Comparative Analysis of Culture Conditions for the Optimization of Carotenoid Production in Several Strains of the Picoeukaryote Ostreococcus
Abstract
:1. Introduction
2. Results
2.1. Growth Rates of Ostreococcus Strains under Abiotic Stresses
2.2. Total Carotenoid Content under Various Stress Conditions
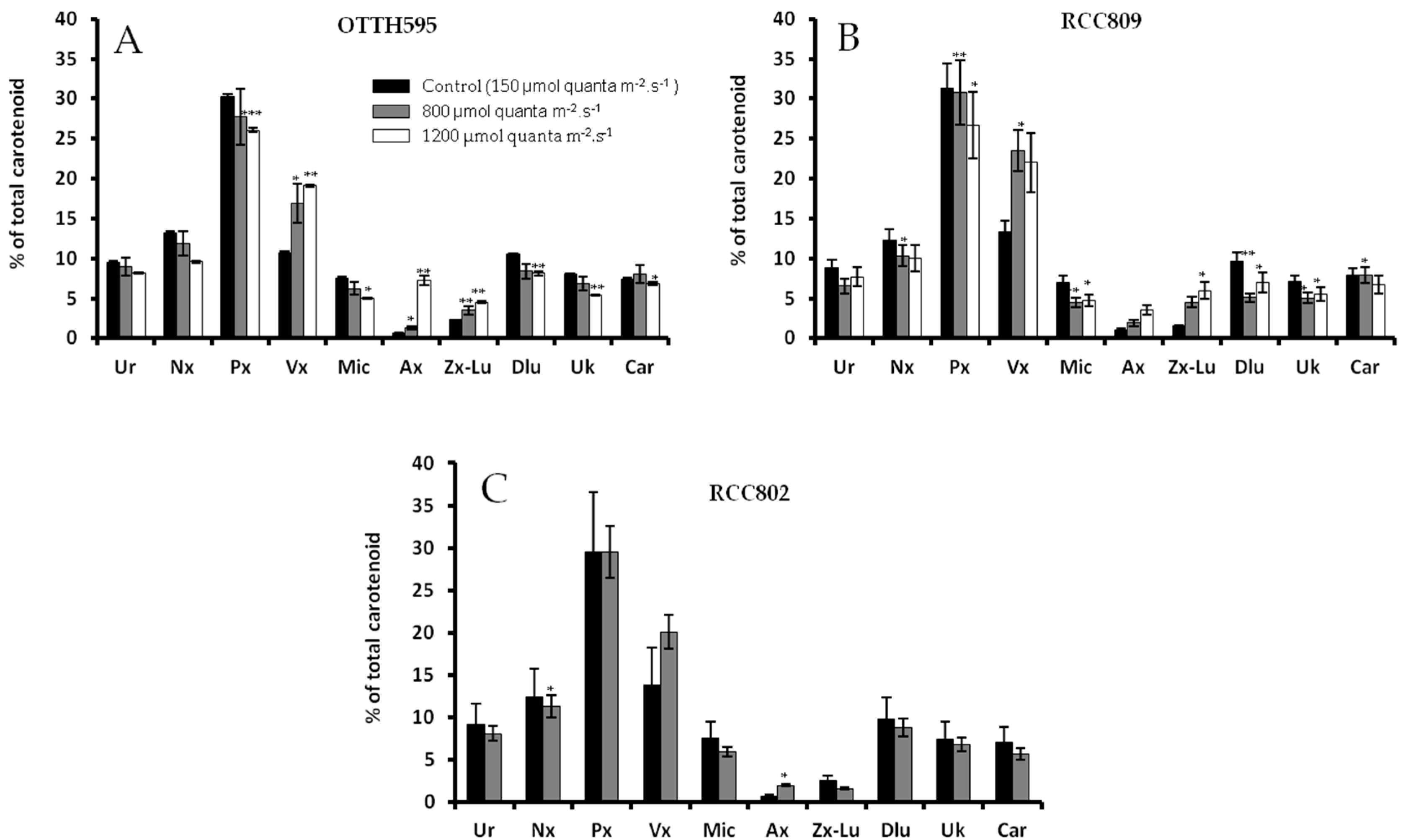
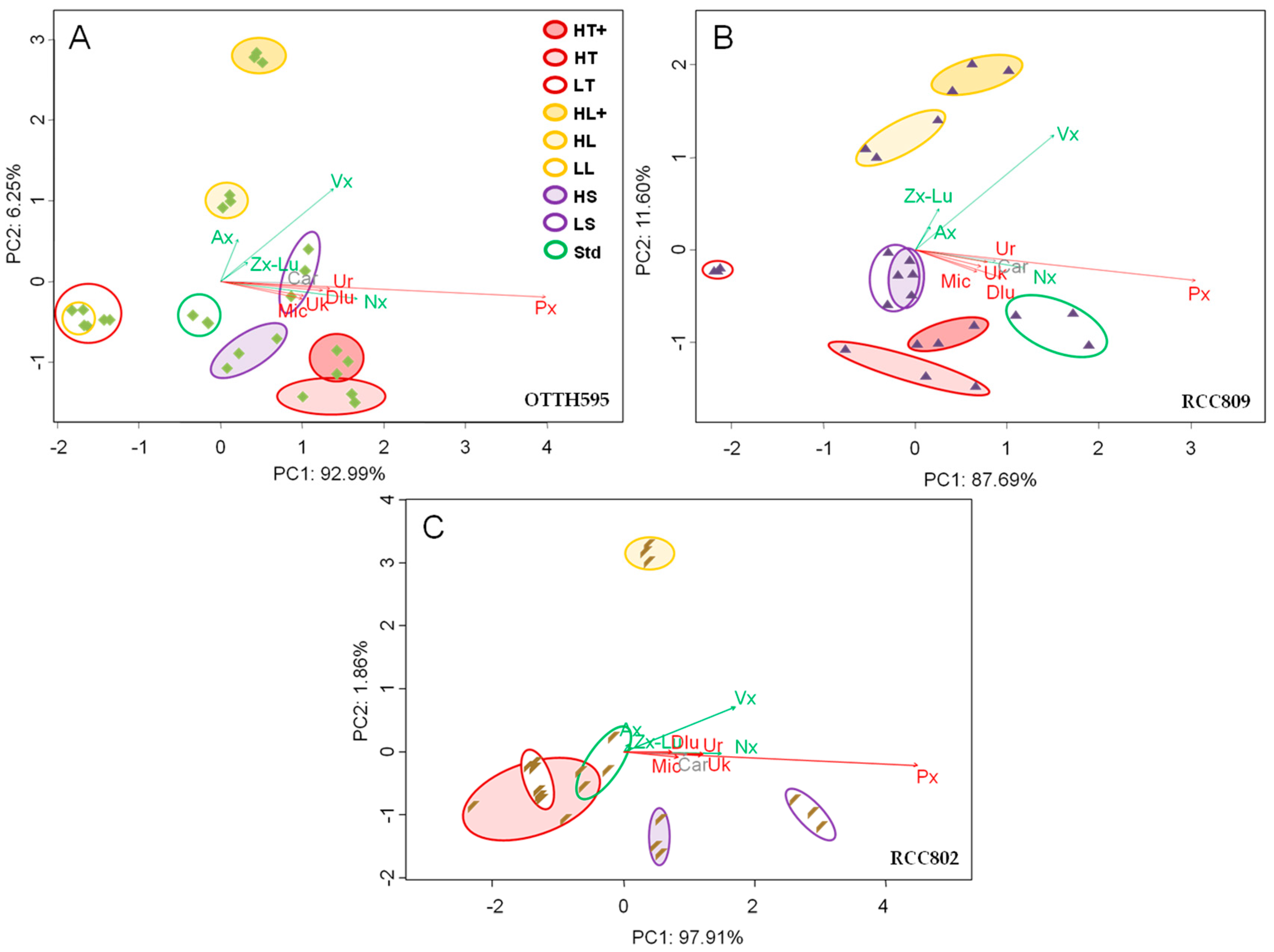
2.3. Effect of Stress Conditions on Carotenoid Profiles
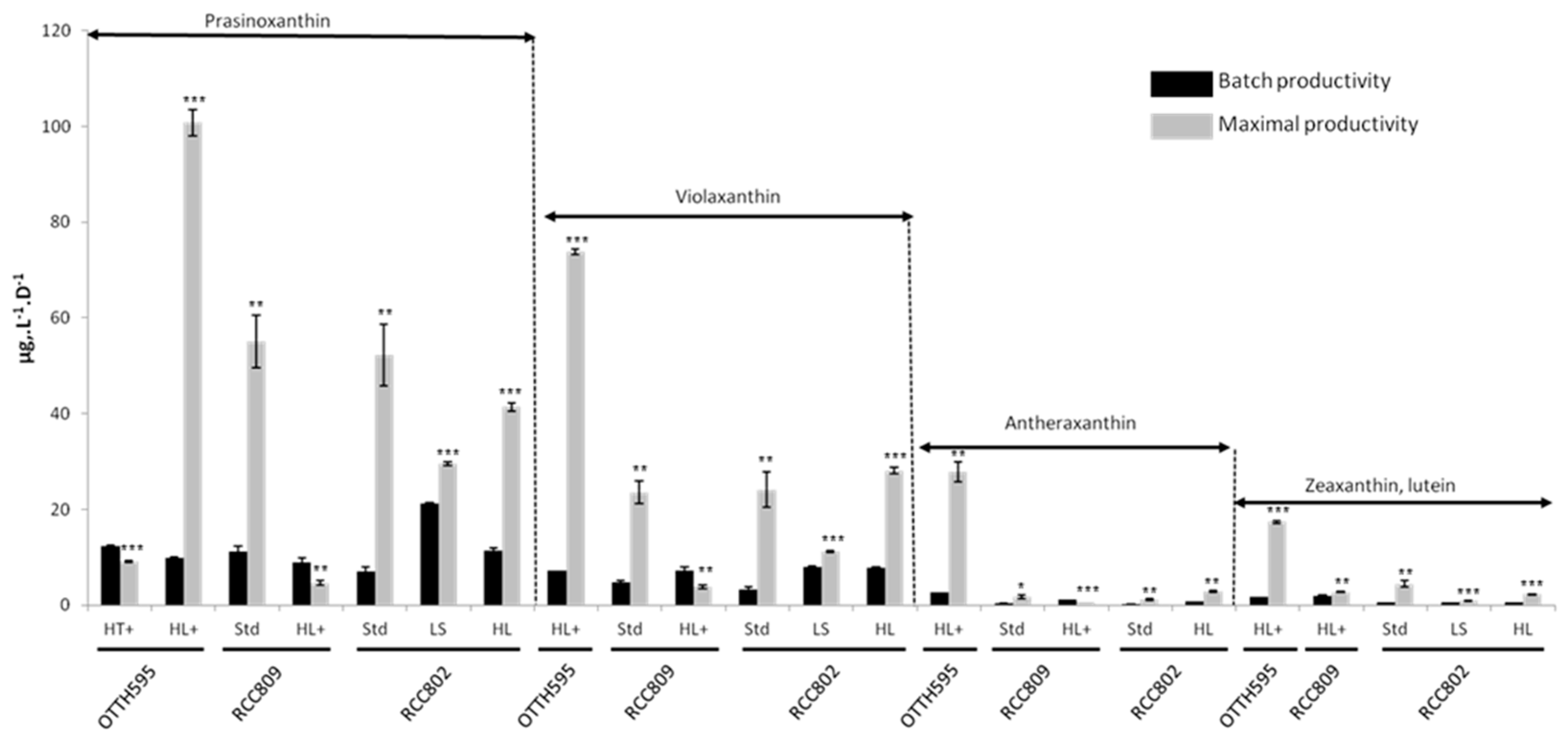
2.4. Effect of Stress Conditions on Carotenoid Productivities
3. Discussion
4. Materials and Methods
4.1. Algal Culture and Harvesting
4.2. Carotenoids Pigment Extraction and Analysis
4.3. Determination of Growth Rates and Theoretical Maximal Productivities
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of high added-value compounds—A brief review of recent work. Biotechnol. Prog. 2011, 27, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Henson, S.A.; Cole, H.S.; Hopkins, J.; Martin, A.P.; Yool, A. Detection of climate change-driven trends in phytoplankton phenology. Glob. Chang. Biol. 2018, 24, e101–e111. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, M.E.; Couso, I.; Crespo, J.L. Carotenoid deficiency triggers autophagy in the model green alga Chlamydomonas reinhardtii. Autophagy 2012, 8, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and industrial potential. Mar. Drugs 2014, 12, 3487–3515. [Google Scholar] [CrossRef] [PubMed]
- Régnier, P.; Bastias, J.; Rodriguez-Ruiz, V.; Caballerocasero, N.; Caballo, C.; Sicilia, D.; Fuentes, A.; Maire, M.; Crepin, M.; Letourneur, D.; et al. Astaxanthin from Haematococcus pluvialis prevents oxidative stress on human endothelial cells without toxicity. Mar. Drugs 2015, 13, 2857–2874. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.J.; Hazen, S.L.; Lockwood, S.F. Seven day oral supplementation with CardaxTM (disodium disuccinate astaxanthin) provides significant cardioprotection and reduces oxidative stress in rats. Mol. Cell. Biochem. 2006, 283, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Vonshak, A. Recent advances in microalgal biotechnology. Biotechnol. Adv. 1990, 8, 709–727. [Google Scholar] [CrossRef]
