Molecular Characterization of a Novel N-Acetylneuraminate Lyase from a Deep-Sea Symbiotic Mycoplasma
Abstract
:1. Introduction
2. Results
2.1. Cloning, Expression, and Purification of MyNal
2.2. Biochemical Characterization of Recombinant MyNal
2.3. Kinetic Parameters of MyNal
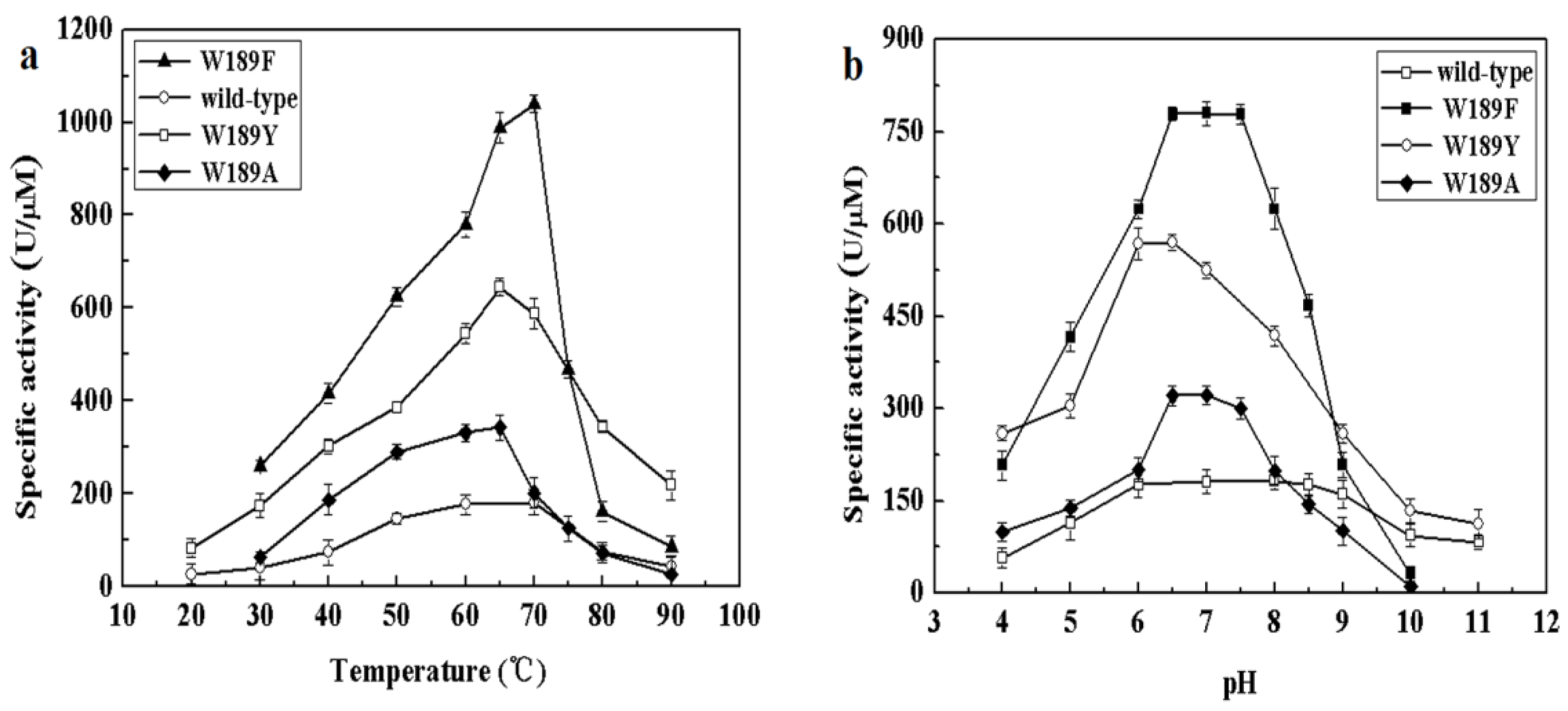
2.4. Mutagenetic Analyses of Recombinant MyNal
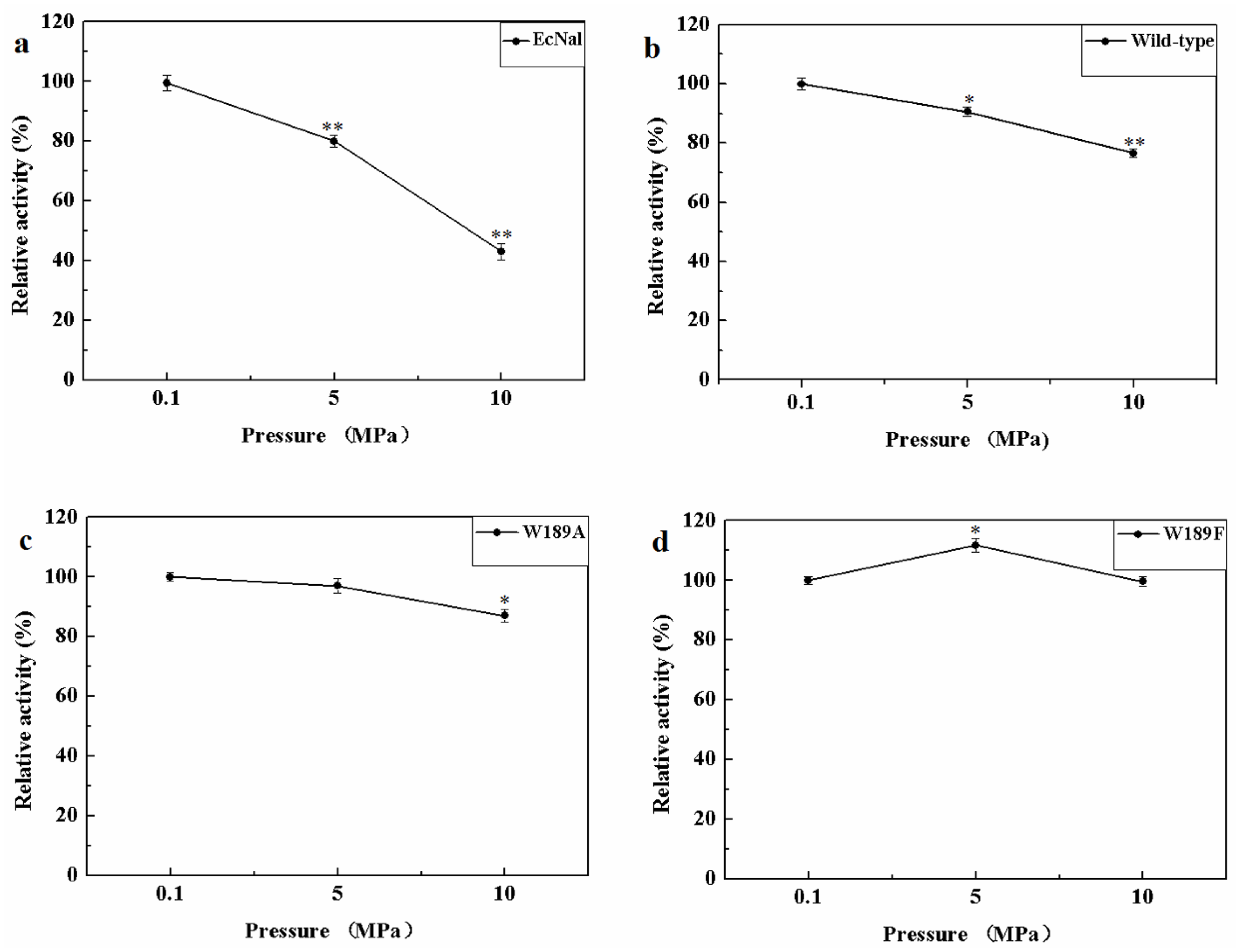
2.5. Effect of Pressure on Activity of MyNal
3. Discussion
4. Materials and Methods
4.1. Strains, Plasmids and Chemicals
4.2. Cloning and Mutagenesis
4.3. Expression and Purification of MyNal
4.4. Enzyme Assay of MyNal
4.5. Enzyme Characterization of MyNal
4.6. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Inoue, Y.; Inoue, S. Diversity in sialic and polysialic acid residues and related enzymes. Pure Appl. Chem. 1999, 71, 789–800. [Google Scholar] [CrossRef]
- Buschiazzo, A.; Alzari, P.M. Structural insights into sialic acid enzymology. Curr. Opin. Chem. Biol. 2008, 12, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Diversity in the sialic acids. Glycobiology 1992, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Varki, A. Advances in the Biology and Chemistry of Sialic Acids. ACS Chem. Biol. 2010, 5, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147. [Google Scholar] [CrossRef] [PubMed]
- Kelm, S.; Schauer, R. Sialic acids in molecular and cellular interactions. Int. Rev. Cytol. 1997, 175, 137. [Google Scholar] [PubMed]
- Maru, I.; Ohnishi, J.; Ohta, Y.; Tsukada, Y. Why Is Sialic Acid Attracting Interest Now? Complete Enzymatic Synthesis of Sialic Acid with N-Acylglucosamine 2-Epimerase. J. Biosci. Bioeng. 2002, 93, 258–265. [Google Scholar] [CrossRef]
