Bacteriophages with Potential for Inactivation of Fish Pathogenic Bacteria: Survival, Host Specificity and Effect on Bacterial Community Structure
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Water Properties
2.1.2. Phages Isolation and Classification
2.1.3. Phage Host Range Analysis
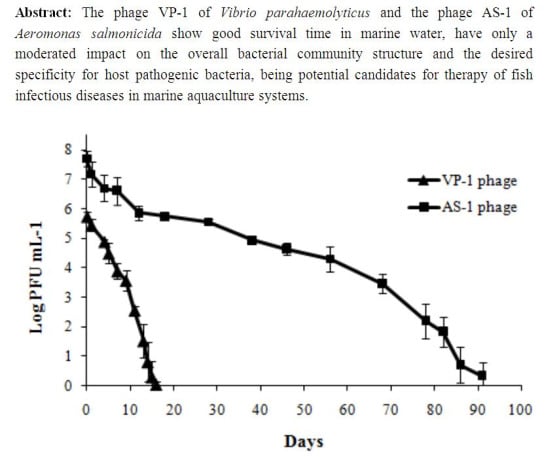
2.1.4. Determination of Phage Survival
2.1.5. Effects of the Phages on the Bacterial Community Structure
2.2. Discussion
3. Experimental Section
3.1. Study Area and Sampling
3.2. Water Properties
3.3. Microorganisms and Growth Conditions
3.4. Bacteriophages Isolation and Purification
3.5. Sample Preparation for Transmission Electron Microscopy (TEM)
3.6. Concentration of Bacteriophage Particles with PEG for Nucleic Acid Extraction
3.7. Nucleic Acid Extraction and Amplification of Phage DNA Using Phi29 DNA Polymerase
3.8. Nucleic Acid Characterization
3.9. Phage Host Range Analysis
3.10. Determination of Phage Survival
3.11. Impact of Phage Addition on Bacterial Community Structure
4. Conclusions
Acknowledgements
- Samples Availability: Available from the authors.
References
- FAO, The State of World Fisheries and Aquaculture—2008; FAO (Fisheries and Aquaculture Department): Rome, Italy, 2009.
- FAO, The State of World Fisheries and Aquaculture; FAO (Fisheries and Aquaculture Department): Rome, Italy, 1998.
- Flegel, T.W. Detection of major penaeid shrimp viruses in Asia, a historical perspective with emphasis on Thailand. Aquaculture 2006, 258, 1–33. [Google Scholar]
- Saksida, S.; Constantine, J.; Karreman, G.A.; Neville, C.; Sweeting, R.; Beamish, R. Evalution of sea lice, Lepeophtheirus salmonis, abundance levels on farmed salmon in British Columbia, Canada. Proceedings from the International Symposium on Veterinary Epidemiology and Economics XI, Cairns, Australia, August 2006.