- Nidhi, B.; Sharavana, G.; Ramaprasad, T.R.; Vallikannan, B. Lutein derived fragments exhibit higher antioxidant and anti-inflammatory properties than lutein in lipopoysaccharide induced inflammation in rats. Food Funct. 2015, 6, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Moran, R.; Beatty, S.; Stack, J.; O’Halloran, A.M.; Feeney, J.; Akuffo, K.O.; Peto, T.; Kenny, R.A.; Nolan, J.M. The relationship between plasma concentrations of lutein and zeaxanthin with self-reported and actual prevalence of AMD in an Irish population-based sample. Curr. Eye Res. 2017, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cordero, B.F.; Couso, I.; Leon, R.; Rodriguez, H.; Vargas, M.A. Isolation and characterization of a lycopene ε-cyclase gene of Chlorella (Chromochloris) zofingiensis. Regulation of the carotenogenic pathway by nitrogen and light. Mar. Drugs 2012, 10, 2069–2088. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.P.; Chuang, L.T.; Chen, C.N. Production of long chain omega-3 fatty acids and carotenoids in tropical areas by a new heat-tolerant microalga Tetraselmis sp. DS3. Food Chem. 2016, 192, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, C.; Mitra, M.; Bhayani, K.; Bharadwaj, S.; Ghosh, T.; Dubey, S.; Mishra, S. Abiotic stresses as tools for metabolites in microalgae. Bioresour. Technol. 2017, 244, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, J.; Sandmann, G.; Chen, F. High-light and sodium chloride stress differentially regulate the biosynthesis of astaxanthin in chlorella zofingiensis (chlorophyceae). J. Phycol. 