- Ogura, H. Development of miracle medicines from sialic acids. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 328. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Zhang, Y.; Ma, C.; Xu, P. Biotechnological production and applications of N-acetyl-d-neuraminic acid: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 87, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Saleem, T. Soluble CD14, Sialic Acid and l-Fucose in Breast Milk and their Role in Increasing the Immunity of Breast-Fed Infants. Am. J. Biochem. Biotechnol. 2011, 7, S35. [Google Scholar]
- Vonitzstein, M.; Wu, W.Y.; Kok, G.B.; Pegg, M.S.; Dyason, J.C.; Jin, B.; Phan, T.V.; Smythe, M.L.; White, H.F.; Oliver, S.W.; et al. Rational design of potent sialidase-based inhibitors of influenza-virus replication. Nature 1993, 363, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Onodera, K.; Hirano, S.; Hayashi, H. Sialic Acid and Related Substances. J. Agric. Chem. Soc. Jpn. 2008, 30, 1170–1172. [Google Scholar]
- Yu, H.; Yu, H.; Karpel, R.; Chen, X. Chemoenzymatic synthesis of CMP-sialic acid derivatives by a one-pot two-enzyme system: Comparison of substrate flexibility of three microbial CMP-sialic acid synthetases. Bioorg. Med. Chem. 2004, 12, 6427–6435. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gao, C.; Zhang, X.; Che, B.; Ma, C.; Qiu, J.; Tao, F.; Xu, P. Production of N-acetyl-d-neuraminic acid by use of an efficient spore surface display system. Appl. Environ. Microbiol. 2011, 77, 3197–3201. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, H.; Wu, B.; Zhang, Y.; Li, H.; Xiao, M.; Wang, P.G. Reassembled Biosynthetic Pathway for a Large-scale Synthesis of CMP-Neu5Ac. Mar. Drugs 2003, 1, 34–45. [Google Scholar] [CrossRef]