- Subasinghe, R.P.; Bondad-Reantaso, M.G.; McGladdery, S.E. Aquaculture in the Millennium. Proceedings of the Conference on Aquaculture in the Third Millennium, Bangkok, Thailand, 2001; Subasinghe, R.P., Bueno, P., Phillips, M.J., Hough, C., McGladdery, S.E., Arthur, J.R., Eds.; [Google Scholar]
- Toranzo, A.E.; Barreiro, S.; Casal, J.F.; Figueras, A.; Magariños, B.; Barja, J.L. Pasteurellosis in cultured gilthead seabream, Sparus aurata: First report in Spain. Aquaculture 1991, 99, 1–15. [Google Scholar]
- Benediktsdóttir, E.; Helgason, S.; Sigurjónsdóttir, H. Vibrio spp. isolated from salmonids with shallow skin lesions and reared at low temperature. J. Fish Dis 1998, 21, 19–28. [Google Scholar]
- Blanch, A.R.; Alsina, M.; Simón, M.; Jofre, J. Determination of bacteria associated with reared turbot (Scophthalmus maximus) larvae. J. Appl. Microbiol 1997, 82, 729–734. [Google Scholar]
- Eguchi, M.; Fujiwara, E.; Miyamoto, N. Survival of Vibrio anguillarum in freshwater environments: Adaptation or debilitation? J. Infect. Chemother 2000, 6, 126–129. [Google Scholar]
- Hanna, P.J.; Altmann, K.; Chen, D.; Smith, A.; Cosic, S.; Moon, P. Development of monoclonal antibodies for the rapid identification of epizootic Vibrio species. J. Fish Dis 1991, 15, 63–69. [Google Scholar]
- Sung, H.H.; Li, H.C.; Tsai, F.M.; Ting, Y.Y.; Chao, W.L. Canges in the composition of Vibrio communities in pond water during tiger shrimp (Penaeus monodon) cultivation and in the hepatopancreas of healthy and diseased shrimp. J. Exp. Mar. Biol. Ecol 1999, 239, 261–271. [Google Scholar]
- Noya, M.; Magarinos, B.; Lamas, J. Interactions between peritoneal exudate cells (PECs) of gilthead seabream (Sparus aurata) and Pasteurella piscicida. A morphological study. Aquaculture 1995, 131, 11–21. [Google Scholar]
- Bernoth, E.M. Furunculosis: Multidisciplinary Fish Disease Research; Academic Press: Waltham, MA, USA, 1997. [Google Scholar]
- Almeida, A.; Cunha, A.; Gomes, N.C.M.; Alves, E.; Costa, L.; Faustino, M.A.F. Phage therapy and photodynamic therapy: Low environmental impact approaches to inactivate microorganisms in fish farming plants. Mar. Drugs 2009, 7, 268–313. [Google Scholar]
- Arijo, S.; Rico, R.; Chabrillon, M.; Diaz-Rosales, P.; Martínez-Manzanares, E.; Balebona, M.C.; Magariños, B.; Toranzo, A.E.; Moriñigo, M.A. Effectiveness of a divalent vaccine for sole, Solea senegalensis (Kaup), against Vibrio harveyi and Photobacterium damselae subsppiscicida. J. Fish Dis 2005, 28, 33–38. [Google Scholar]
- Lin, X.; Huang, J.C.; Mitchell, T.G.; Heitman, J. Virulence attributes and hyphal growth of C. neoformans are quantitative traits and the MATα allele enhances filamentation. PLoS Genet 2006, 2, e187:1801–e187:1814. [Google Scholar]
- Reed, P.A.; Francis-Floyd, R. Vibrio Infections of Fish; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 1996. [Google Scholar]
- Romalde, J. Photobacterium damselae subsp. piscicida: An integrated view of a bacterial fish pathogen. Int. Microbiol 2002, 1, 3–9. [Google Scholar]
- Nakai, T.; Park, S.C. Bacteriophage therapy of infectious diseases in aquaculture. Res. Microbiol 2002, 153, 13–18. [Google Scholar]
- Muroga, K. Viral and bacterial diseases of marine fish and shellfish in Japanese hatcheries. Aquaculture 2001, 202, 23–44. [Google Scholar]
- Vadstein, O. The use of immunostimulation in marine larviculture: Possibilities and challenges. Aquaculture 1997, 155, 401–417. [Google Scholar]
