Effect of Magnetic Nanoparticles on Tobacco BY-2 Cell Suspension Culture
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Preparation of Deionised Water and pH Measurement
2.3. Tobacco BY-2 Cell Suspension Culture and Microscopic Observations
2.4. Magnetic Nanoparticles, Their Characterization and Modification
2.5. Sample Preparation
2.5.1. Spectrophotometric Measurements
2.5.2. Chromatographic Measurements
2.6. Spectrophotometric Measurements
2.6.1. Determination of Total Protein Content—Pyrogallol Method
2.6.2. Determination of Total Thiol Compounds and Glutathione-S-Transferase Activity
2.6.2.1. Total Thiols—Ellman’s Reaction
2.6.2.2. Glutathione-S-Transferase Activity
2.6.3. Determination of Antioxidant Activity
2.6.3.1. DPPH Test
2.6.3.2. ABTS Test
2.6.3.3. DMPD Method
2.6.3.4. Blue CrO5 Method
2.7. High Performance Liquid Chromatography with Electrochemical Detection (HPLC-ED)—Determination of Thiol Compounds
2.8. Mathematical Treatment of Data and Estimation of Detection Limits
3. Results and Discussion
3.1. Effect of NPs and MNPs on Cell Viability and Growth
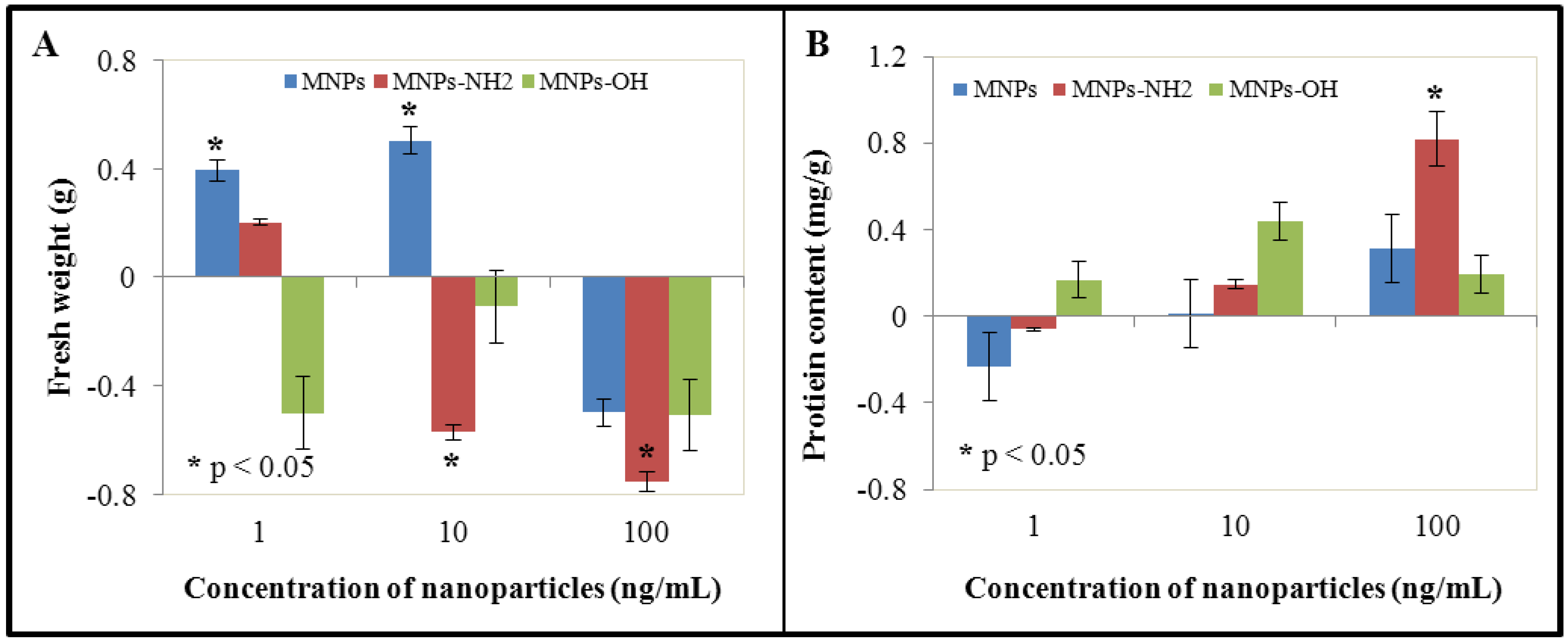
3.2. Effect of NPs and MNPs on Protein Content
3.3. Effect of NPs and MNPs on Intracellular Thiols
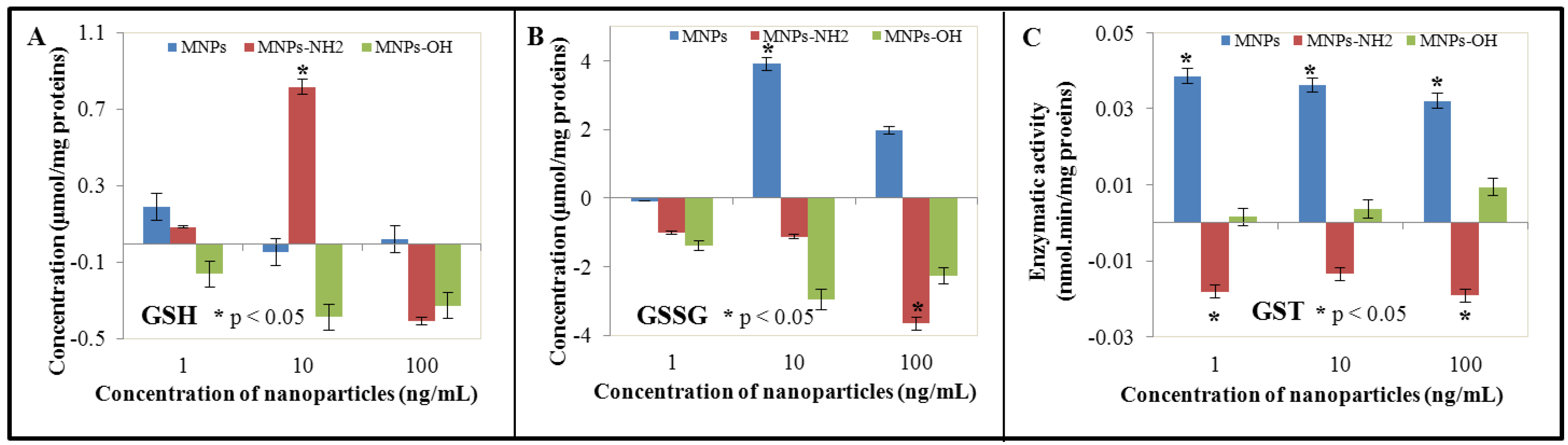