- Huang, S.; Yu, H.; Chen, X. Disaccharides as sialic acid aldolase substrates: Synthesis of disaccharides containing a sialic acid at the reducing end. Angew. Chem. 2007, 119, 2299–2303. [Google Scholar] [CrossRef]
- Comb, D.G.; Roseman, S. The sialic acids. I. The structure and enzymatic synthesis of N-acetylneuraminic acid. J. Biol. Chem. 1960, 235, 2529–2537. [Google Scholar] [PubMed]
- Schauer, R.; Sommer, U.; Krüger, D.; Van, U.H.; Traving, C. The terminal enzymes of sialic acid metabolism: Acylneuraminate pyruvate-lyases. Biosci. Rep. 1999, 19, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R.; Wember, M. Isolation and characterization of sialate lyase from pig kidney. Biol. Chem. 1996, 377, 293. [Google Scholar] [CrossRef]
- Sirbasku, D.A.; Binkley, S.B. Purification and properties of N-acetylneuraminate lyase from beef kidney cortex. Biochim. Biophys. Acta 1970, 206, 479. [Google Scholar] [CrossRef]
- Aisaka, K.; Uwajima, T. Cloning and Constitutive Expression of the N-Acetylneuraminate Lyase Gene of Escherichia coli. Appl. Environ. Microbiol. 1986, 51, 562–565. [Google Scholar] [PubMed]
- Traving, C.; Roggentin, P.; Schauer, R. Cloning, sequencing and expression of the acylneuraminate lyase gene from Clostridium perfringens A99. Glycoconj. J. 1997, 14, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, H.; Cao, H.; Lau, K.; Muthana, S.; Tiwari, V.K.; Son, B.; Chen, X. Pasteurella multocida sialic acid aldolase: A promising biocatalyst. Appl. Microbiol. Biotechnol. 2008, 79, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Lilley, G.G.; Barbosa, J.A.; Pearce, L.A. Expression in Escherichia coli of the putative N-acetylneuraminate lyase gene (nanA) from Haemophilus influenzae: Overproduction, purification, and crystallization. Protein Expr. Purif. 1998, 12, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Meysick, K.C.; Dimock, K.; Garber, G.E. Molecular characterization and expression of a N-acetylneuraminate lyase gene from Trichomonas vaginalis. Mol. Biochem. Parasitol. 1996, 76, 289–292. [Google Scholar] [CrossRef]
