Dynamics of Dissolved and Particulate Polyunsaturated Aldehydes in Mesocosms Inoculated with Different Densities of the Diatom Skeletonema marinoi
Abstract
:1. Introduction
2. Results and Discussion
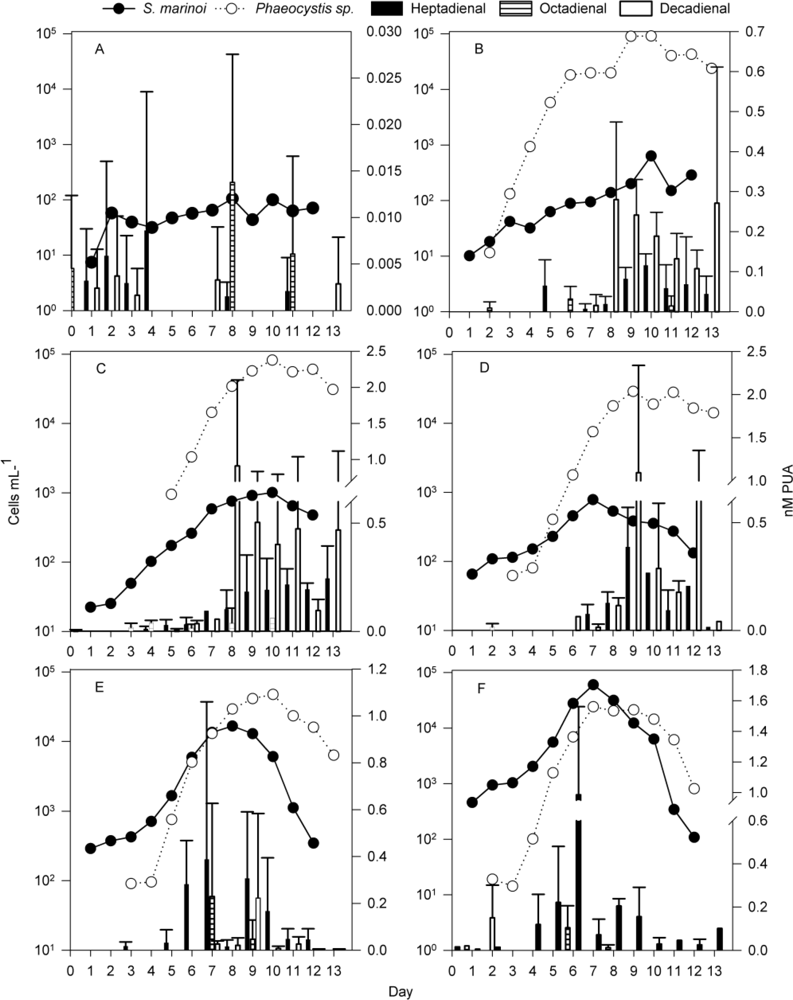
2.1. Development of Communities in the Different Mesocosms
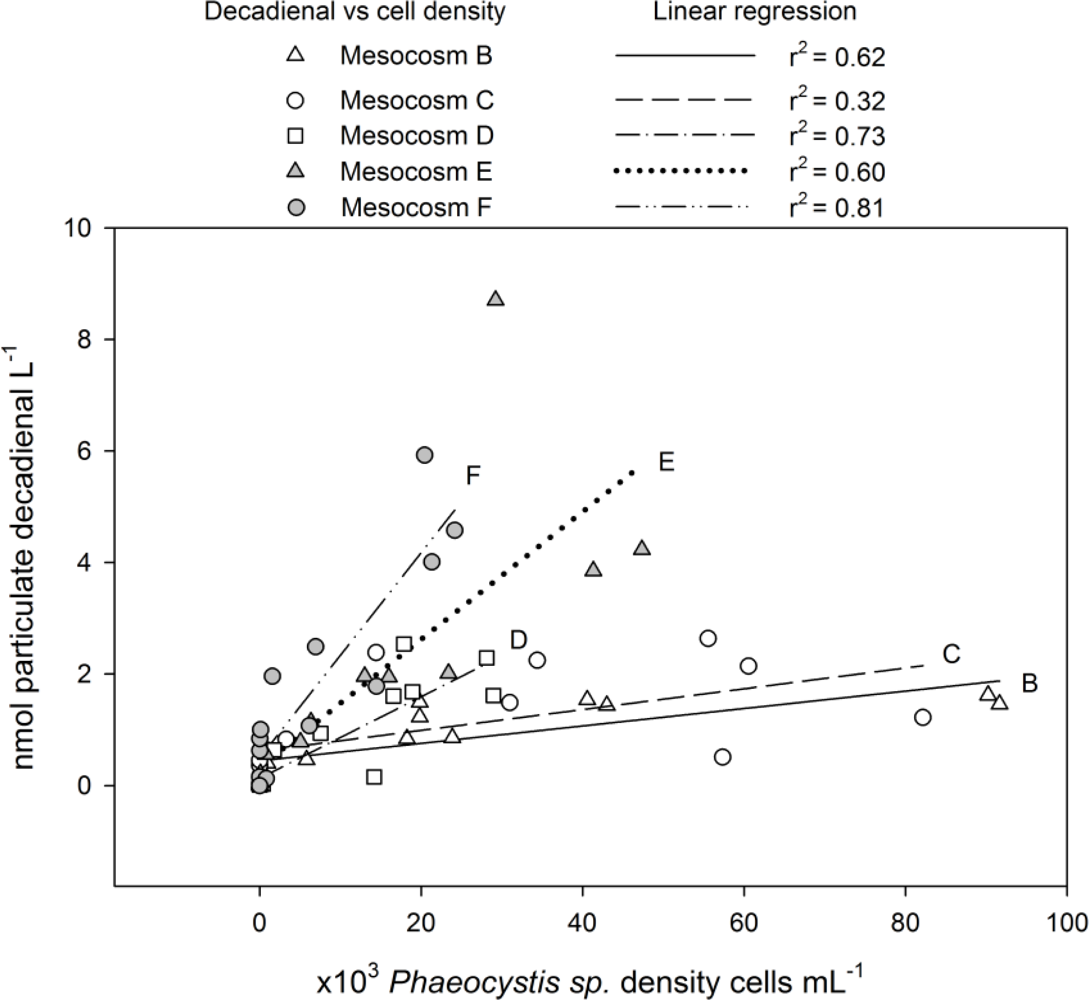
2.2. Particulate PUA
2.3. Dissolved PUA
3. Experimental Section
3.1. Mesocosms
3.2. Sampling
3.3. Apparatus
3.4. Reagents
3.5. Determination of Particulate PUA
3.6. Determination of Dissolved PUA
3.7. Analysis and Quantification of PUA
4. Conclusions
Acknowledgments
References
- Vidoudez, C; Casotti, R; Bastianini, M; Pohnert, G. Quantification of dissolved and particulate polyunsaturated aldehydes in the Adriatic Sea. Mar Drugs 2011. submitted for publication. [Google Scholar]
- Wichard, T; Poulet, SA; Boulesteix, AL; Ledoux, JB; Lebreton, B; Marchetti, J; Pohnert, G. Influence of diatoms on copepod reproduction. II. Uncorrelated effects of diatom-derived alpha,beta,gamma,delta-unsaturated aldehydes and polyunsaturated fatty acids on Calanus helgolandicus in the field. Prog Oceanogr 2008, 77(1), 30–44. [Google Scholar]
- Pohnert, G. Diatom/Copepod interactions in plankton: The indirect chemical defense of unicellular algae. ChemBioChem 2005, 6(6), 946–959. [Google Scholar]
- Wichard, T; Poulet, SA; Halsband-Lenk, C; Albaina, A; Harris, R; Liu, DY; Pohnert, G. Survey of the chemical defence potential of diatoms: Screening of fifty one species for alpha,beta,gamma,delta-unsaturated aldehydes. J Chem Ecol 2005, 31(4), 949–958. [Google Scholar]
- Hansen, E; Ernstsen, A; Eilertsen, HC. Isolation and characterisation of a cytotoxic polyunsaturated aldehyde from the marine phytoplankter Phaeocystis pouchetii (Hariot) Lagerheim. Toxicology 2004, 199(2–3), 207–217. [Google Scholar]
- Ianora, A; Miralto, A; Poulet, SA; Carotenuto, Y; Buttino, I; Romano, G; Casotti, R; Pohnert, G; Wichard, T; Colucci-D’Amato, L; Terrazzano, G; Smetacek, V. Aldehyde suppression of copepod recruitment in blooms of a ubiquitous planktonic diatom. Nature 2004, 429(6990), 403–407. [Google Scholar]