- Alderman, D.J. Geographical spread of bacterial and fungal diseases of crustaceans. Rev. Sci. Tech. IOE 1996, 15, 603–632. [Google Scholar]
- Shao, Z.J. Aquaculture pharmaceuticals and biologicals: Current perspectives and future possibilities. Adv. Drug Deliv. Rev 2001, 50, 229–243. [Google Scholar]
- Wahli, T.; Knuesel, R.; Bernet, D.; Segner, H.; Pugovkin, D.; Burkhardt-Holm, P.; Escher, M.; Schmidt-Posthaus, H. Proliferative kidney disease in Switzerland: Current state of knowledge. J. Fish Dis 2002, 25, 491–500. [Google Scholar]
- Abedon, S.T. Bacteriophage Ecology: Population Growth, Evolution, and Impact of Bacterial Viruses; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Biswas, B.; Adhya, S.; Washart, P.; Paul, B.; Trostel, A.N.; Powell, B.; Carlton, R.; Merril, C.R. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect. Immun 2002, 70, 204–210. [Google Scholar]
- Matsuzaki, S.; Yasuda, M.; Nishikawa, H.; Kuroda, M.; Ujihara, T.; Shuin, T.; Shen, Y.; Jin, Z.; Fujimoto, S.; Nasimuzzaman, M.A.D.; et al. Experimental protection of mice against lethal Staphylococcus aureus infection by novel bacteriophage phi MR11. J. Infect. Dis 2003, 187, 613–624. [Google Scholar]
- Wills, Q.F.; Kerrigan, C.; Soothill, J.S. Experimental bacteriophage protection against Staphylococcus aureus abscesses in a rabbit model. Antimicrob. Agents Chemother 2005, 49, 1220–1221. [Google Scholar]
- Park, S.; Nakai, T. Bacteriophage control of Pseudomonas plecoglossicida infection in ayu Plecoglossus altivelis. Dis. Aquat. Organ 2003, 53, 33–39. [Google Scholar]
- Skurnik, M.; Pajunen, M.; Kiljunen, S. Biotechnological challenges of phage therapy. Biotechnol. Lett 2007, 29, 995–1003. [Google Scholar]
- Imbeault, S.; Parent, S.; Lagace, M.; Uhland, C.F.; Blais, J.F. Using Bacteriophages to prevent furunculosis caused by Aeromonas salmonicida in farmed brook trout. J. Aquat. Anim. Health 2006, 18, 203–214. [Google Scholar]
- Karunasagar, I.; Shivu, M.M.; Girisha, S.K.; Krohne, G.; Karunasagar, I. Biocontrol of pathogens in shrimp hatcheries using bacteriophages. Aquaculture 2007, 268, 288–292. [Google Scholar]
- Nakai, T.; Sugimoto, R.; Park, K.H.; Matsuoka, S.; Mori, K.; Nishioka, T.; Maruyama, K. Protective effects of bacteriophage an experimental Lactococcus gavieae infection in yellowtail. Dis. Aquat. Organ 1999, 37, 33–41. [Google Scholar]
- Vinod, M.G.; Shivu, M.M.; Umesha, K.R.; Rajeeva, B.C.; Krohne, G.; Karunasagar, I.; Karunasagar, I. Isolation of Vibrio harveyi bacteriophage with a potential for biocontrol of luminous vibriosis in hatchery environments. Aquaculture 2006, 255, 117–124. [Google Scholar]
- Suttle, C.A.; Chan, A.M. Dynamics and distribution of cyanophages and their effect on marine Synechococcus spp. Appl. Environ. Microbiol 1994, 60, 3167–3174. [Google Scholar]
- Suttle, C.A.; Chen, F. Mechanisms and rates of decay of marine viruses in seawater. Appl. Environ. Microbiol 1992, 58, 3721–3729. [Google Scholar]
- Cottrell, M.T.; Suttle, C.A. Dynamics of a lytic virus infecting the photosynthetic marine picoflagellate Micromonas pusilla. Limnol. Oceanogr 1995, 40, 730–739. [Google Scholar]
- De Paepe, M.; Taddei, F. Viruses’ life history: Towards a mechanistic basis of a trade-off between survival and reproduction among phages. PLoS Biol 2006, 4, e193:1248–e193:1256. [Google Scholar]
