1.60, m | 41.6 | 23 | - | 136.5 |
| 8 | 2.20, m | 34.5 | 24 | 2.56, m; 2.83, dt (2.5,18.9) | 29.8 |
| 9 | 3.49, dd (5.1, 10.5) | 84.8 | 25 | - | 84.1 |
| 10 | 2.10, t (9.8) | 38.5 | 26 | - | 201.5 |
| 11 | 5.74, d (10.2) | 126.1 | 27 | 1.63, s | 15.2 |
| 12 | 5.42, m | 126.2 | 28 | 0.64, d (6.0) | 22.1 |
| 13 | 3.28, m | 54.3 | 29 | 1.09, d (7.2) | 14.1 |
| 14 | - | 136.1 | 30 | 1.34, s | 14.5 |
| 15 | 5.16, m | 123.1 | 31 | 1.53, s | 16.2 |
| 16 | 2.28, m; 1.59, m | 30.8 | 32 | 9.58, s | 192.6 |
| Sugars | |||||
| A-1 | 4.44, dd (9.6, 1.8) | 96.5 | C-1 | 4.88, brd (3.0) | 92.7 |
| A-2 | 2.72, brd (9.6); 1.64, m | 36.1 | C-2 | 1.88, m; 1.75, m | 29.6 |
| A-3 | - | 91.6 | C-3 | 2.03, m; 1.97, m | 26.4 |
| A-4 | 4.36, dd (10.2, 2.4) | 53.8 | C-4 | 3.21, td (9.6, 4.8) | 81.3 |
| A-4-NH | 5.07, d (10.2) | C-5 | 3.70, m | 68.1 | |
| A-5 | 3.48, m | 69.4 | C-6 | 1.16, d (6.6) | 18.2 |
| A-6 | 1.15, d (6.6) | 17.1 | D-1 | 4.90, dd (9.6, 1.8) | 99.5 |
| A3-CH3 | 1.60, s | 25.4 | D-2 | 2.15, dt (14.4, 1.8);1.67, m | 37.1 |
| A4-NHCOOCH3 | 3.71, s | 53.0 | D-3 | 4.25, m | 64.0 |
| A4-NHCOOCH3 | - | 157.4 | D-4 | 3.46, dd (9.6, 3.0) | 75.3 |
| B-1 | 4.92, d (4.8) | 98.9 | D-5 | 3.85, dq (9.6, 6.0) | 67.9 |
| B-2 | 2.24, dd (14.4, 3.0); 1.79, m | 31.2 | D-6 | 1.32, d (6.0) | 19.0 |
| B-3 | 4.23, m | 66.5 | E-1 | 4.91, brs | 92.0 |
| B-4 | 4.83, dd (10.5, 3.0) | 69.5 | E-2 | 1.83, 2H, m | 29.8 |
| B-5 | 4.50, m | 64.6 | E-3 | 1.90, m; 1.74, m | 27.5 |
| B-6 | 4.32, dd(12.0, 5.4); | 63.5 | E-4 | 3.30, td (9.6, 4.8) | 71.8 |
| 4.12, dd (12.0, 1.8) | |||||
| B4-OCOCH3 | 2.08, s | 20.9 | E-5 | 3.63, dq (9.6, 6.0) | 70.4 |
| B4-OCOCH3 | - | 170.2 | E-6 | 1.23, d (6.0) | 17.8 |
| B6-OCOCH3 | 2.07, s | 21.0 | - | - | - |
| B6-OCOCH3 | - | 170.9 | - | - | - |
| Compounds | MICs (μM) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Ampicillin | |
| B. subitlis ATCC 63501 | 12.5 | <0.048 | 0.5 | 1.562 | 50 | 0.048 | >400 | 3.125 |
| Compounds | IC50 (μM) | ||||
|---|---|---|---|---|---|
| A549 | BGC823 | HCT116 | HepG2 | U87 MG | |
| 1 | >50.0 | 28.3 | 32.4 | 49.3 | 13.3 |
| 2 | 5.71 | 7.45 | 5.97 | 18.2 | 0.50 |
| 3 | 19.2 | 25.4 | 28.2 | >50.0 | 11.0 |
| 4 | 27.1 | 27.4 | 27.3 | >50.0 | 21.3 |
| 5 | >50.0 | >50.0 | >50.0 | >50.0 | 44.7 |
| 6 | 5.33 | 19.7 | 6.53 | 18.8 | 2.42 |
| 7 | >50.0 | >50.0 | >50.0 | >50.0 | >50.0 |
| paclitaxel a | 0.001 | 0.01 | 0.01 | 0.07 | - |
| gefitinib b | - | - | - | - | 8.30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, T.; Zhen, X.; Li, X.-L.; Chen, J.-J.; Chen, T.-J.; Yang, J.-L.; Zhu, P. Tetrocarcin Q, a New Spirotetronate with a Unique Glycosyl Group from a Marine-Derived Actinomycete Micromonospora carbonacea LS276. Mar. Drugs 2018, 16, 74. https://doi.org/10.3390/md16020074
Gong T, Zhen X, Li X-L, Chen J-J, Chen T-J, Yang J-L, Zhu P. Tetrocarcin Q, a New Spirotetronate with a Unique Glycosyl Group from a Marine-Derived Actinomycete Micromonospora carbonacea LS276. Marine Drugs. 2018; 16(2):74. https://doi.org/10.3390/md16020074
Chicago/Turabian StyleGong, Ting, Xin Zhen, Xing-Lun Li, Jing-Jing Chen, Tian-Jiao Chen, Jin-Ling Yang, and Ping Zhu. 2018. "Tetrocarcin Q, a New Spirotetronate with a Unique Glycosyl Group from a Marine-Derived Actinomycete Micromonospora carbonacea LS276" Marine Drugs 16, no. 2: 74. https://doi.org/10.3390/md16020074





