Green Synthesis of Silver Nanoparticles, Their Characterization, Application and Antibacterial Activity †
Abstract
:1. Introduction
2. Materials and Methods
| Scientific name | Common name |
|---|---|
| Actaea racemosa | Black cohosh |
| Magnolia grandiflora | Magnolia |
| Aloe sp. | “Tingtinkie” |
| Eucalyptusangophoroides | Eucalyptus |
| Sansevieria trifasciata | Mother-in-laws’ tongue |
| Impatiens balsamina | Rose Balsam |
| Pelargonium graveolens | Geranium |
3. Results and Discussion
3.1. Nanoparticles Production and Characterization
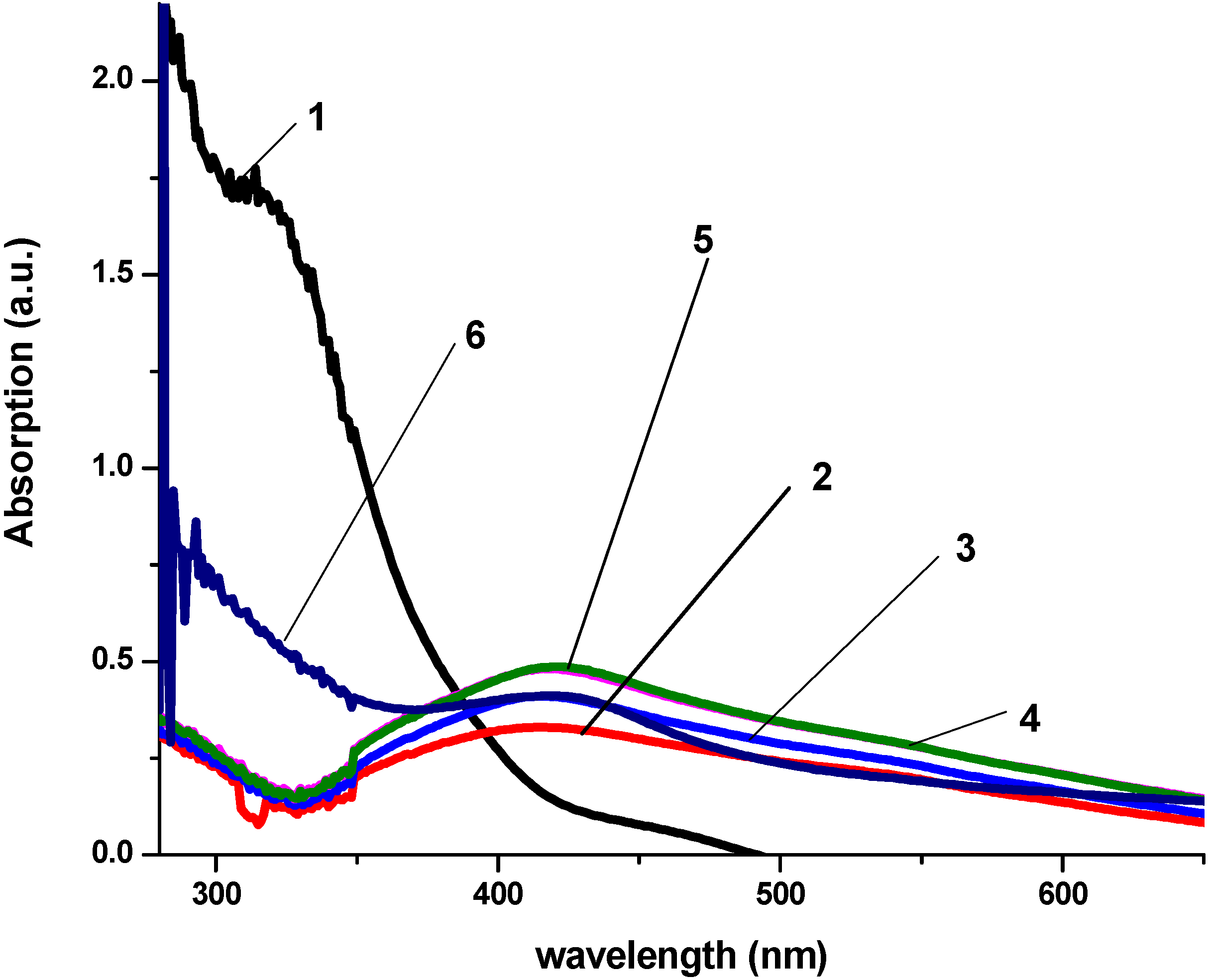
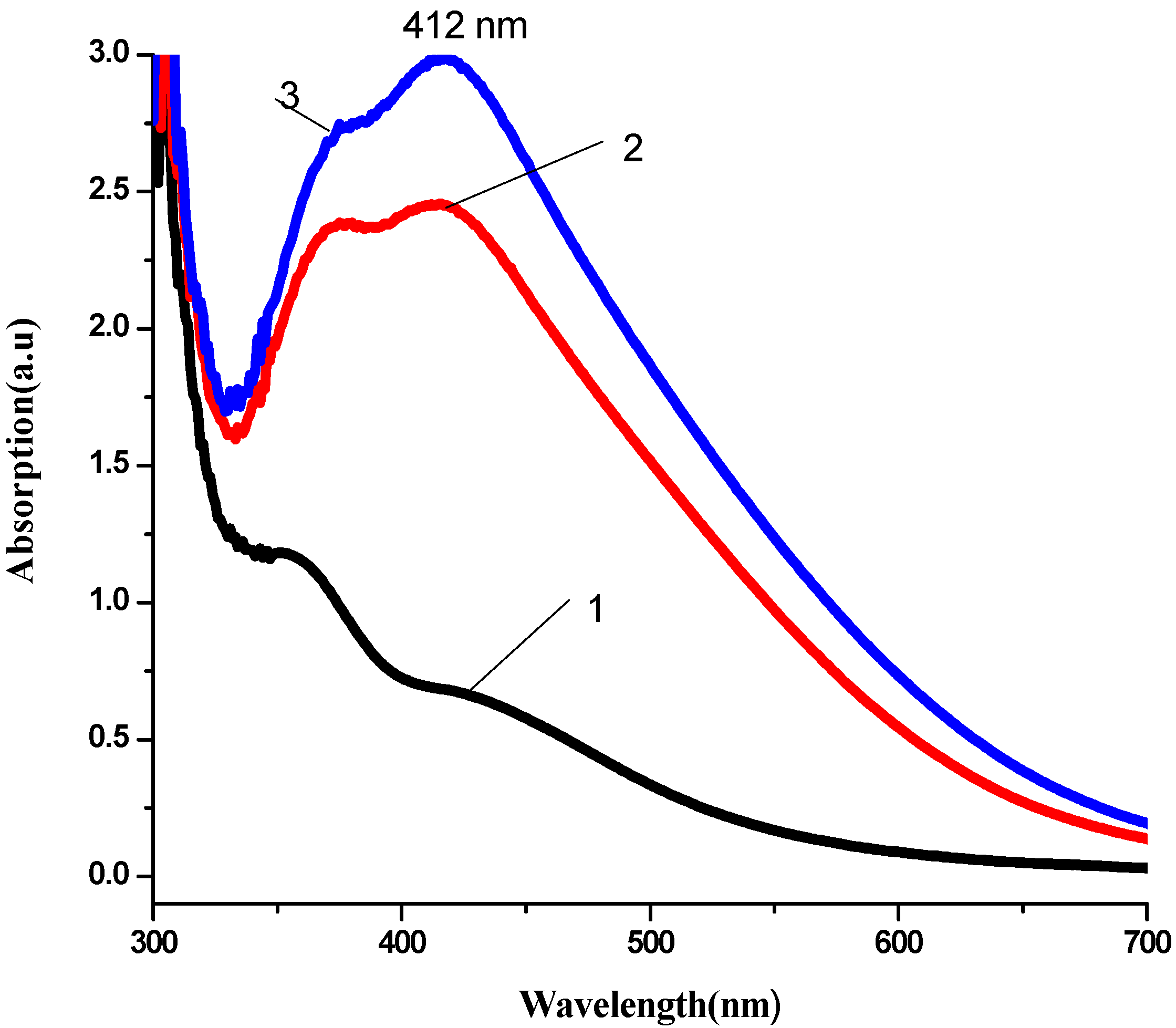
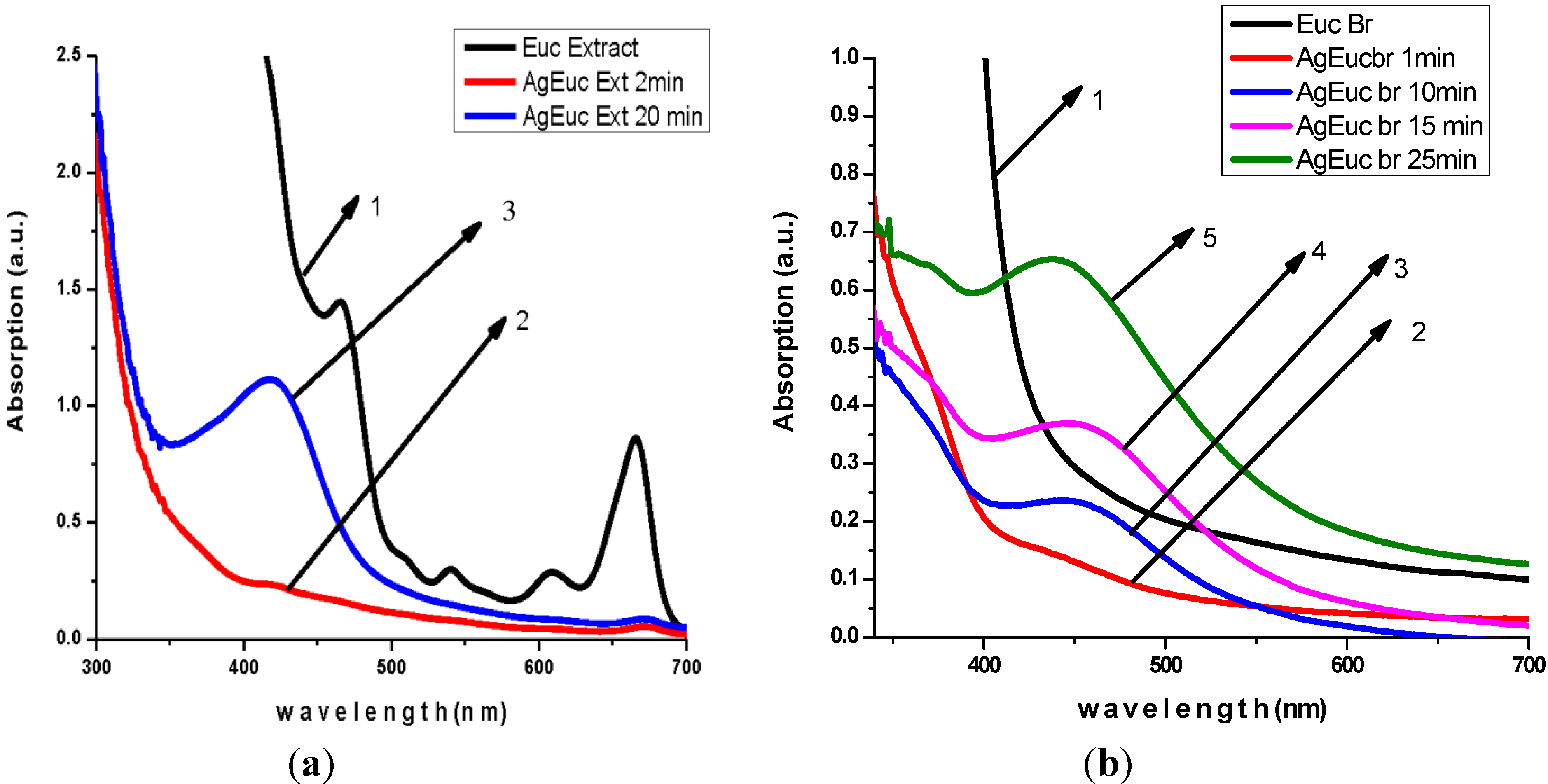
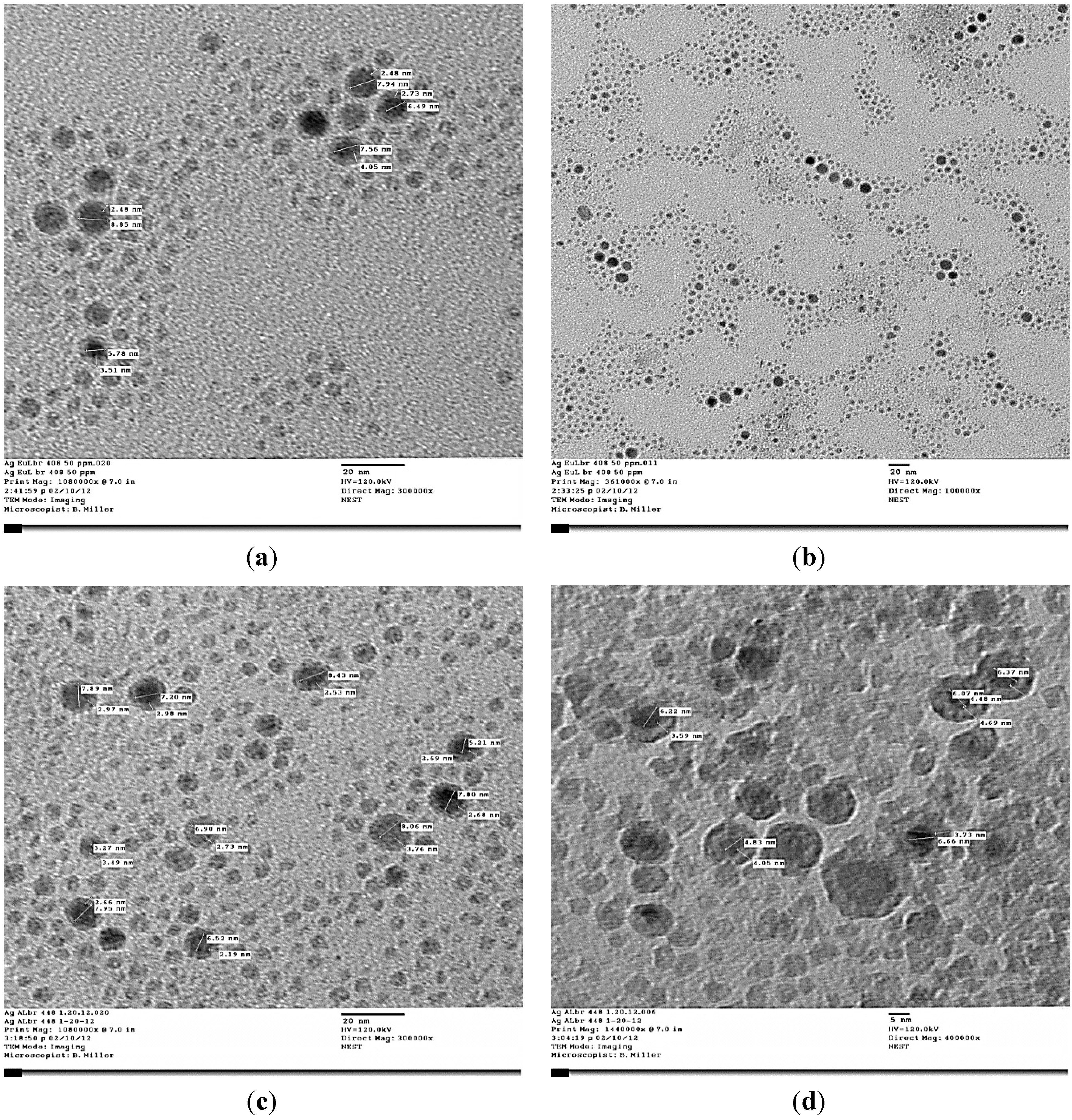
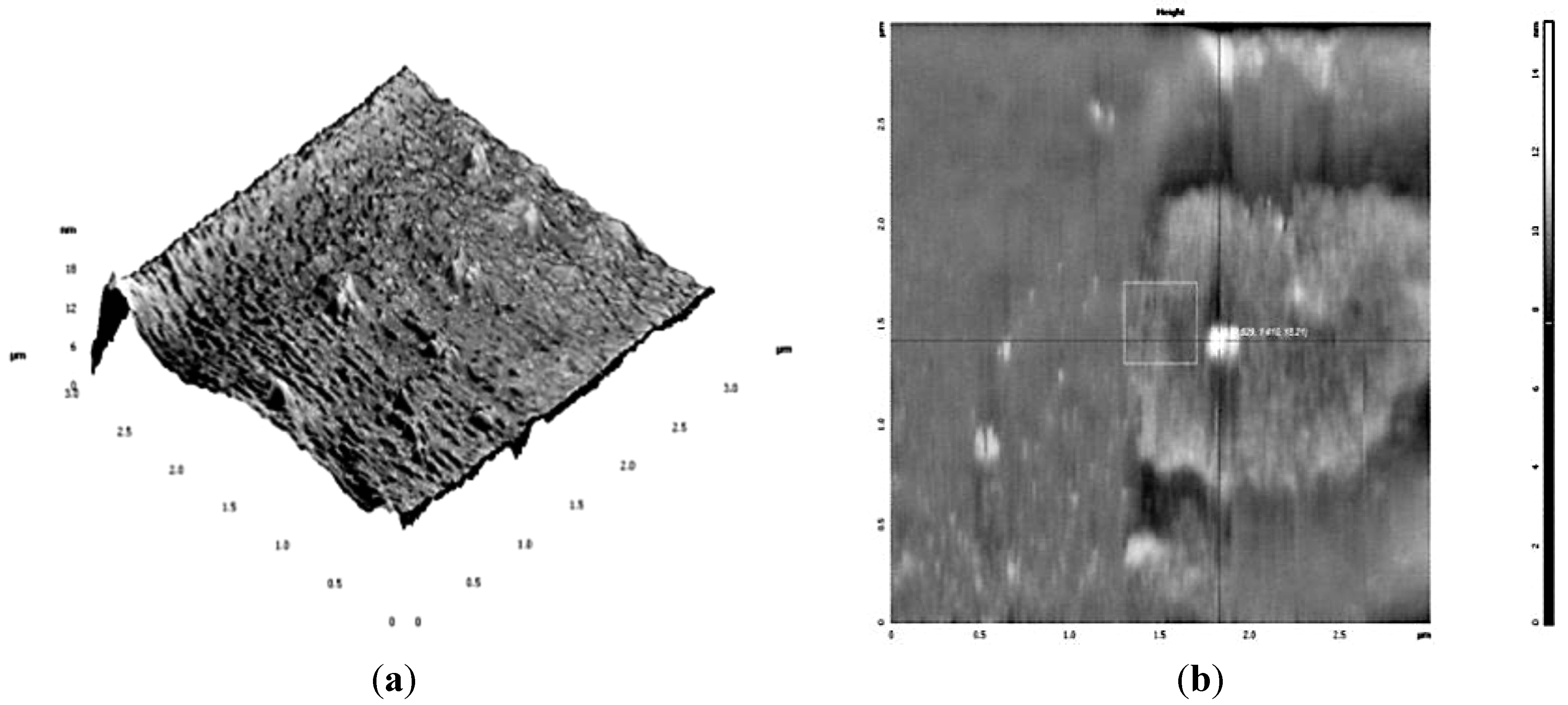
3.2. Antibacterial Activity of Silver Nanoparticles
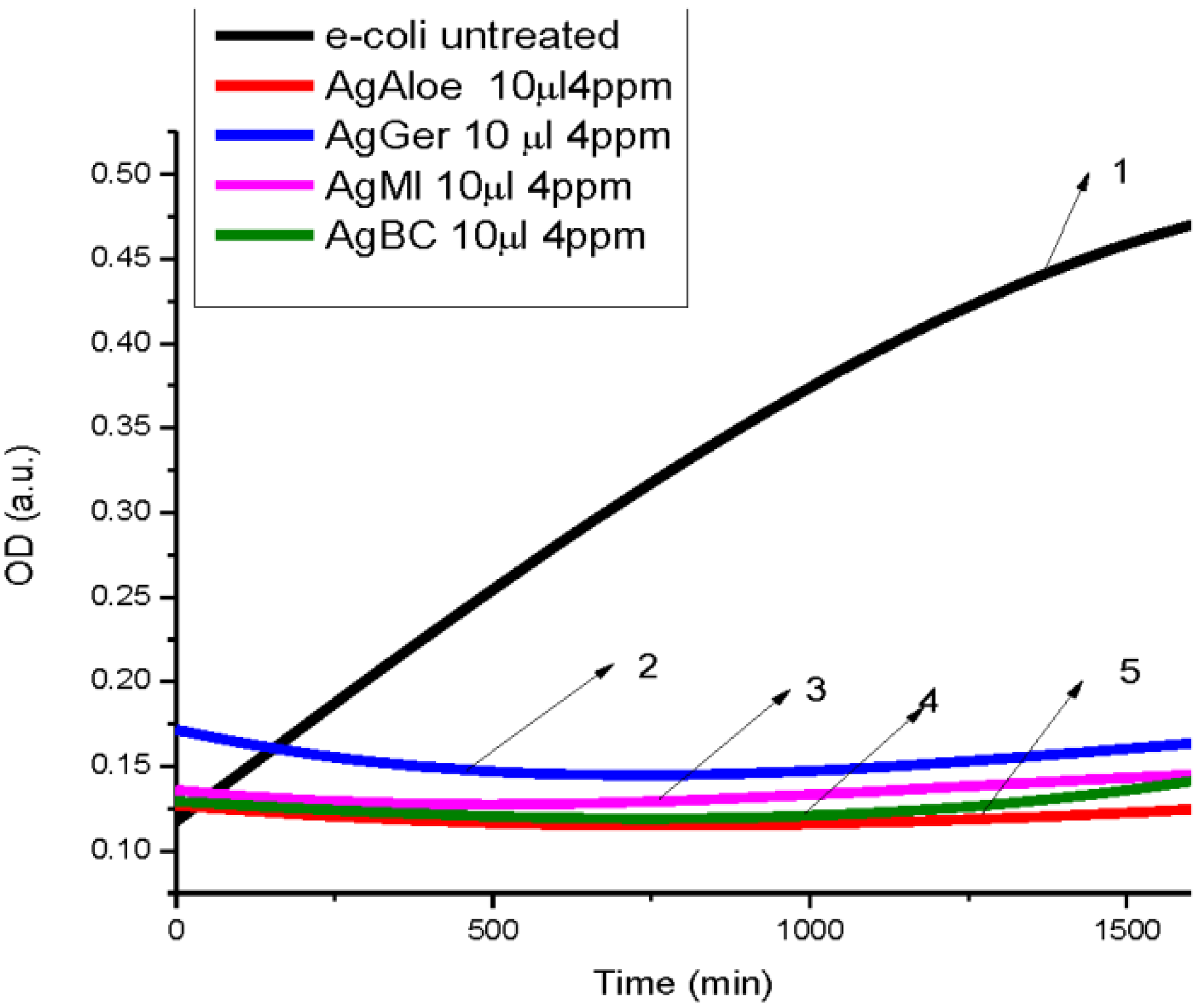
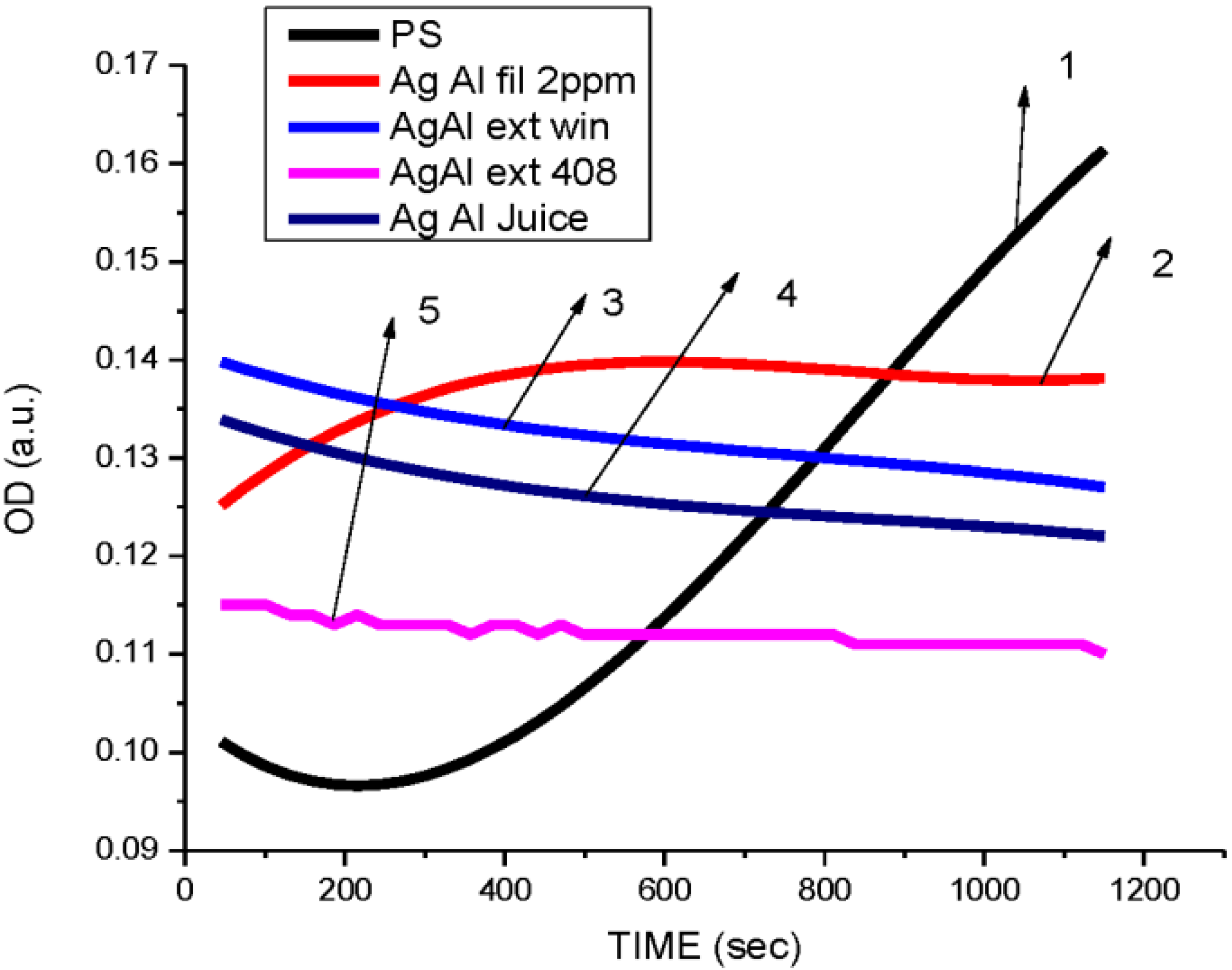
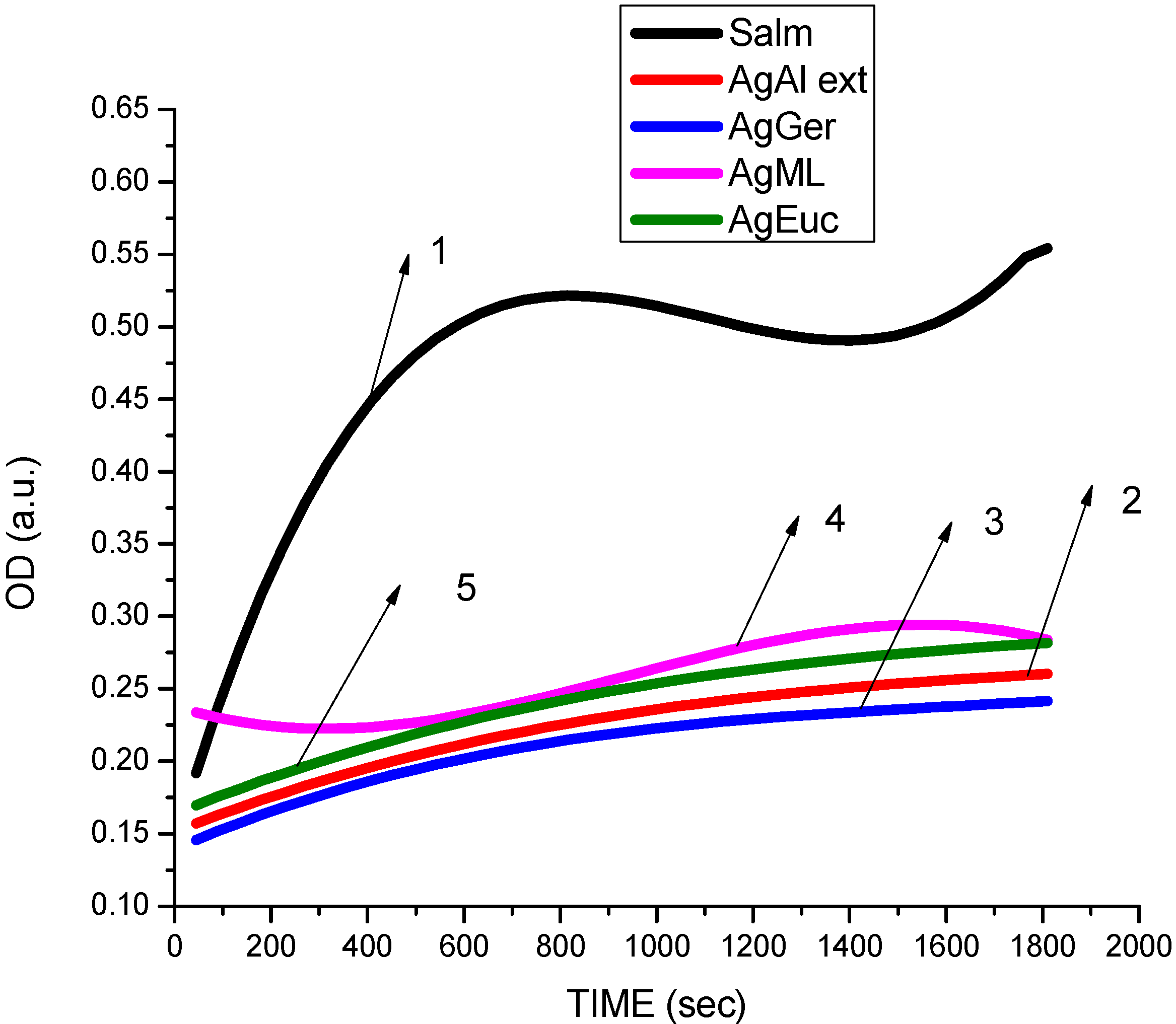
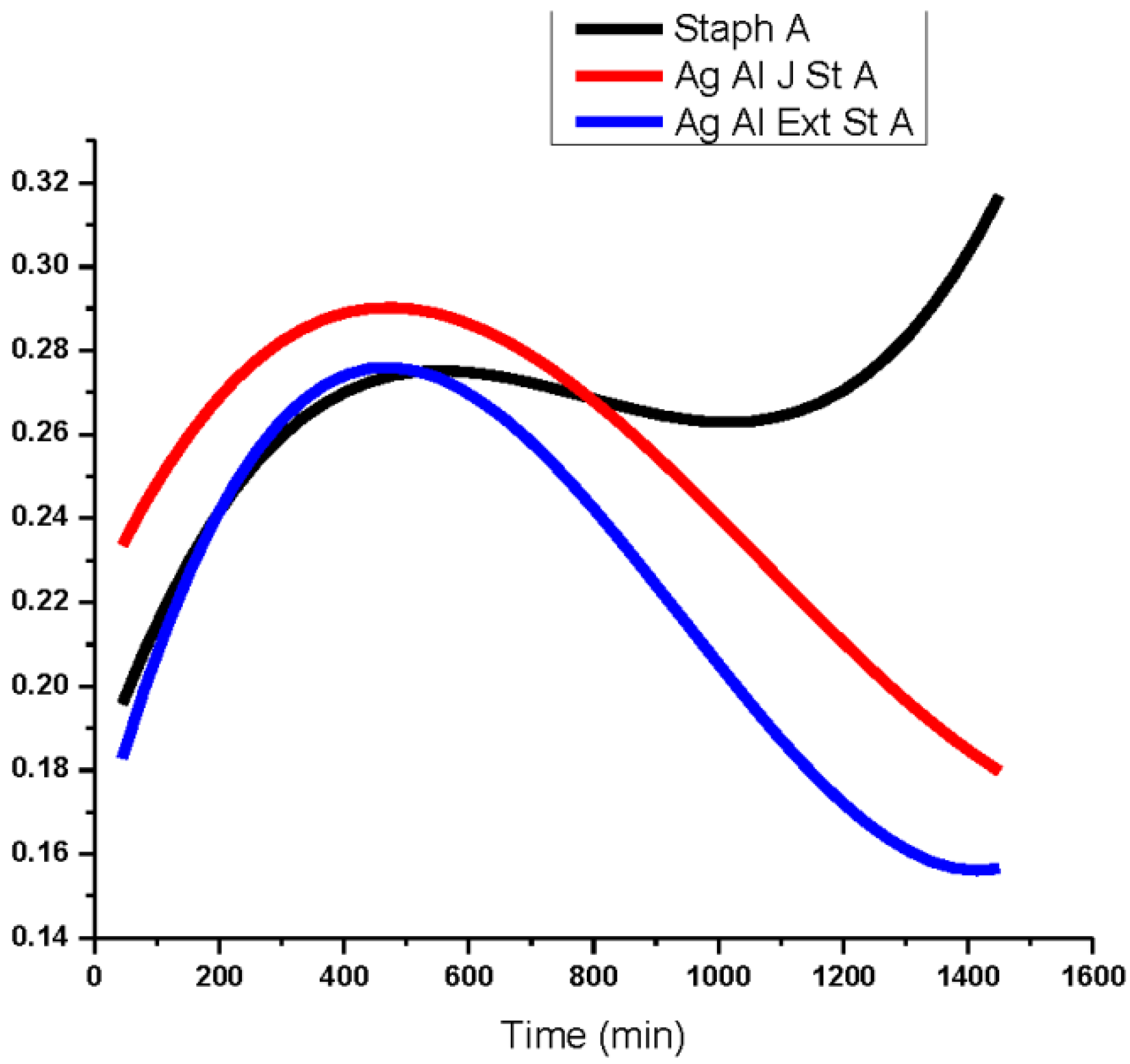
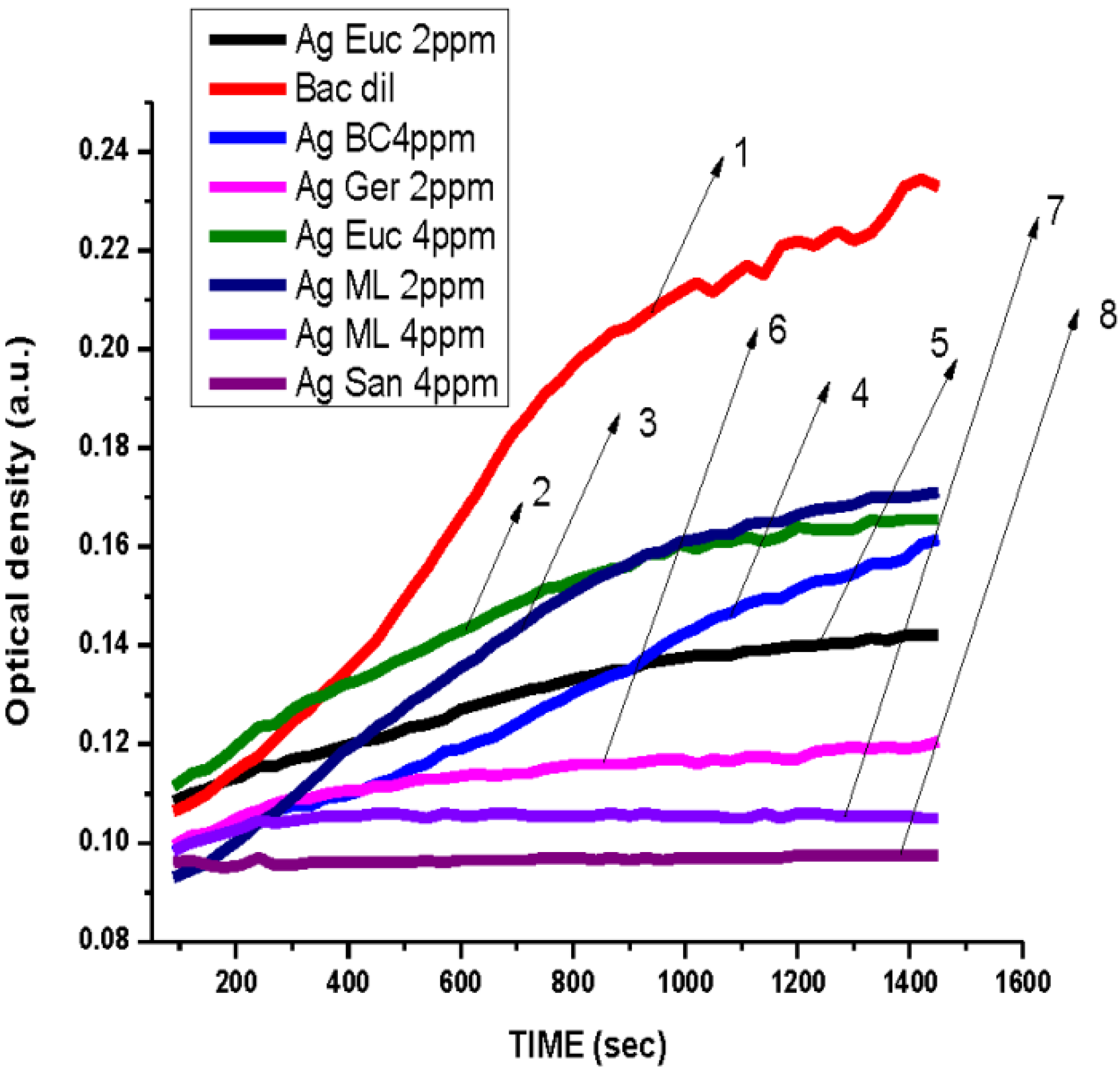