- Cho, B.C.; Azam, F. Biogeochemical significance of bacterial biomass in the ocean’s euphotic 17 zone. Mar. Ecol. Prog. Ser 1990, 63, 253–259. [Google Scholar]
- Pomeroy, L.R. Status and Future Needs in Protozoan Ecology. In Protozoan and Their Role in Marine Processes, NATO ASI Series G: Ecological 20 Sciences; Reid, P.C., Turley, C.M., Burkhill, P.H., Eds.; Springer-Verlag: Berlin, Heidelberg, Germany, 1991; Volume 25, pp. 475–492. [Google Scholar]
- Holmfeldt, K.; Middelboe, M.; Nybroe, O.; Riemann, L. Large variabilities in host strain susceptibility and phage host range govern interactions between lytic marine phages and their Flavobacterium hosts. Appl. Environ. Microbiol 2007, 73, 6730–6739. [Google Scholar]
- Ackermann, H.W. Bacteriophage observations and evolution. Res. Microbiol 2003, 154, 245–251. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. Primer v5: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2001; p. 9. [Google Scholar]
- Hagens, S.; Habel, A.; von Uwe, A.; von Gabain, A.; Blasi, U. Therapy of experimental pseudomonas infections with a nonreplicating genetically modified phage. Antimicrob. Agents Chemother 2004, 48, 3817–3822. [Google Scholar]
- Watanabe, R.; Matsumoto, T.; Sano, G.; Ishii, Y.; Tateda, K.; Sumiyama, Y.; Uchiyama, J.; Sakurai, S.; Matsuzaki, S.; Imai, S.; et al. Efficacy of bacteriophage therapy against gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob. Agents Chemother 2007, 51, 446–452. [Google Scholar]
- Fuhrman, J.A. Marine viruses and their biogeochemical and ecological effects. Nature 1999, 399, 541–548. [Google Scholar]
- Mathur, M.; Vidhani, S.; Mehndiratta, P. Bateriophage therapy: An alternative to conventional antibiotics. J. Assoc. Physicians India 2003, 51, 593–596. [Google Scholar]
- Thingstad, T.F. Elements of a theory for the mechanisms controlling abundance, diversity, and biogeochemical role of lytic bacterial viruses in aquatic systems. Limnol Oceanogr 2000, 45, 1320–1328. [Google Scholar]
- Miller, E.S.; Heidelberg, J.F.; Eisen, J.A.; Nelson, W.C.; Durkin, A.S.; Ciecko, A.; Feldblyum, T.V.; White, O.; Paulsen, I.T.; Nierman, W.C.; et al. Complete genome sequence of the broad-hostrange vibriophage KVP40: Comparative genomics of a t4-related Bacteriophage. J. Bacteriol 2003, 17, 5220–5233. [Google Scholar]
- Pereira, C. Use of Bacteriophages on the Inactivation of Pathogenic Bacteria in Aquaculture System; University of Aveiro: Aveiro, Portugal, 2009. [Google Scholar]
- Fogg, G.E.; Calvario-Martinez, O. Effects of bottle size in determinations of primary productivity by phytoplankton. Hydrobiologia 1989, 173, 89–94. [Google Scholar]
- Pereira, C.; Salvador, S.; Arrojado, C.; Silva, Y.; Santos, A.L.; Cunha, A.; Gomes, N.C.M.; Almeida, A. Evaluating seasonal dynamics of bacterial communities in marine fish aquaculture: A preliminary study before applying phage therapy. J. Environ. Monit 2011, 13, 1053–1058. [Google Scholar]
- Costa, L.; Alves, E.; Carvalho, C.; Tomé, J.; Faustino, M.; Neves, M.; Tomé, A.; Cavaleiro, J.; Cunha, Â.; Almeida, A. Sewage bacteriophage photoinactivation by cationic porphyrins: A study of charge effect. Photochem. Photobiol. Sci 2008, 7, 415–422. [Google Scholar]
- Louvado, A. Isolamento e Caracterização de Bactérias Resistentes a Surfactantes; University of Aveiro: Aveiro, Portugal, 2010. [Google Scholar]
- Carey-Smith, G.V.; Billington, C.; Cornelius, A.J.; Hudson, J.A.; Heinemann, J.A. Isolation and characterization of bacteriophages infecting Salmonella spp. FEMS Microbiol. Lett 2006, 258, 182–186. [Google Scholar]