- Jeong, H.G.; Oh, M.H.; Kim, B.S.; Lee, M.Y.; Han, H.J.; Choi, S.H. The capability of catabolic utilization of N-acetylneuraminic acid, a sialic acid, is essential for Vibrio vulnificus pathogenesis. Infect. Immun. 2009, 77, 3209–3217. [Google Scholar] [CrossRef] [PubMed]
- Vimr, E.R.; Troy, F.A. Regulation of sialic acid metabolism in Escherichia coli: Role of N-acylneuraminate pyruvate-lyase. J. Bacteriol. 1985, 164, 854–860. [Google Scholar] [PubMed]
- Rodríguezaparicio, L.B.; Ferrero, M.A.; Reglero, A. N-acetyl-d-neuraminic acid synthesis in Escherichia coli K1 occurs through condensation of N-acetyl-d-mannosamine and pyruvate. Biochem. J. 1995, 308, 501–505. [Google Scholar] [CrossRef]
- Schauer, R. Chemistry, metabolism, and biological functions of sialic acids. Adv. Carbohydr. Chem. Biochem. 1982, 40, 131–234. [Google Scholar] [PubMed]
- Uchida, Y.; Tsukada, Y.; Sugimori, T. Purification and properties of N-acetylneuraminate lyase from Escherichia coli. J. Biochem. 1984, 96, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Nees, S.; Schauer, R.; Mayer, F. Purification and characterization of N-acetylneuraminate lyase from Clostridium perfringens. Hoppe-Seyler’s Z. Physiol. Chem. 1976, 357, 839. [Google Scholar] [CrossRef] [PubMed]
- Sánchezcarrón, G.; Garcíagarcía, M.I.; Lópezrodríguez, A.B.; Jiménezgarcía, S.; Solacarvajal, A.; Garcíacarmona, F.; Sánchezferrer, A. Molecular Characterization of a Novel N-Acetylneuraminate Lyase from Lactobacillus plantarum WCFS1. Appl. Environ. Microbiol. 2011, 77, 2471–2478. [Google Scholar] [CrossRef] [PubMed]