2009, 45, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Kao, A.L.; Tsai, Z.C.; Shen, Y.M.; Kao, P.H.; Ng, I.S.; Chang, J.S. Expression of synthetic phytoene synthase gene to enhance β-carotene production in Scenedesmus sp. CPC2. Biotechnol. J. 2017, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Blatt, A.; Bauch, M.E.; Pörschke, Y.; Lohr, M. A lycopene β-cyclase/lycopene ε-cyclase/light-harvesting complex-fusion protein from the green alga Ostreococcus lucimarinus can be modified to produce α-carotene and β-carotene at different ratios. Plant J. 2015, 82, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.C.; Schatt, P.; Botebol, H.; Vergé, V.; Lesuisse, E.; Blain, S.; Carré, I.A.; Bouget, F.Y. Efficient gene targeting and removal of foreign DNA by homologous recombination in the picoeukaryote Ostreococcus. Plant J. 2014, 78, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Courties, C.; Vaquer, A.; Troussellier, M.; Lautier, J.; Chretiennot-dinet, M.J.; Neveux, J.; Machado, C.; Claustre, H. Smallest eukaryotic organism. Nature 1994, 370, 255. [Google Scholar] [CrossRef]
- Derelle, E.; Ferraz, C.; Rombauts, S.; Rouzé, P.; Worden, A.Z.; Robbens, S.; Partensky, F.; Degroeve, S.; Echeynié, S.; Cooke, R.; et al. Genome analysis of the smallest free-living eukaryote Ostreococcus tauri unveils many unique features. Proc. Natl. Acad. Sci. USA 2006, 103, 11647–11652. [Google Scholar] [CrossRef] [PubMed]
- Corellou, F.; Schwartz, C.; Motta, J.-P.; Djouani-Tahri, E.B.; Sanchez, F.; Bouget, F.-Y. Clocks in the green lineage: Comparative functional analysis of the circadian architecture of the picoeukaryote Ostreococcus. Plant Cell 2009, 21, 3436–3449. [Google Scholar] [CrossRef] [PubMed]
- O-Neill, J.S.; Van Ooijen, G.; Dixon, L.E.; Troein, C.; Corellou, F.; Bouget, F.; Reddy, A.B.; Millar, A.J. Circadian rhythms persist without transcription in a eukaryote. Nature 2011, 469, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Moulager, M.; Monnier, A.; Jesson, B.; Bouvet, R.; Mosser, J.; Schwartz, C.; Garnier, L.; Corellou, F.; Bouget, F.Y. Light-dependent regulation of cell division in Ostreococcus: Evidence for a major transcriptional input. Plant Physiol. 