- Flynn, KJ; Irigoien, X. Aldehyde-induced insidious effects cannot be considered as a diatom defence mechanism against copepods. Mar Ecol Prog Ser 2009, 377, 79–89. [Google Scholar]
- Miralto, A; Barone, G; Romano, G; Poulet, SA; Ianora, A; Russo, L; Buttino, I; Mazzarella, G; Laabir, M; Cabrini, M. The insidious effect of diatoms on copepod reproduction. Nature 1999, 402, 173–176. [Google Scholar]
- Adolph, S; Poulet, SA; Pohnert, G. Synthesis and biological activity of alpha,beta,gamma,delta-unsaturated aldehydes from diatoms. Tetrahedron 2003, 59(17), 3003–3008. [Google Scholar]
- Ianora, A; Miralto, A. Toxigenic effects of diatoms on grazers, phytoplankton and other microbes: a review. Ecotoxicology 2010, 19(3), 493–511. [Google Scholar]
- Vardi, A; Formiggini, F; Casotti, R; De Martino, A; Ribalet, F; Miralto, A; Bowler, C. A stress surveillance system based on calcium and nitric oxide in marine diatoms. PLoS Biol 2006, 4(3), 411–419. [Google Scholar]
- Vidoudez, C; Pohnert, G. Growth phase specific release of polyunsaturated aldehydes by the diatom Skeletonema marinoi. J Plankton Res 2008, 30(11), 1305–1313. [Google Scholar]
- Pohnert, G. Wound-activated chemical defense in unicellular planktonic algae. Angew Chem Int Ed 2000, 39(23), 4352–4354. [Google Scholar]
- Ribalet, F; Vidoudez, C; Cassin, D; Pohnert, G; Ianora, A; Miralto, A; Casotti, R. High plasticity in the production of diatom-derived polyunsaturated aldehydes under nutrient limitation: physiological and ecological implications. Protist 2009, 160(3), 444–451. [Google Scholar]
- Ribalet, F; Wichard, T; Pohnert, G; Ianora, A; Miralto, A; Casotti, R. Age and nutrient limitation enhance polyunsaturated aldehyde production in marine diatoms. Phytochemistry 2007, 68(15), 2059–2067. [Google Scholar]