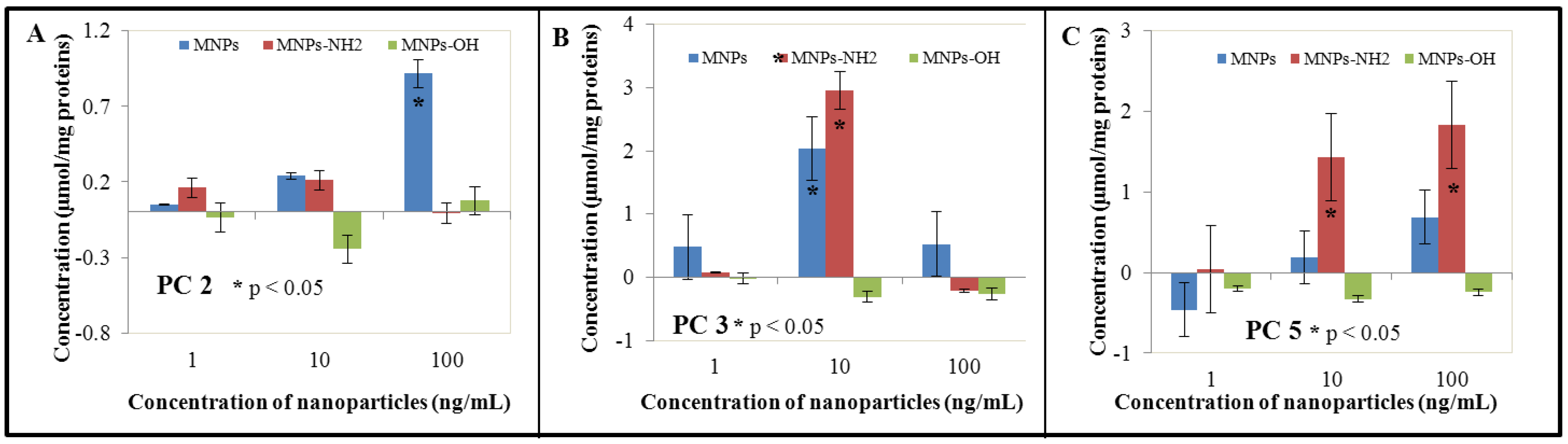
3.4. Effect of NPs and MNPS on Antioxidant Activity

3.5. Microscopical Observations
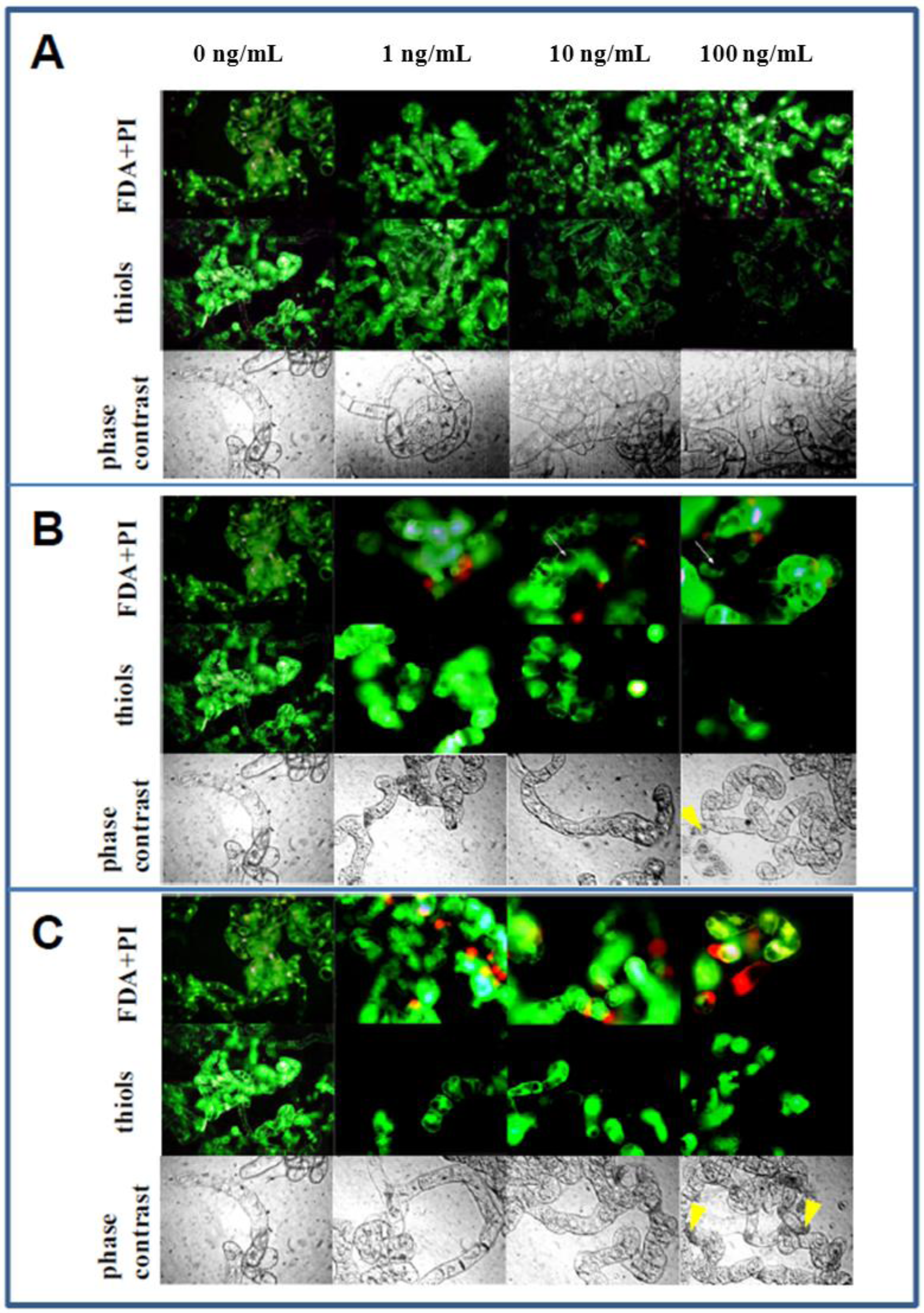
4. Conclusions
Acknowledgements
References
- Wang, J.; Asbach, C.; Fissan, H.; Hulser, T.; Kuhlbusch, T.A.J.; Thompson, D.; Pui, D.Y.H. How can nanobiotechnology oversight advance science and industry: Examples from environmental, health, and safety studies of nanoparticles (nano-EHS). J. Nanopart. Res. 2011, 13, 1373–1387. [Google Scholar] [CrossRef]
- Hoshino, A.; Manabe, N.; Fujioka, K.; Suzuki, K.; Yasuhara, M.; Yamamoto, K. Use of fluorescent quantum dot bioconjugates for cellular imaging of immune cells, cell organelle labeling, and nanomedicine: Surface modification regulates biological function, including cytotoxicity. J. Artif. Organs 2007, 10, 149–157. [Google Scholar] [CrossRef]
- Bhaskar, S.; Tian, F.R.; Stoeger, T.; Kreyling, W.; de la Fuente, J.M.; Grazu, V.; Borm, P.; Estrada, G.; Ntziachristos, V.; Razansky, D. Multifunctional nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: Perspectives on tracking and neuroimaging. Part. Fibre Toxicol. 2010, 7, 1–25. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Bhaumik, J.; Karver, M.R.; Erdem, S.S.; Weissleder, R. Targeted nanoagents for the detection of cancers. Mol. Oncol. 2010, 4, 511–528. [Google Scholar] [CrossRef]