2007, 144, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Botebol, H.; Lelandais, G.; Six, C.; Lesuisse, E.; Meng, A.; Bittner, L.; Lecrom, S.; Sutak, R.; Lozano, J.C.; Schatt, P.; et al. Acclimation of a low iron adapted Ostreococcus strain to iron limitation through cell biomass lowering. Sci. Rep. 2017, 7, 327. [Google Scholar] [CrossRef] [PubMed]
- Bork, P.; Bowler, C.; De Vargas, C.; Gorsky, G.; Karsenti, E.; Wincker, P. Tara Oceans studies plankton at planetary scale. Science 2015, 348, 873. [Google Scholar] [CrossRef] [PubMed]
- Palenik, B.; Grimwood, J.; Aerts, A.; Rouzé, P.; Salamov, A.; Putnam, N.; Dupont, C.; Jorgensen, R.; Derelle, E.; Rombauts, S.; et al. The tiny eukaryote Ostreococcus provides genomic insights into the paradox of plankton speciation. Proc. Natl. Acad. Sci. USA 2007, 104, 7705–7710. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, F.; Derelle, E.; Guillou, L.; Le Gall, F.; Vaulot, D.; Moreau, H. Ecotype diversity in the marine picoeukaryote Ostreococcus (Chlorophyta, Prasinophyceae). Environ. Microbiol. 2005, 7, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Six, C.; Sherrard, R.; Lionard, M.; Roy, S.; Campbell, D.A. Photosystem II and pigment dynamics among ecotypes of the green alga Ostreococcus. Plant Physiol. 2009, 151, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Domergue, F.; Abbadi, A.; Zähringer, U.; Moreau, H.; Heinz, E. In vivo characterization of the first acyl-CoA Delta6-desaturase from a member of the plant kingdom, the microalga Ostreococcus tauri. Biochem. J. 2005, 9, 83–490. [Google Scholar] [CrossRef]
- Degraeve-Guilbault, C.; Bréhélin, C.; Haslam, R.; Sayanova, O.; Marie-Luce, G.; Jouhet, J.; Corellou, F. Glycerolipid characterization and nutrient deprivation-associated changes in the green picoalga Ostreococcus tauri. Plant Physiol. 2017, 173, 2060–2080. [Google Scholar] [CrossRef] [PubMed]