- Poulet, SA; Escribano, R; Hidalgo, P; Cueff, A; Wichard, T; Aguilera, V; Vargas, CA; Pohnert, G. Collapse of Calanus chilensis reproduction in a marine environment with high diatom concentration. J Exp Mar Biol Ecol 2007, 352(1), 187–199. [Google Scholar]
- Kooistra, W; Sarno, D; Balzano, S; Gu, HF; Andersen, RA; Zingonea, A. Global diversity and biogeography of Skeletonema species (Bacillariophyta). Protist 2008, 159(2), 177–193. [Google Scholar]
- Yamasaki, Y; Nagasoe, S; Matsubara, T; Shikata, T; Shimasaki, Y; Oshima, Y; Honjo, T. Allelopathic interactions between the bacillariophyte Skeletonema costatum and the raphidophyte Heterosigma akashiwo. Mar Ecol Prog Ser 2007, 339, 83–92. [Google Scholar]
- Ask, J; Reinikainen, M; Bamstedt, U. Variation in hatching success and egg production of Eurytemora affinis (Calanoida, Copepoda) from the Gulf of Bothnia, Baltic Sea, in relation to abundance and clonal differences of diatoms. J Plankton Res 2006, 28(7), 683–694. [Google Scholar]
- d’Ippolito, G; Romano, G; Iadicicco, O; Miralto, A; Ianora, A; Cimino, G; Fontana, A. New birth-control aldehydes from the marine diatom Skeletonema costatum: characterization and biogenesis. Tetrahedron Lett 2002, 43(35), 6133–6136. [Google Scholar]
- Pohnert, G; Lumineau, O; Cueff, A; Adolph, S; Cordevant, C; Lange, M; Poulet, S. Are volatile unsaturated aldehydes from diatoms the main line of chemical defence against copepods. Mar Ecol Prog Ser 2002, 245, 33–45. [Google Scholar]
- Nejstgaard, JC; Frischer, ME; Verity, PG; Anderson, JT; Jacobsen, A; Zirbel, MJ; Larsen, A; Martinez-Martinez, J; Sazhin, AF; Walters, T; Bronk, DA; Whipple, SJ; Borrett, SR; Patten, BC; Long, JD. Plankton development and trophic transfer in seawater enclosures with nutrients and Phaeocystis pouchetii added. Mar Ecol Prog Ser 2006, 321, 99–121. [Google Scholar]
- Barofsky, A; Simonelli, P; Vidoudez, C; Troedsson, C; Nejstgaard, JC; Jakobsen, HH; Pohnert, G. Growth phase of the diatom Skeletonema marinoi influences the metabolic profile of the cells and the selective feeding of the copepod Calanus spp. J Plankton Res 2010, 32(3), 263–272. [Google Scholar]