- Kaounides, L.; Yu, H.; Harper, T. Nanotechnology innovation and apptications in textites industry: Current markets and future growth trends. Mater. Technol. 2007, 22, 209–237. [Google Scholar]
- Mokhatab, S.; Towler, B.F. Nanomaterials hold promise in natural gas industry. Int. J. Nanotechnol. 2007, 4, 680–690. [Google Scholar]
- Schmid, K.; Riediker, M. Use of nanoparticles in swiss industry: A targeted survey. Environ. Sci. Technol. 2008, 42, 2253–2260. [Google Scholar] [CrossRef]
- Kitisriworaphan, T.; Sawangdee, Y. Nanotechnology Divides: Development Indicators and Thai Construction Industry. In Nanotechnology in Construction 3; Springer: Berlin, Germany, 2009; pp. 251–259. [Google Scholar]
- Lee, J.; Mahendra, S.; Alvarez, P.J.J. Nanomaterials in the construction industry: A review of their applications and environmental health and safety considerations. ACS Nano 2010, 4, 3580–3590. [Google Scholar] [CrossRef]
- Weiter, M.; Vala, M.; Vynuchal, J.; Kubac, L.; Ltd, T. Development of New Organic Semiconductors and Their Applications in Organic Electronics and Photonics. In Proceedings ofNanocon 20102nd International Conference, Olomouc, Czech Republic, 12–14 October 2010; pp. 114–119.