- Cardol, P.; Bailleul, B.; Rappaport, F.; Derelle, E.; Béal, D.; Breyton, C.; Bailey, S.; Wollman, F.A.; Grossman, A.; Moreau, H.; et al. An original adaptation of photosynthesis in the marine green alga Ostreococcus. Proc. Natl. Acad. Sci. USA 2008, 105, 7881–7886. [Google Scholar] [CrossRef] [PubMed]
- Six, C.; Worden, A.Z.; Rodríguez, F.; Moreau, H.; Partensky, F. New insights into the nature and phylogeny of prasinophyte antenna proteins: Ostreococcus tauri, a case study. Mol. Biol. Evol. 2005, 22, 2217–2230. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tu, W.; Liu, C.; Rao, Y.; Gao, Z.; Yang, C. 9-cis-Neoxanthin in light harvesting complexes of photosystem II regulates the binding of violaxanthin and canthophyll cycle. Plant Physiol. 2017, 174, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.E.; Selivanova, E.A.; Chekanov, K.A.; Sidorov, R.A.; Nemtseva, N.V.; Lobakova, E.S. Induction of secondary carotenogenesis in new halophile microalgae from the genus dunaliella (chlorophyceae). Biochemistry 2015, 80, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Chekanov, K.; Lobakova, E.; Selyakh, I.; Semenova, L.; Sidorov, R.; Solovchenko, A. Accumulation of astaxanthin by a new Haematococcus pluvialis strain BM1 from the white sea coastal rocks (Russia). Mar. Drugs 2014, 12, 4504–4520. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Bassi, A. Investigation of chlorella vulgaris UTEX 265 cultivation under light and low temperature stressed conditions for lutein production in flasks and the coiled tree photo-bioreactor (CTPBR). Appl. Biochem. Biotechnol. 2017, 183, 652–671. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; Moreno, J.; Rodríguez, H.; Angeles Vargas, M.; Rivas, J.; Guerrero, M.G. Carotenoid content of chlorophycean microalgae: Factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Carotenoids from marine microalgae: A valuable natural source for the prevention of chronic diseases. Mar. Drugs 2015, 13, 5128–5155. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Morishita, Y.; Suzui, M.; Kojima, T.; Okumura, A.; Mori, H. Chemoprevention of mouse urinary-bladder carcinogenesis by the naturally-occurring carotenoid astaxanthin. Carcinogenesis 1994, 15, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Yasui, Y.; Hosokawa, M.; Mikami, N.; Miyashita, K.; Tanaka, T. Dietary astaxanthin inhibits colitis and colitis-associated colon carcinogenesis in mice via modulation of the inflammatory cytokines. Chem. Biol. Interact. 2011, 193, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, W.E.; Hu, L.; Zhao, L.; Huang, J. Carotenoids inhibit proliferation and regulate expression of peroxisome proliferators-activated receptor gamma (PPARγ) in K562 cancer cells. Arch. Biochem. Biophys. 2011, 512, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Dahmen-Ben Moussa, I.; Chtourou, H.; Karray, F.; Sayadi, S.; Dhouib, A. Nitrogen or phosphorus repletion strategies for enhancing lipid or carotenoid production from Tetraselmis marina. Bioresour. Technol. 2017, 238, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ras, J.; Claustre, H.; Uitz, J. Spatial variability of phytoplankton pigment distributions in the Subtropical South Pacific Ocean: Comparison between in situ and predicted data. Biogeosciences 2008, 5, 353–369. [Google Scholar] [CrossRef]

| Strain Name | OTTH595 | Eum16BBL_clonal | PROSOTOPE_44_clonal |
|---|---|---|---|
| #RCC | 745 | 809 | 802 |
| Species | Ostreococcus tauri | Ostreococcus sp. | Ostreococcus lucimarinus |
| Clade | C | B | A |
| Isolation date | 03/05/1995 | 01/10/1991 | 18/09/1999 |
| Latitude | +43°24′ | +21°2′ | +36°29′ |
| Longitude | +3°36′ | −31°8′ | +13°19′ |
| Depth (m) | Surface | 105 | 65 |