- García García, M.I.; Sola, C.A.; García, C.F.; Sánchez, F.Á. Characterization of a novel N-acetylneuraminate lyase from Staphylococcus carnosus TM300 and its application to N-acetylneuraminic acid production. J. Agric. Food Chem. 2012, 60, 7450–7456. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Sun, W.; Feng, J.; Song, T.; Zhang, D.; Ouyang, P.; Gu, Z.; Xie, J. Characterization of a novel N-acetylneuraminic acid lyase favoring N-acetylneuraminic acid synthesis. Sci. Rep. 2015, 5, 9341. [Google Scholar] [CrossRef] [PubMed]
- Izard, T.; Lawrence, M.C.; Malby, R.L.; Lilley, G.G.; Colman, P.M. The three-dimensional structure of N-acetylneuraminate lyase from Escherichia coli. Structure 1994, 2, 361. [Google Scholar] [CrossRef]
- Lawrence, M.C.; Barbosa, J.A.R.G.; Smith, B.J.; Hall, N.E.; Pilling, P.A.; Ooi, H.C.; Marcuccio, S.M. Structure and mechanism of a sub-family of enzymes related to N-acetylneuraminate lyase. J. Mol. Biol. 1997, 266, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Aisaka, K.; Igarashi, A.; Yamaguchi, K.; Uwajima, T. Purification, crystallization and characterization of N-acetylneuraminate lyase from Escherichia coli. Biochem. J. 1991, 276, 541. [Google Scholar] [CrossRef] [PubMed]
- Michels, P.C.; Clark, D.S. Pressure-Enhanced Activity and Stability of a Hyperthermophilic Protease from a Deep-Sea Methanogen. Appl. Environ. Microbiol. 1997, 63, 3985. [Google Scholar] [PubMed]
- Zhang, J.; Lin, S.; Zeng, R. Cloning, expression, and characterization of a cold-adapted lipase gene from an antarctic deep-sea psychrotrophic bacterium, Psychrobacter sp. 7195. J. Microbiol. Biotechnol. 2007, 17, 604. [Google Scholar] [PubMed]