- Yang, E.H. Engineered low-dimensional nanomaterials for sensors, actuators, and electronics. J. Micro-Nanolithogr. MEMS MOEMS 2010, 9, 1–6. [Google Scholar]
- Das, R.N.; Lin, H.T.; Lauffer, J.M.; Markovich, V.R. Printable electronics: Towards materials development and device fabrication. Circuit World 2011, 37, 38–45. [Google Scholar] [CrossRef]
- Lahiri, I.; Das, S.; Kang, C.; Choi, W. Application of carbon nanostructures-energy to electronics. JOM 2011, 63, 70–76. [Google Scholar]
- Musee, N.; Thwala, M.; Nota, N. The antibacterial effects of engineered nanomaterials: Implications for wastewater treatment plants. J. Environ. Monit. 2011, 13, 1164–1183. [Google Scholar] [CrossRef]
- Westerhoff, P.; Song, G.X.; Hristovski, K.; Kiser, M.A. Occurrence and removal of titanium at full scale wastewater treatment plants: Implications for TiO2 nanomaterials. J. Environ. Monit. 2011, 13, 1195–1203. [Google Scholar] [CrossRef]
- Musthaba, S.M.; Ahmad, S.; Ahuja, A.; Ali, J.; Baboota, S. Nano approaches to enhance pharmacokinetic and pharmacodynamic activity of plant origin drugs. Curr. Nanosci. 2009, 5, 344–352. [Google Scholar] [CrossRef]
- Vance, D.; Martin, J.; Patke, S.; Kane, R.S. The design of polyvalent scaffolds for targeted delivery. Adv. Drug Deliv. Rev. 2009, 61, 931–939. [Google Scholar] [CrossRef]
- Biju, V.; Itoh, T.; Ishikawa, M. Delivering quantum dots to cells: Bioconjugated quantum dots for targeted and nonspecific extracellular and intracellular imaging. Chem. Soc. Rev. 2010, 39, 3031–3056. [Google Scholar] [CrossRef]
- Patra, C.R.; Bhattacharya, R.; Mukhopadhyay, D.; Mukherjee, P. Fabrication of gold nanoparticles for targeted therapy in pancreatic cancer. Adv. Drug Deliv. Rev. 2010, 62, 346–361. [Google Scholar] [CrossRef]
- Phillips, M.A.; Gran, M.L.; Peppas, N.A. Targeted nanodelivery of drugs and diagnostics. Nano Today 2010, 5, 143–159. [Google Scholar] [CrossRef]
- Lee, W.M.; An, Y.J.; Yoon, H.; Kweon, H.S. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (phaseolus radiatus) and wheat (triticum aestivum): Plant agar test for water-insoluble nanoparticles. Environ. Toxicol. Chem. 2008, 27, 1915–1921. [Google Scholar] [CrossRef]
- Judy, J.D.; Unrine, J.M.; Bertsch, P.M. Evidence for biomagnification of gold nanoparticles within a terrestrial food chain. Environ. Sci. Technol. 2011, 45, 776–781. [Google Scholar] [CrossRef]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar]
- Poelman, E.H.; van Loon, J.J.A.; Dicke, M. Consequences of variation in plant defense for biodiversity at higher trophic levels. Trends Plant. Sci. 2008, 13, 534–541. [Google Scholar] [CrossRef]
- Mishra, V.K.; Kumar, A. Impact of metal nanoparticles on the plant growth promoting rhizobacteria. Dig. J. Nanomater. Biostruct. 2009, 4, 587–592. [Google Scholar]
- Gardea-Torresdey, J.L.; Gomez, E.; Peralta-Videa, J.; Parsons, J.; Tiemann, K.; Troiani, H.; Yacaman, M.J. Use of XAS and TEM to determine the uptake of gold and silver and nanoparticle formation by living alfalfa plants. Abstr. Pap. Am. Chem. Soc. 2003, 225, U837–U837. [Google Scholar]
- Harris, A.T.; Bali, R. On the formation and extent of uptake of silver nanoparticles by live plants. J. Nanopart. Res. 2008, 10, 691–695. [Google Scholar] [CrossRef]
- Lin, S.J.; Reppert, J.; Hu, Q.; Hudson, J.S.; Reid, M.L.; Ratnikova, T.A.; Rao, A.M.; Luo, H.; Ke, P.C. Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small 2009, 5, 1128–1132. [Google Scholar]
- Hernandez-Viezcas, J.A.; Castillo-Michel, H.; Servin, A.D.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Spectroscopic verification of zinc absorption and distribution in the desert plant prosopis juliflora-velutina (velvet mesquite) treated with ZnO nanoparticles. Chem. Eng. J. 2011, 170, 346–352. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; de Silva, K.; Nedosekin, D.A.; Dervishi, E.; Biris, A.S.; Shashkov, E.V.; Galanzha, E.I.; Zharov, V.P. Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proc. Natl. Acad. Sci. USA. 2011, 108, 1028–1033. [Google Scholar]
- Pavel, I.E.; Monahan, J.; Markopoulos, M.M.; Gagnon, Z.E.; NeJame, B. The Bioaccumulation and Toxicity of Silver Nanoparticles in Animal and Plant Tissues. Available online: acs.confex.com/acs/cerm09/webprogram/Paper71449.html (accessed on 1 December 2012).