- Adams, N.A. Bacteriophages; John Wiley and Sons Inc: New York, NY, USA, 1959. [Google Scholar]
- Almeida, M.A.; Cunha, M.A.; Alcântara, F. Loss of estuarine bacteria by viral infection and predation in microcosm conditions. Microb. Ecol 2001, 42, 562–571. [Google Scholar]
- Bratbak, G.; Heldal, M. Total Counts of Viruses in Aquatic Environments. In Aquatic Microbial Ecology; Kemp, P.F., Sherr, B.F., Sherr, E.B., Cole, J.J, Eds.; Handbook of Methods in Lewis Publishers: New York, NY, USA, 1993; pp. 135–138. [Google Scholar]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Griffiths, R.I.; Whiteley, A.S.; O’Donnell, A.G.; Bailey, M.J. Rapid Method for Coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA and rRNA-based microbial community composition. Appl. Environ. Microbiol 2000, 66, 5488–5491. [Google Scholar]
- Henriques, I.S.; Almeida, A.; Cunha, A.; Correia, A. Molecular sequence analysis of prokariotic diversity in the middle and outer sections of the Portuguese estuary Ria de Aveiro. FEMS Microbiol. Ecol 2004, 49, 269–279. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol 1991, 173, 697–703. [Google Scholar]
- Nubel, U.; Engelen, B.; Felske, A.; Snaidr, J.; Wieshuber, A.; Amann, R.I.; Ludwig, W.; Backhaus, H. Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J. Bacteriol 1996, 178, 5636–5643. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Smalla, K.; Wieland, G.; Buchner, A.; Zock, A.; Parzy, J.; Kaiser, S.; Roskot, N.; Heuer, H.; Berg, G. Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel e1ectrophoresis: Plant-dependent enrichment and seasonal shifts revealed. Appl. Environ. Microb 2001, 67, 4742–4751. [Google Scholar]





| FISH PATHOGENIC BACTERIA | PHAGES | |
|---|---|---|
| AS-1 | VP-1 | |
| V. anguillarium | 98.87 | 83.27 |
| V. parahaemolyticus | 96.03 | 100 |
| V. fischeri | 0 | 0 |
| A. salmonicida | 100 | 64.75 |
| P. damselae subsp. damselae | 0 | 0 |
| P. damselae subsp. piscicida | 0 | 0 |
| E. coli | 0 | 0 |
| P. aeruginosa | 0 | 0 |
| P. fluorescens | 0 | 0 |
| P. putida | 0 | 0 |
| P. segetis | 0 | 0 |
| P. gingeri | 0 | 0 |
| Groups | R | |
|---|---|---|
| AS-1 | VP-1 | |
| WT0, WT10 | 0.037 | 0.333 |
| WT0, TSB-CL | 0.667 | 0.778 |
| WT0, P100 | 0.333 | 0.556 |
| TSB-CL, P100 | 0.630 | 0.519 |
| TSB-CL, WT10 | 0.963 | 0.407 |
| P100, WT10 | 0.556 | 0.185 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pereira, C.; Silva, Y.J.; Santos, A.L.; Cunha, Â.; Gomes, N.C.M.; Almeida, A. Bacteriophages with Potential for Inactivation of Fish Pathogenic Bacteria: Survival, Host Specificity and Effect on Bacterial Community Structure. Mar. Drugs 2011, 9, 2236-2255. https://doi.org/10.3390/md9112236
Pereira C, Silva YJ, Santos AL, Cunha Â, Gomes NCM, Almeida A. Bacteriophages with Potential for Inactivation of Fish Pathogenic Bacteria: Survival, Host Specificity and Effect on Bacterial Community Structure. Marine Drugs. 2011; 9(11):2236-2255. https://doi.org/10.3390/md9112236
Chicago/Turabian StylePereira, Carla, Yolanda J. Silva, Ana L. Santos, Ângela Cunha, Newton C. M. Gomes, and Adelaide Almeida. 2011. "Bacteriophages with Potential for Inactivation of Fish Pathogenic Bacteria: Survival, Host Specificity and Effect on Bacterial Community Structure" Marine Drugs 9, no. 11: 2236-2255. https://doi.org/10.3390/md9112236