- Zhang, Y.; Hao, J.; Zhang, Y.Q.; Chen, X.L.; Xie, B.B.; Shi, M.; Zhou, B.C.; Zhang, Y.Z.; Li, P.Y. Identification and Characterization of a Novel Salt-Tolerant Esterase from the Deep-Sea Sediment of the South China Sea. Front. Microbiol. 2017, 8, 441. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, J.M.; Wang, S.L.; Gao, Z.M.; Zhang, A.Q.; Danchin, A.; He, L.S. Genomic characterization of symbiotic mycoplasmas from the stomach of deep-sea isopod bathynomus sp. Environ. Microbiol. 2016, 18, 2646–2659. [Google Scholar] [CrossRef] [PubMed]
- Krüger, D.; Schauer, R.; Traving, C. Characterization and mutagenesis of the recombinant N-acetylneuraminate lyase from Clostridium perfringens: Insights into the reaction mechanism. Eur. J. Biochem. 2001, 268, 3831–3839. [Google Scholar] [CrossRef] [PubMed]
- Butman, C.A.; Carlton, J.T. Marine biological diversity: Some important issues, opportunities and critical research needs. Rev. Geophys. 1995, 33, 1201–1209. [Google Scholar] [CrossRef]
- Barbosa, J.A.; Smith, B.J.; Degori, R.; Ooi, H.C.; Marcuccio, S.M.; Campi, E.M.; Jackson, W.R.; Brossmer, R.; Sommer, M.; Lawrence, M.C. Active site modulation in the N-acetylneuraminate lyase sub-family as revealed by the structure of the inhibitor-complexed Haemophilus influenzae enzyme. J. Mol. Biol. 2000, 303, 405. [Google Scholar] [CrossRef] [PubMed]
- Brindley, A.A.; Pickersgill, R.W.; Partridge, J.C.; Dunstan, D.J.; Hunt, D.M.; Warren, M.J. Enzyme sequence and its relationship to hyperbaric stability of artificial and natural fish lactate dehydrogenases. PLoS ONE 2008, 3, e2042. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, J.P., Jr.; Siebenaller, J.F. Pressure-adaptive differences in proteolytic inactivation of M4-lactate dehydrogenase homologues from marine fishes. J. Exp. Zool. 1987, 241, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ohmae, E.; Murakami, C.; Tate, S.; Gekko, K.; Hata, K.; Akasaka, K.; Kato, C. Pressure dependence of activity and stability of dihydrofolate reductases of the deep-sea bacterium Moritella profunda and Escherichia coli. Biochim. Biophys. Acta 2012, 1824, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Ohmae, E.; Gekko, K.; Kato, C. Environmental Adaptation of Dihydrofolate Reductase from Deep-Sea Bacteria. Subcell. Biochem. 2015, 72, 423–442. [Google Scholar] [PubMed]
- Somero, G.N. Protein adaptations to temperature and pressure: Complementary roles of adaptive changes in amino acid sequence and internal milieu. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 136, 577. [Google Scholar] [CrossRef]
- Comb, D.G.; Roseman, S. N-acetylneuraminic acid aldolase: N-Acetylneuraminic acid = N-Acetyl-d-mannosamine + pyruvate. Methods Enzymol. 1962, 5, 391–394. [Google Scholar]
Sample Availability: The plasmid pET32a-MyNal and its mutants are available from the authors, Proteomic and Molecular Biological Research Laboratory, Institute of Deep-sea Science and Engineering, Chinese Academy of Sciences. |
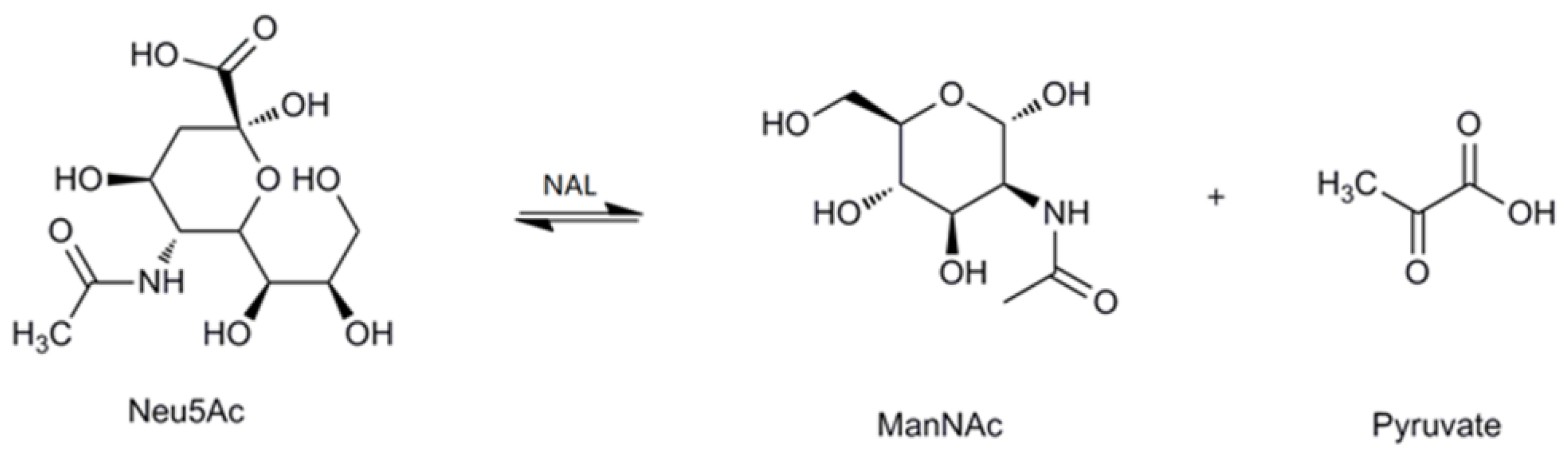
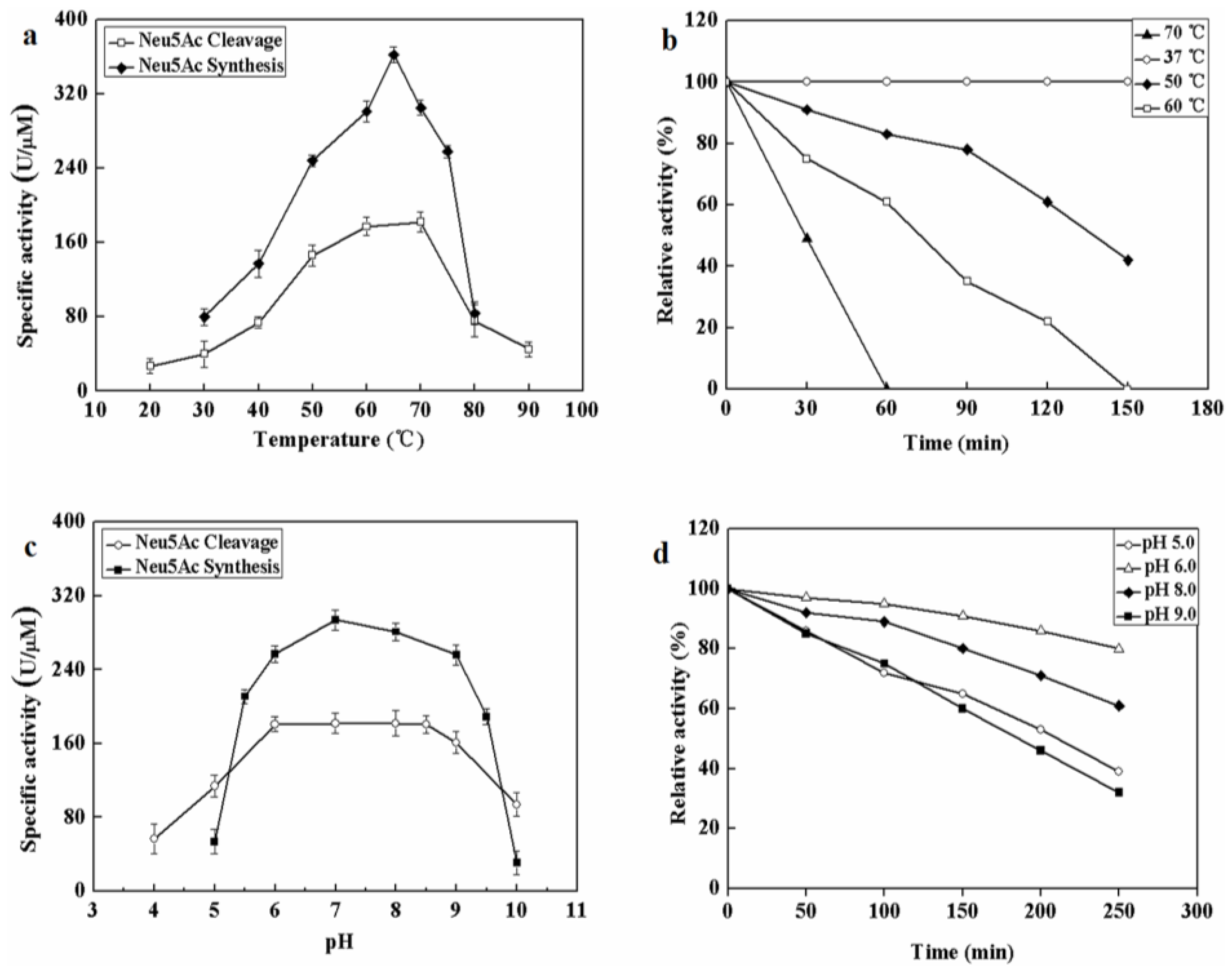
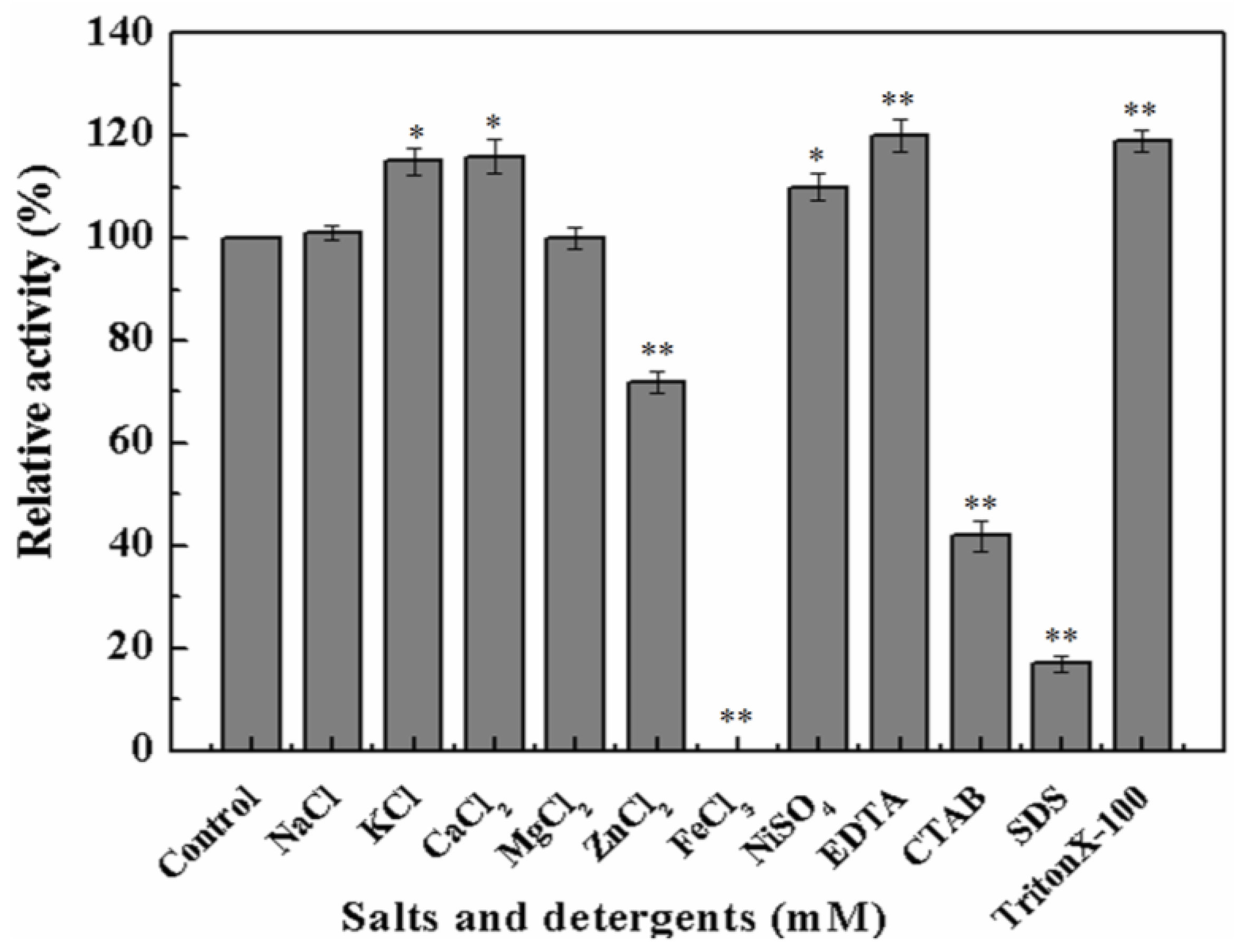
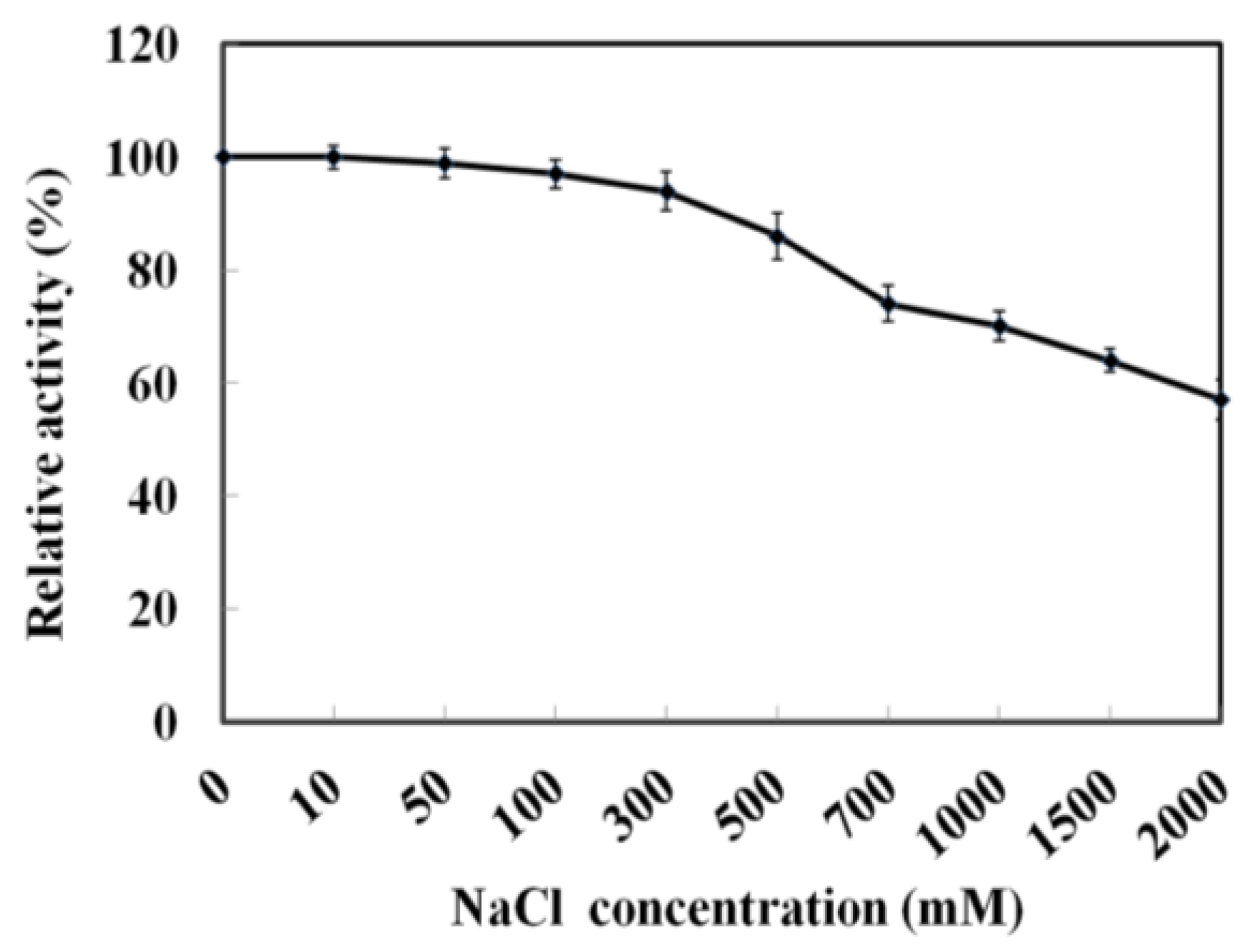
| Species | Neu5Ac Cleavage | Neu5Ac Synthesis | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neu5Ac | ManNAc | Pyruvate | |||||||||
| Km (mM) | kcat/Km (s−1mM−1) | Optimum pH | Optimum Temp (°C) | Km (mM) | kcat/Km (s−1mM−1) | Km (mM) | kcat/Km (s−1mM−1) | Optimum pH | Optimum Temp (°C) | ||