| Region | Thau lagoon, France | Tropical Atlantic Ocean | Sicily channel, Italy |
| Trophic level | Meso/eutrophic | Oligotrophic | Oligotrophic |
| Temperature | 4 to 30 °C | 18 to 20 °C | 14 to 16 °C |
| Condition | OTTH595 | RCC809 | RCC802 | |
|---|---|---|---|---|
| Temperature (°C) | 10 | 0 | 0 | 0.18 ** ± 0.059 |
| 12 | 0.31 ± 0.029 | 0.38 ** ± 0.008 | 0.37 ** ± 0.062 | |
| 15 | 0.41 ± 0.032 | 0.47 ** ± 0.051 | 0.50 ** ± 0.020 | |
| 20 | 0.82 ± 0.067 | 0.78 ± 0.017 | 0.84 ± 0.003 | |
| 25 | 0.74 * ± 0.030 | 0.59 ± 0.139 | 0.72 ± 0.139 | |
| 27.5 | 0.72 ± 0.085 | 0.55 ± 0.069 | 0.41 ± 0.206 | |
| 30 | 0.70 ± 0.041 | 0.46 *** ± 0.051 | 0 | |
| 32 | 0.59 ± 0.011 | 0.32 ** ± 0.017 | 0 | |
| Light (μmol quanta m−2·s−1) | 50 | 0.49 ± 0.05 | 0 | 0 |
| 150 | 1.66 ± 0.089 | 1.64 ± 0.086 | 1.52 ± 0.092 | |
| 500 | 1.65 ± 0.077 | 1.29 * ± 0.066 | 1.19 ± 0.082 | |
| 800 | 1.36 * ± 0.041 | 0.77 * ± 0.156 | 1.16 ± 0.350 | |
| 1200 | 1.37 * ± 0.026 | 0.72 ** ± 0.091 | 0 | |
| 1500 | 1.12 * ± 0.037 | 0.66 *** ± 0.043 | 0 | |
| 2000 | 0 | 0 | 0 | |
| Salinity (0–70‰) | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | |
| 7.5 | 0.64 * ± 0.123 | 0 | 0 | |
| 10 | 0.79 ± 0.039 | 0.83 ± 0.105 | 0 | |
| 15 | 0.83 * ± 0.057 | 0.91 ± 0.072 | 0.86 * ± 0.036 | |
| 36 | 0.96 ± 0.065 | 0.84 ± 0.005 | 1.12 ± 0.111 | |
| 57.5 | 0.68 * ± 0.018 | 0.67 * ± 0.048 | 1.07 ± 0.024 | |
| 60 | 0.65 * ± 0.018 | 0.51** ± 0.033 | 0.77 ± 0.222 | |
| 62.5 | 0.62 * ± 0.042 | 0.61* ± 0.074 | 0.52 ± 0.153 | |
| 65 | 0.44 ** ± 0.022 | 0.40 * ± 0.136 | 0.43 * ± 0.048 | |
| 70 | 0 | 0 | 0 | |
| Total Carotenoid (µg·L−1) | Treatments | ||||||||||
| Microalgae | Control | LL | HL | HL+ | LT− | LT | HT | HT+ | LS | HS 50 g/L | HS 60 g/L |
| OTTH595 | 179.443 ± 13.198 | 33.331 ** ± 2.492 | 168.001 ± 11.862 | 266.256 * ± 6.563 | 18.500 ** ± 2.899 | 60.000 ** ± 2.808 | 225.603 ± 8.454 | 278.981 ** ± 6.082 | 265.402 * ± 14.606 | / | 230.762 ± 37.176 |