- Chen, R.; Ratnikova, T.A.; Stone, M.B.; Lin, S.; Lard, M.; Huang, G.; Hudson, J.S.; Ke, P.C. Differential uptake of carbon nanoparticles by plant and mammalian cells. Small 2010, 6, 612–617. [Google Scholar] [CrossRef]
- Hischemoller, A.; Nordmann, J.; Ptacek, P.; Mummenhoff, K.; Haase, M. In-vivo imaging of the uptake of upconversion nanoparticles by plant roots. J. Biomed. Nanotechnol. 2009, 5, 278–284. [Google Scholar] [CrossRef]
- Lopez-Moreno, M.L.; de la Rosa, G.; Hernandez-Viezcas, J.A.; Castillo-Michel, H.; Botez, C.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (glycine max) plants. Environ. Sci. Technol. 2010, 44, 7315–7320. [Google Scholar]
- Parsons, J.G.; Lopez, M.L.; Gonzalez, C.M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Toxicity and biotransformation of uncoated and coated nickel hydroxide nanoparticles on mesquite plants. Environ. Toxicol. Chem. 2010, 29, 1146–1154. [Google Scholar]
- de la Rosa, G.; Lopez-Moreno, M.L.; Hernandez-Viezcas, J.; Montes, M.O.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Toxicity and biotransformation of ZnO nanoparticles in the desert plants prosopis juliflora-velutina, salsola tragus and parkinsonia florida. Int. J. Nanotechnol. 2011, 8, 492–506. [Google Scholar] [CrossRef]
- Eichert, T.; Kurtz, A.; Steiner, U.; Goldbach, H.E. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol. Plant. 2008, 134, 151–160. [Google Scholar] [CrossRef]
- Basiuk, E.V.; Ochoa-Olmos, O.E.; de la Mora-Estrada, L.F. Ecotoxicological effects of carbon nanomaterials on algae, fungi and plants. J. Nanosci. Nanotechnol. 2011, 11, 3016–3038. [Google Scholar] [CrossRef]
- Corredor, E.; Testillano, P.S.; Coronado, M.J.; Gonzalez-Melendi, P.; Fernandez-Pacheco, R.; Marquina, C.; Ibarra, M.R.; de la Fuente, J.M.; Rubiales, D.; Perez-De-Luque, A.; et al. Nanoparticle penetration and transport in living pumpkin plants: In situ subcellular identification. BMC Plant. Biol. 2009, 9, 1–11. [Google Scholar] [CrossRef]
- Ovecka, M.; Lang, I.; Baluska, F.; Ismail, A.; Illes, P.; Lichtscheidl, I.K. Endocytosis and vesicle trafficking during tip growth of root hairs. Protoplasma 2005, 226, 39–54. [Google Scholar] [CrossRef]
- Zhu, H.; Han, J.; Xiao, J.Q.; Jin, Y. Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J. Environ. Monit. 2008, 10, 713–717. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; He, X.; Zhang, H.F.; Ma, Y.H.; Zhang, P.; Ding, Y.Y.; Zhao, Y.L. Uptake and distribution of ceria nanoparticles in cucumber plants. Metallomics 2011, 3, 816–822. [Google Scholar] [CrossRef]
- Lopez-Moreno, M.L.; de la Rosa, G.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J. Agric. Food Chem. 2010, 58, 3689–3693. [Google Scholar]
- Ma, X.M.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef]
- Lu, C.M.; Zhang, C.Y.; Wen, J.Q.; Wu, G.R.; Tao, M.X. Research of the effect of nanometer materials on germination and growth enhancement of glycine max and its mechanism. Soybean Sci. 2002, 21, 168–172. [Google Scholar]
- Gao, F.Q.; Liu, C.; Qu, C.X.; Zheng, L.; Yang, F.; Su, M.G.; Hong, F.H. Was improvement of spinach growth by nano-TiO2 treatment related to the changes of rubisco activase? Biometals 2008, 21, 211–217. [Google Scholar] [CrossRef]
- Yang, L.; Watts, D.J. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol. Lett. 2005, 158, 122–132. [Google Scholar] [CrossRef]
- Hund-Rinke, K.; Simon, M. Ecotoxic effect of photocatalytic active nanoparticles TiO2 on algae and daphnids. Environ. Sci. Pollut. Res. 2006, 13, 225–232. [Google Scholar] [CrossRef]
- Lin, D.H.; Xing, B.S. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Ma, Y.H.; Kuang, L.L.; He, X.; Bai, W.; Ding, Y.Y.; Zhang, Z.Y.; Zhao, Y.L.; Chai, Z.F. Effects of rare earth oxide nanoparticles on root elongation of plants. Chemosphere 2010, 78, 273–279. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Josko, I.; Xing, B.S. The toxicity to plants of the sewage sludges containing multiwalled carbon nanotubes. J. Hazard. Mater. 2011, 186, 436–442. [Google Scholar] [CrossRef]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef]