| Symbiont Mycoplasma sp | 1.8 | 3.66 | 6.0–8.5 | 70 | 6.9 | 1.86 | 5.9 | 1.12 | 7 | 65 | This study |
| Corynebacterium glutamicum ATCC 13032 | 33.50 | 0.28 | 8.4–8.8 | 40 | 53.30 | 0.11 | 14.70 | 0.41 | 8.2–8.4 | 40 | [34] |
| Escherichia coli | 3.50 | 23.75 | 287.1 | 0.07 | 206.1 | 0.11 | [34] | ||||
| Escherichia coli | 3.60 | 23.60 | 7.7 | 75 | 7.7 | [30] | |||||
| Pasteurella multocida P1059 | 4.90 | 3.27 | 7.5–8.0 | 220 | 0.05 | 23 | 0.08 | 7.5–8.0 | [23] | ||
| Clostridium perfringens | 3.20 | 5.01 | 7.6 | 65–70 | [42] | ||||||
| Staphylococcus carnosus TM300 | 2 | 2 | 7 | 60–70 | 149 | 0.03 | 14 | 0.21 | 7 | 50 | [33] |
| Lactobacillus plantarum WCFS1 | 1.80 | 5.60 | 7.5 | 70 | 160 | 0.03 | 19.90 | 0.11 | 7.5 | 60 | [32] |
| Enzyme | Km (mM) | kcat (s−1) | kcat/Km (s−1mM−1) |
|---|---|---|---|
| Wild-type | 1.8 ± 0.2 | 6.59 ± 0.3 | 3.66 |
| W189A | 1.03 ± 0.3 | 11.37 ± 0.4 | 11.04 |
| W189Y | 0.5 ± 0.1 | 12.11 ± 0.3 | 24.21 |
| W189F | 0.36 ± 0.2 | 12.51 ± 0.1 | 34.76 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-l.; Li, Y.-l.; Han, Z.; Chen, X.; Chen, Q.-j.; Wang, Y.; He, L.-s. Molecular Characterization of a Novel N-Acetylneuraminate Lyase from a Deep-Sea Symbiotic Mycoplasma. Mar. Drugs 2018, 16, 80. https://doi.org/10.3390/md16030080
Wang S-l, Li Y-l, Han Z, Chen X, Chen Q-j, Wang Y, He L-s. Molecular Characterization of a Novel N-Acetylneuraminate Lyase from a Deep-Sea Symbiotic Mycoplasma. Marine Drugs. 2018; 16(3):80. https://doi.org/10.3390/md16030080
Chicago/Turabian StyleWang, Shao-lu, Yun-liang Li, Zhuang Han, Xi Chen, Qi-jia Chen, Yong Wang, and Li-sheng He. 2018. "Molecular Characterization of a Novel N-Acetylneuraminate Lyase from a Deep-Sea Symbiotic Mycoplasma" Marine Drugs 16, no. 3: 80. https://doi.org/10.3390/md16030080