- Adolph, S; Bach, S; Blondel, M; Cueff, A; Moreau, M; Pohnert, G; Poulet, SA; Wichard, T; Zuccaro, A. Cytotoxicity of diatom-derived oxylipins in organisms belonging to different phyla. J Exp Biol 2004, 207(17), 2935–2946. [Google Scholar]
- Hansen, E; Even, Y; Genevière, A-M. The alpha, beta, gamma, delta-unsaturated aldehyde 2-trans-4-trans-decadienal disturbs DNA replication and mitotic events in early sea urchin embryos. Toxicol Sci 2004, 81(1), 190–197. [Google Scholar]
- Ianora, A; Miralto, A. Toxigenic effects of diatoms on grazers, phytoplankton and other microbes: a review. Ecotoxicology 2010, 19(3), 493–511. [Google Scholar]
- Wichard, T; Poulet, SA; Pohnert, G. Determination and quantification of alpha,beta,gamma,delta-unsaturated aldehydes as pentafluorobenzyl-oxime derivates in diatom cultures and natural phytoplankton populations: application in marine field studies. J Chrom B 2005, 814(1), 155–161. [Google Scholar]


© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vidoudez, C.; Nejstgaard, J.C.; Jakobsen, H.H.; Pohnert, G. Dynamics of Dissolved and Particulate Polyunsaturated Aldehydes in Mesocosms Inoculated with Different Densities of the Diatom Skeletonema marinoi. Mar. Drugs 2011, 9, 345-358. https://doi.org/10.3390/md9030345
Vidoudez C, Nejstgaard JC, Jakobsen HH, Pohnert G. Dynamics of Dissolved and Particulate Polyunsaturated Aldehydes in Mesocosms Inoculated with Different Densities of the Diatom Skeletonema marinoi. Marine Drugs. 2011; 9(3):345-358. https://doi.org/10.3390/md9030345
Chicago/Turabian StyleVidoudez, Charles, Jens Christian Nejstgaard, Hans Henrik Jakobsen, and Georg Pohnert. 2011. "Dynamics of Dissolved and Particulate Polyunsaturated Aldehydes in Mesocosms Inoculated with Different Densities of the Diatom Skeletonema marinoi" Marine Drugs 9, no. 3: 345-358. https://doi.org/10.3390/md9030345