| RCC 809 | 250.50 ± 27.286 | / | 147.481* ± 32.157 | 233.55 ± 25.957 | / | 17.247 ** ± 1.522 | 128.403 * ± 31.104 | 124.815 * ± 9.689 | 142.657 * ± 13.689 | / | 141.464 * ± 7.367 |
| RCC 802 | 167.613 ± 22.826 | / | 273.278 * ± 10.180 | / | 71.633 * ± 5.402 | 102.833 * ± 1.833 | 103.541 * ± 8.401 | / | 431.08 ** ± 7.605 | 277.513 ** ± 6.238 | / |
| Carotenoid Content (pg·cell−1) | Treatments | ||||||||||
| Microalgae | Control | LL | HL | HL+ | LT− | LT | HT | HT+ | LS | HS 50 g/L | HS 60 g/L |
| OTTH595 | 2.01 ± 0.029 | 0.515 *** ± 0.027 | 2.769 ± 0.36 | 3.520 *** ± 0.069 | 0.812 ** ± 0.127 | 0.800 ** ± 0.160 | 11.09 ** ± 0.776 | 7.559 ** ± 0.458 | 5.443 *** ± 0.206 | / | 3.063 * ± 0.229 |
| RCC 809 | 6.236 ± 0.670 | / | 2.599 ** ± 0.327 | 3.470 * ± 0.563 | / | 0.320 ** ± 0.058 | 5.551 ± 0.313 | 7.703 ± 1.305 | 3.242 * ± 0.233 | / | 3.946 * ± 0.523 |
| RCC 802 | 4.621 ± 1.223 | / | 3.567 ± 0.380 | / | 3.378 ± 0.148 | 1.376 ± 0.168 | 5.161 ± 0.420 | / | 9.268 * ± 1.203 | 3.514 ± 0.125 | / |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guyon, J.-B.; Vergé, V.; Schatt, P.; Lozano, J.-C.; Liennard, M.; Bouget, F.-Y. Comparative Analysis of Culture Conditions for the Optimization of Carotenoid Production in Several Strains of the Picoeukaryote Ostreococcus. Mar. Drugs 2018, 16, 76. https://doi.org/10.3390/md16030076
Guyon J-B, Vergé V, Schatt P, Lozano J-C, Liennard M, Bouget F-Y. Comparative Analysis of Culture Conditions for the Optimization of Carotenoid Production in Several Strains of the Picoeukaryote Ostreococcus. Marine Drugs. 2018; 16(3):76. https://doi.org/10.3390/md16030076
Chicago/Turabian StyleGuyon, Jean-Baptiste, Valérie Vergé, Philippe Schatt, Jean-Claude Lozano, Marion Liennard, and François-Yves Bouget. 2018. "Comparative Analysis of Culture Conditions for the Optimization of Carotenoid Production in Several Strains of the Picoeukaryote Ostreococcus" Marine Drugs 16, no. 3: 76. https://doi.org/10.3390/md16030076