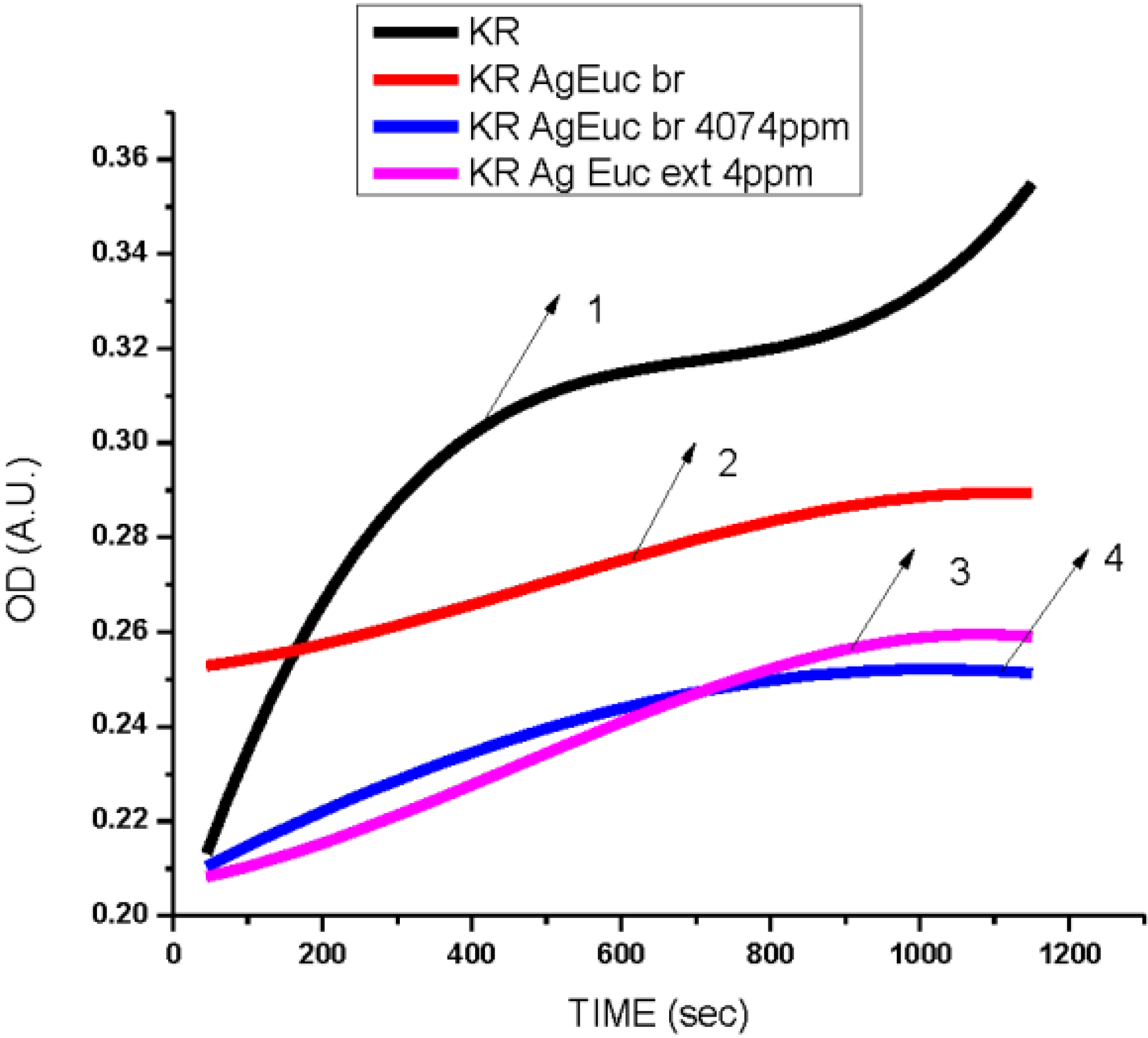
3.3. Cytotoxicity of Biosynthesized Noble Nanoparticles on Healthy Human Cells
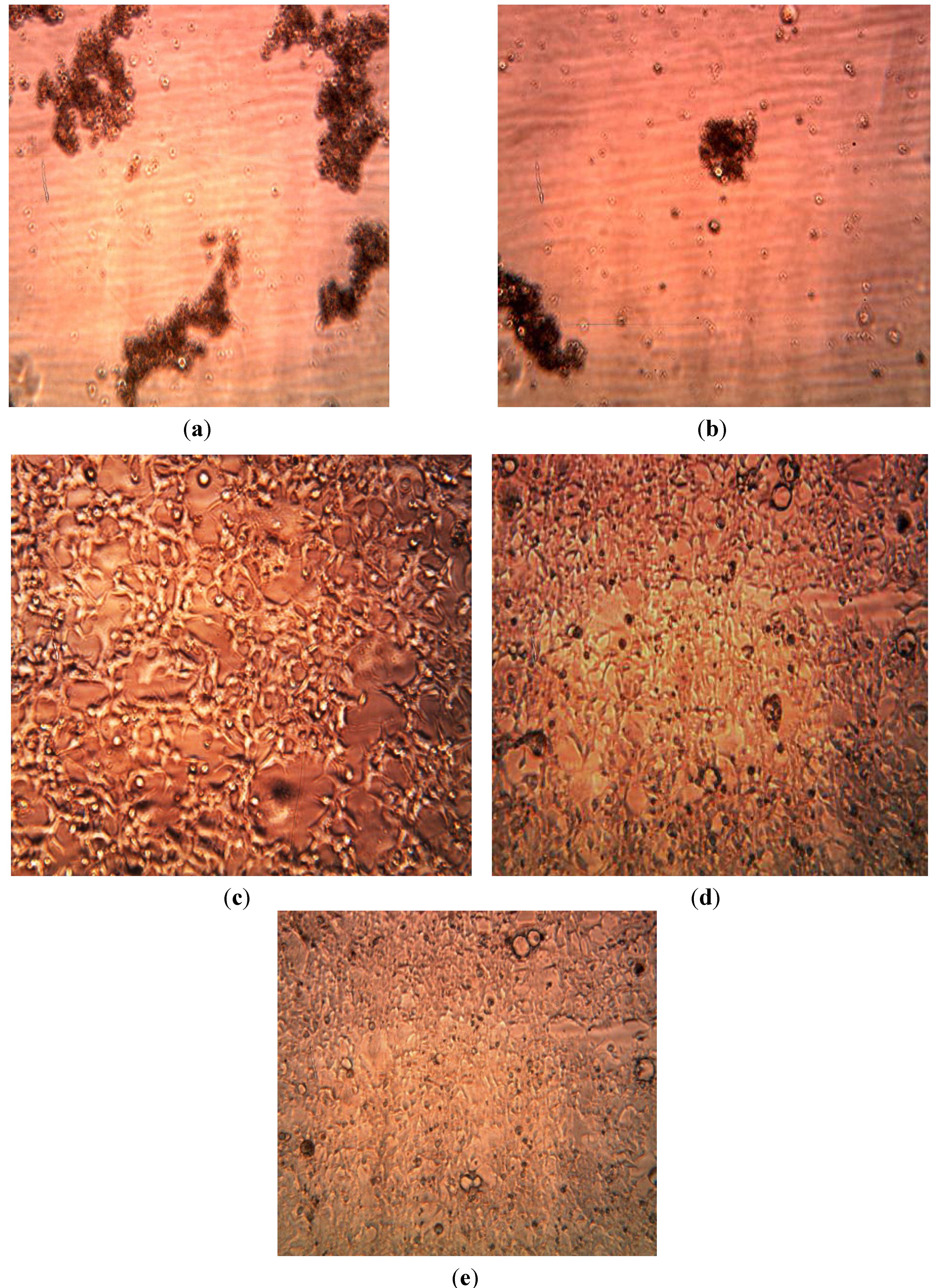
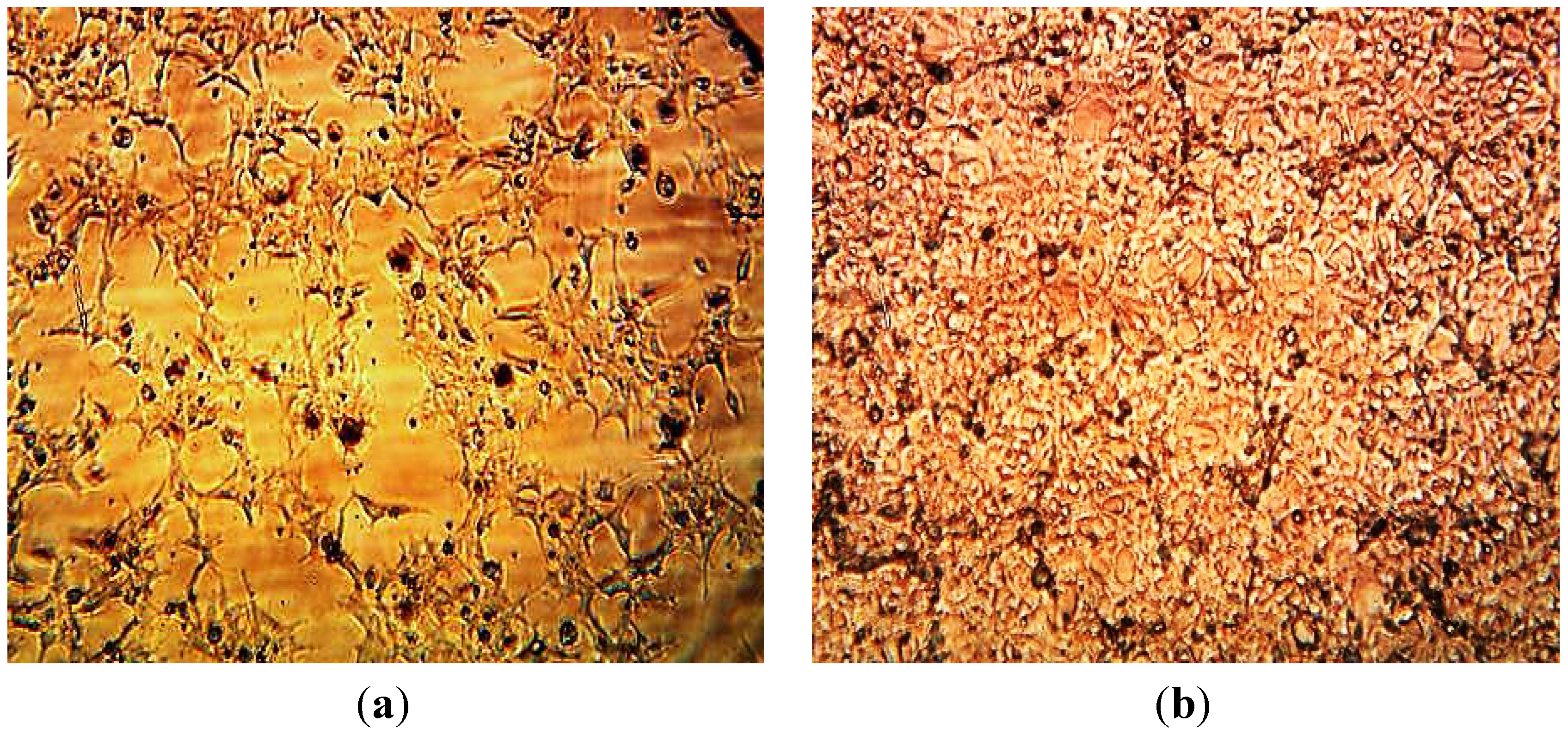
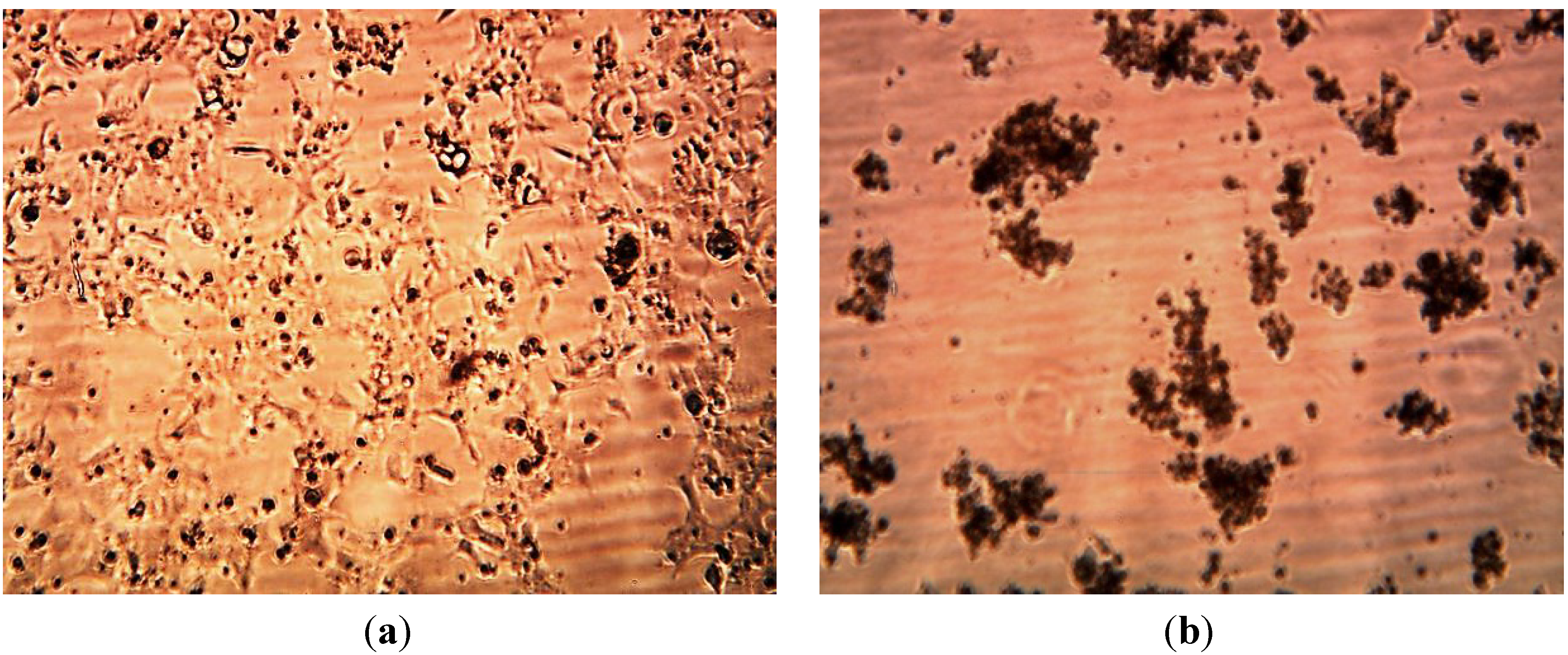
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Jiang, Z.J.; Liu, C.Y.; Sun, L.W. Catalytic properties of silver nanoparticles supported on silica spheres. J. Am. Chem. Soc. 2004, 71, 2341–2343. [Google Scholar]