- Carriere, M.; Simon-Dekers, A.; Larue, C.; Mayne-L’Hermite, M.; Herlin-Boime, N.; Reynaud, C. Investigation of nanoparticles and carbon nanotubes toxicity and transfer in bacteria and plants. Geochim. Cosmochim. Acta 2009, 73, A194–A194. [Google Scholar]
- Chomoucka, J.; Drbohlavova, J.; Hubalek, J.; Babula, P.; Adam, V.; Kizek, R. Toxicity of nanoparticles for plants. Listy Cukrov. Reparske 2010, 126, 400–401. [Google Scholar]
- Nagata, T.; Nemoto, Y.; Hasezawa, S. Tobacco BY-2 cell-line as the hela-cell in the cell biology of higher-plants. Int. Rev. Cytol. 1992, 132, 1–30. [Google Scholar] [CrossRef]
- Vitecek, J.; Adam, V.; Petrek, J.; Vacek, J.; Kizek, R.; Havel, L. Esterases as a marker for growth of BY-2 tobacco cells and early somatic embryos of the norway spruce. Plant. Cell. Tissue Organ. Cult. 2004, 79, 195–201. [Google Scholar] [CrossRef]
- Vitecek, J.; Adam, V.; Petrek, J.; Babula, P.; Novotna, P.; Kizek, R.; Havel, L. Application of fluorimetric determination of esterases in plant material. Chem. Listy 2005, 99, 496–501. [Google Scholar]
- Synek, P.; Jasek, O.; Zajickova, L.; David, B.; Kudrle, V.; Pizurova, N. Plasmachemical synthesis of maghemite nanoparticles in atmospheric pressure microwave torch. Mater. Lett. 2011, 65, 982–984. [Google Scholar] [CrossRef]
- Riener, C.K.; Kada, G.; Gruber, H.J. Quick measurement of protein sulfhydryls with ellman’s reagent and with 4,4'-dithiodipyridine. Anal. Bioanal. Chem. 2002, 373, 266–276. [Google Scholar] [CrossRef]
- Sochor, J.; Ryvolova, M.; Krystofova, O.; Salas, P.; Hubalek, J.; Adam, V.; Trnkova, L.; Havel, L.; Beklova, M.; Zehnalek, J.; et al. Fully automated spectrometric protocols for determination of antioxidant activity: Advantages and disadvantages. Molecules 2010, 15, 8618–8640. [Google Scholar]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.R.; Watanabe, F.; Biris, A.S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 2009, 3, 3221–3227. [Google Scholar] [CrossRef]
- Trojan, V.; Chomoucka, J.; Krystofova, O.; Hubalek, J.; Babula, P.; Kizek, R. Quantum dots (CdSe) modified by glutathione and their localization of tobacco BY-2 cells. J. Biotechnol. 2010, 150, S479–S479. [Google Scholar]
- Serag, M.F.; Kaji, N.; Gaillard, C.; Okamoto, Y.; Terasaka, K.; Jabasini, M.; Tokeshi, M.; Mizukami, H.; Bianco, A.; Baba, Y. Trafficking and subcellular localization of multiwalled carbon nanotubes in plant cells. ACS Nano 2011, 5, 493–499. [Google Scholar] [CrossRef]
- Babula, P.; Opatrilova, R.; Krystofova, O.; Zehnalek, J.; Adam, V.; Havel, L.; Kizek, R. The importance and effects of copper on plants. Listy Cukrov. Reparske 2010, 126, 397–398. [Google Scholar]
- Kuthanova, A.; Gemperlova, L.; Zelenkova, S.; Eder, J.; Machackova, I.; Opatrny, Z.; Cvikrova, M. Cytological changes and alterations in polyamine contents induced by cadmium in tobacco BY-2 cells. Plant. Physiol. Biochem. 2004, 42, 149–156. [Google Scholar] [CrossRef]
- Hemmerlin, A.; Reents, R.; Mutterer, J.; Feldtrauer, J.F.; Waldmann, H.; Bach, T.J. Monitoring farnesol-induced toxicity in tobacco BY-2 cells with a fluorescent analog. Arch. Biochem. Biophys. 2006, 448, 93–103. [Google Scholar] [CrossRef]
- Yin, L.Y.; Huang, J.Q.; Li, W.; Liu, Y.D. Microcystin-RR-induced apoptosis in tobacco BY-2 cells. Toxicon 2006, 48, 204–210. [Google Scholar] [CrossRef]
- Huang, W.M.; Xing, W.; Li, D.H.; Liu, Y.D. Microcystin-RR induced apoptosis in tobacco BY-2 suspension cells is mediated by reactive oxygen species and mitochondrial permeability transition pore status. Toxicol. Vitro 2008, 22, 328–337. [Google Scholar] [CrossRef]
- Babula, P.; Adam, V.; Kizek, R.; Sladly, Z.; Havel, L. Naphthoquinones as allelochemical triggers of programmed cell death. Environ. Exp. Bot. 2009, 65, 330–337. [Google Scholar] [CrossRef]
- Cobbett, C.S. Heavy metal detoxification in plants: Phytochelatin biosynthesis and function. IUBMB Life 2001, 51, 183–188. [Google Scholar] [CrossRef]