- Wijnhoven, S.W.P.; Peijnenburg, W.J.G.M.; Herberts, C.A.; Hagens, W.I.; Oomen, A.G.; Heugens, E.H.W.; Roszek, B.; Bisschops, J.; Gosens, I.; van De Meent, D.; et al. Nano-silver—A review ofavailable data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
- Scheringer, M.; MacLeod, M.; Behra, T.; Sigg, L.; Hungerbuhler, K. Environmental risks associated with nanoparticulate silver used as biocide. Household Pers. Care Today 2010, 1, 34–37. [Google Scholar]
- Nowack, B. Nanosilver revisited downstream. Science 2010, 330, 1054–1055. [Google Scholar] [CrossRef]
- Benn, T.M.; Westerhoff, P. Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef]
- Geranio, L.; Heuberger, M.; Nowack, B. Behavior of silver nanotextiles during washing. Environ. Sci. Technol. 2009, 43, 8113–8118. [Google Scholar] [CrossRef]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of silver nanoparticles to chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef]
- Kaegi, R.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Mueller, E.; Vonbank, R.; Boller, M.; Burkhardt, M. Release of silver nanoparticles from outdoor facades. Environ. Pollut. 2010, 158, 2900–2905. [Google Scholar] [CrossRef]
- Rai, M.; Duran, N. Metal Nanoparticles in Microbiology; Springer: Berlin, Germany, 2011. [Google Scholar]
- Bonsak, J.; Mayandi, J.; Thøgersen, A.; Marstein, E.S.; Mahalingam, U. Chemical synthesis of silver nanoparticles for solar cell applications. Phys. Status Solidi C 2011, 8, 924–927. [Google Scholar] [CrossRef]
- Nanoparticles Inspire Plasmonic Solar Cell. Available online: http://images.iop.org/objects/ntw/news/8/3/48/pdf.pdf (accessed on 21 March 2009).
- Technical Articles & Reports on Plastic Industry: Silver Nanoparticles Can Increase Electrical Power Generation of Polymer Solar Cells. Available online: http://www.plastemart.com/Plastic-TechnicalArticle.asp?LiteratureID=1436&Paper=silver-nanoparticles-increase-electrical-power-generation-polymer-solar-cells (accessed on 30 June 2010).