- Maughan, S.; Foyer, C.H. Engineering and genetic approaches to modulating the glutathione network in plants. Physiol. Plant. 2006, 126, 382–397. [Google Scholar] [CrossRef]
- Szalai, G.; Kellos, T.; Galiba, G.; Kocsy, G. Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J. Plant. Growth Regul. 2009, 28, 66–80. [Google Scholar] [CrossRef]
- Cummins, I.; Dixon, D.P.; Freitag-Pohl, S.; Skipsey, M.; Edwards, R. Multiple roles for plant glutathione transferases in xenobiotic detoxification. Drug Metab. Rev. 2011, 43, 266–280. [Google Scholar] [CrossRef]
- Mohsenzadeh, S.; Esmaeili, M.; Moosavi, F.; Shahrtash, M.; Saffari, B.; Mohabatkar, H. Plant glutathione s-transferase classification, structure and evolution. Afr. J. Biotech. 2011, 10, 8160–8165. [Google Scholar]
- Kumar, C.; Igbaria, A.; D’Autreaux, B.; Planson, A.G.; Junot, C.; Godat, E.; Bachhawat, A.K.; Delaunay-Moisan, A.; Toledano, M.B. Glutathione revisited: A vital function in iron metabolism and ancillary role in thiol-redox control. EMBO J. 2011, 30, 2044–2056. [Google Scholar] [CrossRef]
- Richie, J.P.; Kleinman, W.; Marina, P.; Abraham, P.; Wynder, E.L.; Muscat, J.E. Blood iron, glutathione, and micronutrient levels and the risk of oral cancer. Nutr. Cancer 2008, 60, 474–482. [Google Scholar] [CrossRef]
- Kaur, D.; Lee, D.; Ragapolan, S.; Andersen, J.K. Glutathione depletion in immortalized midbrain-derived dopaminergic neurons results in increases in the labile iron pool: Implications for parkinson’s disease. Free Radic. Biol. Med. 2009, 46, 593–598. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, B.; Saxena, R. Glutathione s-transferase gene deletions and their effect on iron status in hbe/beta thalassemia patients. Ann. Hematol. 2010, 89, 411–414. [Google Scholar] [CrossRef]
- Arakawa, Y.; Masaoka, Y.; Sakai, J.; Higo, H.; Higo, K. An alfalfa gene similar to glutathione s-transferase is induced in root by iron deficiency. Soil Sci. Plant. Nutr. 2002, 48, 111–116. [Google Scholar] [CrossRef]
- Zaharieva, T.B.; Abadia, J. Iron deficiency enhances the levels of ascorbate, glutathione, and related enzymes in sugar beet roots. Protoplasma 2003, 221, 269–275. [Google Scholar]
- Yamaguchi, Y.; Yamamoto, Y.; Ikegawa, H.; Matsumoto, H. Protective effect of glutathione on the cytotoxicity caused by a combination of aluminum and iron in suspension-cultured tobacco cells. Physiol. Plant. 1999, 105, 417–422. [Google Scholar] [CrossRef]
- Tepe, M.; Harms, H. Influence of abiotic stress on the GSH/GSSG system of plant-cell cultures. Zeitschrift Pflanzen. Boden. 1995, 158, 75–78. [Google Scholar] [CrossRef]
- Pahlich, E.; Muller, C.; Jager, H.J. New insights into the dynamics of the glutathione-ascorbate redox system of plants. J. Appl. Bot. Food Qual. Angew. Bot. 2007, 81, 110–120. [Google Scholar]
- Cuypers, A.; Vangronsveld, J.; Clijsters, H. The redox status of plant cells (AsA and GSH) is sensitive to zinc imposed oxidative stress in roots and primary leaves of Phaseolus vulgaris. Plant. Physiol. Biochem. 2001, 39, 657–664. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Corpas, F.J.; Rodriguez-Serrano, M.; Gomez, M.; del Rio, L.A.; Sandalio, L.M. Differential expression and regulation of antioxidative enzymes by cadmium in pea plants. J. Plant. Physiol. 2007, 164, 1346–1357. [Google Scholar] [CrossRef]
- Kondo, N.; Imai, K.; Isobe, M.; Goto, T.; Murasugi, A.; Wadanakagawa, C.; Hayashi, Y. Cadystin-a and cadystin-b, major unit peptides comprising cadmium binding peptides induced in a fission yeast—Separation, revision of structures and synthesis. Tetrahedron Lett. 1984, 25, 3869–3872. [Google Scholar] [CrossRef]
- Clemens, S.; Persoh, D. Multi-tasking phytochelatin synthases. Plant. Sci. 2009, 177, 266–271. [Google Scholar] [CrossRef]
- Kneer, R.; Zenk, M.H. The formation of Cd-phytochelatin complexes in plant cell cultures. Phytochemistry 1997, 44, 69–74. [Google Scholar] [CrossRef]