- McFarland, A.D.; van Duyne, R.P. Single silver nanoparticles as real-time optical sensors with zeptomole sensitivity. Nano Lett. 2003, 3, 1057–1062. [Google Scholar] [CrossRef]
- Dwivedi, P.; Narvi, S.S.; Tewari, R.P. Green route to a novel Ag/PLGA bionanocomposite: A self-sterilizing surgical suture biomaterial. Int. J. Adv. Eng. Sci. Technol. 2012, 2, 236–243. [Google Scholar]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 168–171. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, K.D. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011. [Google Scholar] [CrossRef]
- Illingworth, B.; Bianco, R.W.; Weisberg, S. In vivo efficacy of silver-coated fabric against Staphylococcus epidermidis. J. Heart Valve Dis. 2000, 9, 135–141. [Google Scholar]
- Hoffmann, S. Silver sulfadiazine: An antibacterial agent for topical use in burns: A review of the literature. J. Plast. Surg. Hand Surg. 1984, 18, 119–126. [Google Scholar] [CrossRef]
- Lara, H.H.; Ayala-Nunez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010, 8. [Google Scholar] [CrossRef]
- Petrus, E.M.; Tinakumari, S.; Chai, L.C.; Ubong, A.; Tunung, R.; Elexson, N.; Chai, L.F.; Son, R. A study on the minimum inhibitory concentration and minimum bactericidal concentration of nano colloidal silver on food-borne pathogens. Int. Food Res. J. 2011, 18, 55–66. [Google Scholar]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Malik, A.; Sultan, A.; Shahid, M.; Shujatullah, F.; Azam, A. Evaluation of antibacterial activity of silver nanoparticles against MSSA and MRSA on isolates from skin infections. Biol. Med. 2011, 3, 141–146. [Google Scholar]
- Prakash, A.; Sharma, S.; Ahmad, N.; Ghosh, A.; Sinha, P. Synthesis of AgNPs by Bacillus Cereus bacteria and their antimicrobial potential. J.Biomater.Nanobiotechnol. 2011, 2, 156–162. [Google Scholar]
- Egorova, E.M. Interaction of silver nanoparticles with biological objects: Antimicrobial properties and toxicity for the other living organisms. J. Phys. 2011, 291. [Google Scholar] [CrossRef]
- Sadowski, Z. Biosynthesis and Application of Silver and Gold Nanoparticles. Available online: www.intechopen.com/books/silver-nanoparticles/biosynthesis-and-application-of-silver-and-gold-nanoparticles (accessed on 18 December 2012).
- Sathyavathi1, R.; Krishna, M.B.; Rao, S.V.; Saritha, R.; Rao, D.N. Biosynthesis of silver nanoparticles using coriandrum sativum leaf extract and their application in nonlinear optics. Adv. Sci. Lett. 2010, 3, 1–6. [Google Scholar] [CrossRef]
- Virender, K.S.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Gomez, E.; Peralta-Videa, J.R.; Parsons, J.G.; Troiani, H.Y. Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachtaindica (Neem) leaves MJ. Langmuir 2003, 19, 237–246. [Google Scholar]
- Umashankari, J.; Inbakandan, D.; Ajithkumar, T.T.; Balasubramanian, T. Mangrove plant, Rhizophora mucronata (Lamk, 1804) mediated one pot green synthesis of silver nanoparticles and its antibacterial activity against aquatic pathogens. Aquat. Biosy. 2012. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Controlling the optical properties of lemongrass extract synthesized gold nanotriangles and potential application in infrared-absorbing optical coatings. Chem. Mater. 2005, 17, 566–572. [Google Scholar] [CrossRef]
- Richardson, A.; Chan, B.C.; Crouch, R.D.; Janiec, A.; Chan, B.C.; Crouch, R.D. Synthesis of silver nanoparticles: An undergraduate laboratory using green approach. Chem. Educ. 2006, 11, 331–333. [Google Scholar]
- Vaidyanathan, R.; Kalishwaralal, K.; Gopalram, S.; Gurunathan, S. Nanosilver—The burgeoning therapeutic molecule and its green synthesis. Biotechnol. Adv. 2009, 27, 924–937. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Satyajit, D.; Sarker, Y. Maruyama Magnolia: The Genus Magnolia (Medicinal and Aromatic Plants—Industrial Profiles); Taylor& Francis: New York, NY, USA, 2003. [Google Scholar]
- Wolverton, B.C.; Johnson, A.; Bounds, K. Interior Landscape Plants for Indoor Air Pollution Abatement NASA. Available online: http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19930073077_1993073077.pdf (accessed on 18 September 2012).
- Albert, V.A.; Williams, S.E.; Chase, M.W. Carnivorous plants: Phylogeny and structural evolution. Science 1992, 257, 1491–1495. [Google Scholar]
- Shankar, S.S.; Ahmad, A.; Sastry, M. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol. Prog. 2003, 19, 1627–1631. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 2009, 84, 151–157. [Google Scholar] [CrossRef]
- Growth Curves USA. Available online: www.growthcurvesusa.com (accessed on 28 December 2008).
- Labrenz, M.; Druschel, G.K.; Thomsen, E.T.; Gilbert, B.; Welch, S.A.; Kemner, K.M. Formation of sphalerite (ZnS) deposits in natural biofilms of sulfate-reducing bacteria. Science 2000, 1, 744–747. [Google Scholar]
- Roh, Y.; Bai, J.; Lauf, R.J.; Mcmillan, A.D.; Phelps, T.J.; Rawn, C.J. Microbial synthesis of metal-substituted magnetites. Solid State Commun. 2001, 11, 529–534. [Google Scholar]
- Zhang, Y.; Yang, D.; Kong, Y.; Wang, X.; Pandoli, O.; Gao, G. Synergetic antibacterial effects of silver nanoparticles@Aloe Vera prepared via a green method. Nano Biomed. Eng. 2010, 2, 252–257. [Google Scholar]
- Gogoi, S.; Gopinath, P.; Paul, A.; Ramesh, A.; Ghosh, S.; Chattopadhyay, A. Green fluorescent protein-expressing Escherichia coli as a model system for investigating the antimicrobial activities of silver nanoparticles. Langmuir 2006, 22, 9322–9328. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, H.S.; Ryu, D.S.; Choi, S.J.; Lee, D.S. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli Korean. J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Duan, S.S.; Ouyang, Y.S.; Chen, Y.B. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Biometals 2011, 24, 135–141. [Google Scholar] [CrossRef]
- Soo-Hwan, J.; Jung, W.L.; Dengteng, G.; Kai, S.; Takuya, N.; Seong, II Y.; Ashish, A.; Yao, L.; Kotov, N.A. Reversible nanoparticle gels with colour switching. J. Mater. Chem. 2011, 21, 11639–11643. [Google Scholar] [CrossRef]
- Kvitek, L.; Panacek, A.; Soukupova, J.; Kolar, M.; Vecerova, R.; Prucek, R. Effect of surfactant and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J. Phys.Chem. 2008, 112, 5825–5834. [Google Scholar]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Sur, I.; Altunbek, M.; Kahraman, M.; Culha, M. The influence of the surface chemistry of silver nanoparticles on cell death. Nanotechnology 2012. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kim, M.; Park, H.-S.; Shin, U.S.; Gong, M.-S.; Kim, H.-W. Size-dependent cellular toxicity of silver nanoparticles. J. Biomed. Mater. Res. A 2012, 100, 1033–1043. [Google Scholar]
- Kawata, K.; Osawa, M.; Okabe, S. In vitro toxicity of silver nanoparticles at noncytotoxic doses to HepG2 human hepatoma cells. Environ. Sci. Technol. 2009, 43, 6046–6051. [Google Scholar] [CrossRef]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. 2008, 112, 13608–13619. [Google Scholar]
- Bhakat, C.; Chetal, G.; Sarkar, P.; Singh, P.; Babu, S.; Reddy, A. Effects of silver nanoparticles synthesize from ficus benjamina on normal cells and cancer cells. IOSR J. Pharm. Biol. Sci. 2012, 1, 33–36. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Okafor, F.; Janen, A.; Kukhtareva, T.; Edwards, V.; Curley, M. Green Synthesis of Silver Nanoparticles, Their Characterization, Application and Antibacterial Activity. Int. J. Environ. Res. Public Health 2013, 10, 5221-5238. https://doi.org/10.3390/ijerph10105221
Okafor F, Janen A, Kukhtareva T, Edwards V, Curley M. Green Synthesis of Silver Nanoparticles, Their Characterization, Application and Antibacterial Activity. International Journal of Environmental Research and Public Health. 2013; 10(10):5221-5238. https://doi.org/10.3390/ijerph10105221
Chicago/Turabian StyleOkafor, Florence, Afef Janen, Tatiana Kukhtareva, Vernessa Edwards, and Michael Curley. 2013. "Green Synthesis of Silver Nanoparticles, Their Characterization, Application and Antibacterial Activity" International Journal of Environmental Research and Public Health 10, no. 10: 5221-5238. https://doi.org/10.3390/ijerph10105221