- Nakazawa, R.; Ikawa, M.; Yasuda, K.; Takenaga, H. Synergistic inhibition of the growth of suspension cultured tobacco cells by simultaneous treatment with cadmium and arsenic in relation to phytochelatin synthesis. Soil Sci. Plant. Nutr. 2000, 46, 271–275. [Google Scholar] [CrossRef]
- Nakazawa, R.; Ozawa, T.; Naito, T.; Kameda, Y.; Takenaga, H. Interactions between cadmium and nickel in phytochelatin biosynthesis and the detoxification of the two metals in suspension-cultured tobacco cells. Biol. Plant. 2001, 44, 627–630. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.U.; Min, S.R.; Jeong, W.J.; Sultana, S.; Choi, K.S.; Lee, Y.; Liu, J.R. Overexpression of AtATM3 in Brassica juncea confers enhanced heavy metal tolerance and accumulation. Plant. Cell. Tissue Organ. Cult. 2011, 107, 69–77. [Google Scholar] [CrossRef]
- Ramos, J.; Naya, L.; Gay, M.; Abian, J.; Becana, M. Functional characterization of an unusual phytochelatin synthase, LjPCS3, of Lotus japonicus. Plant. Physiol. 2008, 148, 536–545. [Google Scholar] [CrossRef]
- Ray, D.; Williams, D.L. Characterization of the phytochelatin synthase of schistosoma mansoni. Plos Neglect. Trop. Dis. 2011, 5, 1–11. [Google Scholar]
- Loscos, J.; Naya, L.; Ramos, J.; Clemente, M.R.; Matamoros, M.A.; Becana, M. A reassessment of substrate specificity and activation of phytochelatin synthases from model plants by physiologically relevant metals. Plant. Physiol. 2006, 140, 1213–1221. [Google Scholar] [CrossRef]
- Zhang, R.; Niu, Y.J.; Li, Y.W.; Zhao, C.F.; Song, B.; Li, Y.; Zhou, Y.K. Acute toxicity study of the interaction between titanium dioxide nanoparticles and lead acetate in mice. Environ. Toxicol. Pharmacol. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol.-Mysore 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Asghar, M.N.; Khan, I.U.; Arshad, M.N.; Sherin, L. Evaluation of antioxidant activity using an improved dmpd radical cation decolorization assay. Acta Chim. Slov. 2007, 54, 295–300. [Google Scholar]
- Gulcin, I. Measurement of antioxidant ability of melatonin and serotonin by the dmpd and cuprac methods as trolox equivalent. J. Enzym. Inhib. Med. Chem. 2008, 23, 871–876. [Google Scholar] [CrossRef]
- Rodriguez-Nogales, J.M.; Vila-Crespo, J.; Gomez, M. Development of a rapid method for the determination of the antioxidant capacity in cereal and legume milling products using the radical cation DMPD. Food Chem. 2011, 129, 1800–1805. [Google Scholar] [CrossRef]
- Charalampidis, P.S.; Veltsistas, P.; Karkabounas, S.; Evangelou, A. Blue CrO5 assay: A novel spectrophotometric method for the evaluation of the antioxidant and oxidant capacity of various biological substances. Eur. J. Med. Chem. 2009, 44, 4162–4168. [Google Scholar] [CrossRef]
- Vitecek, J.; Petrlova, J.; Adam, V.; Havel, L.; Kramer, K.J.; Babula, P.; Kizek, R. A fluorimetric sensor for detection of one living cell. Sensors 2007, 7, 222–238. [Google Scholar] [CrossRef]
- Young, B.; Wightman, R.; Blanvillain, R.; Purcel, S.B.; Gallois, P. pH-sensitivity of YFP provides an intracellular indicator of programmed cell death. Plant. Methods 2010, 6, 1–9. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Krystofova, O.; Sochor, J.; Zitka, O.; Babula, P.; Kudrle, V.; Adam, V.; Kizek, R. Effect of Magnetic Nanoparticles on Tobacco BY-2 Cell Suspension Culture. Int. J. Environ. Res. Public Health 2013, 10, 47-71. https://doi.org/10.3390/ijerph10010047
Krystofova O, Sochor J, Zitka O, Babula P, Kudrle V, Adam V, Kizek R. Effect of Magnetic Nanoparticles on Tobacco BY-2 Cell Suspension Culture. International Journal of Environmental Research and Public Health. 2013; 10(1):47-71. https://doi.org/10.3390/ijerph10010047
Chicago/Turabian StyleKrystofova, Olga, Jiri Sochor, Ondrej Zitka, Petr Babula, Vit Kudrle, Vojtech Adam, and Rene Kizek. 2013. "Effect of Magnetic Nanoparticles on Tobacco BY-2 Cell Suspension Culture" International Journal of Environmental Research and Public Health 10, no. 1: 47-71. https://doi.org/10.3390/ijerph10010047




