Applying Evidence-Based Medicine in Telehealth: An Interactive Pattern Recognition Approximation
Abstract
:1. Introduction
2. Evidence-Based Medicine Principles and Clinical Guidelines
- Intensive use of the biomedical literature: The integration of biomedical literature with daily practice will allow the decisions of physicians to be based on statistical evidence. To allow that integration, it is necessary that this information be accessible to physicians in an easy and practical way.
- Critical reading of the literature based on personal experience: Due to the high variability of human behavior and multi-pathological patients, it is very usual that patients that have the same illness have different responses to the same treatment. Therefore, the evidence taken from the biomedical literature should be used only as a valid complement to the personal experience of the physician.
- Patient-centered care: The EBM advocates for patient involvement in the care process. The empowerment of patients and informal caregivers not only will allow for a more effective self-care of patients, but also allows for better understanding of their illness, allowing them to prevent disease complications.
3. Evidence-Based Medicine in the Interactive Pattern Recognition Framework
3.1. Interactive Pattern Recognition Framework
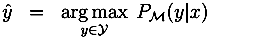
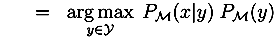
3.2. IPR Approach for EBM
- Post-process approach: The PR system offers a solution, and the expert analyzes and adapts it.
- Interactive approach: The expert is involved in the IPR building process of the solution.
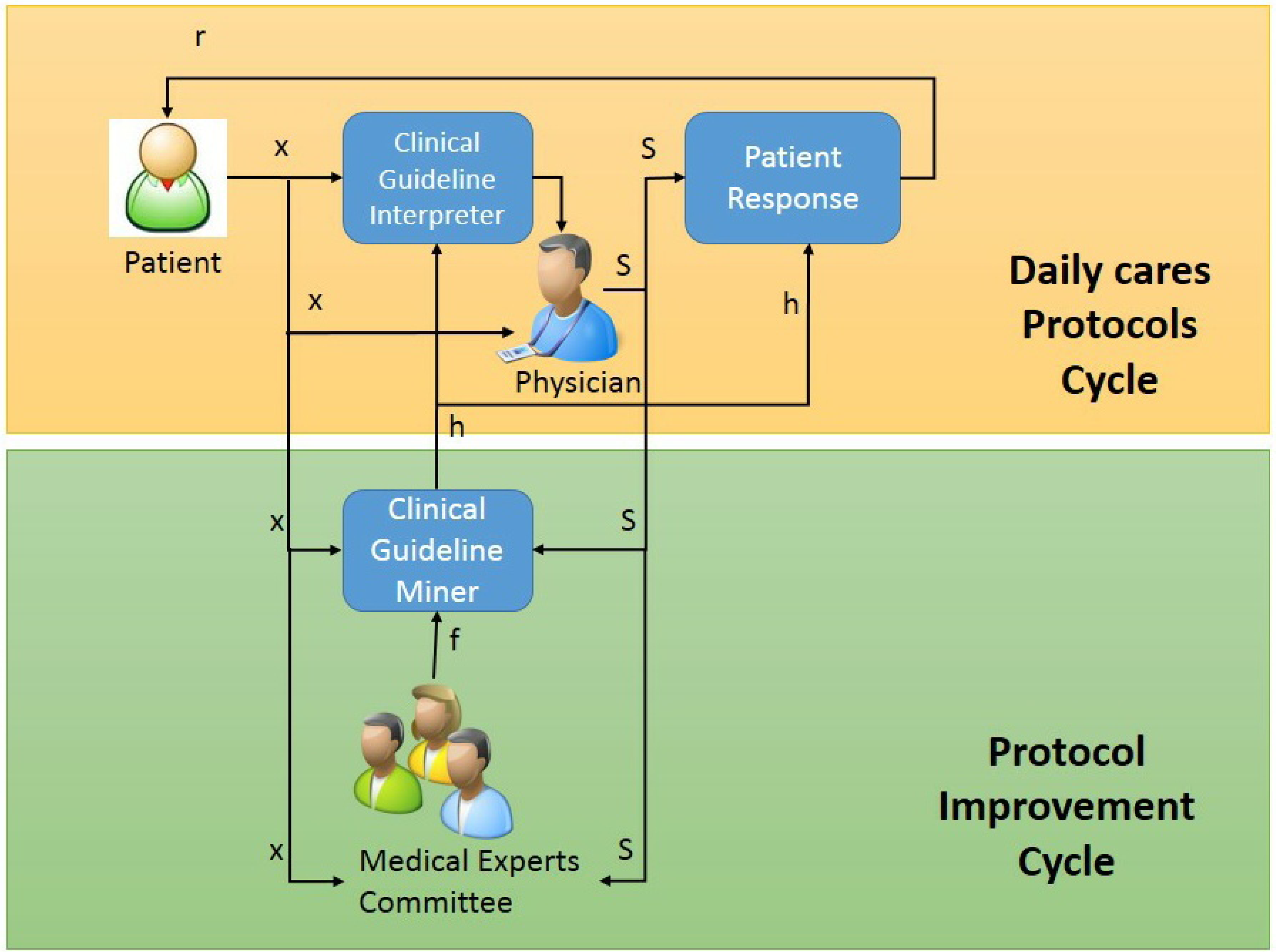
3.2.1. Daily Care Protocol Cycle
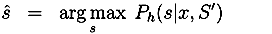
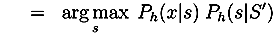
3.2.2. Interactive Protocol Improvement Cycle
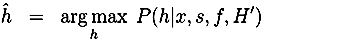
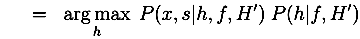
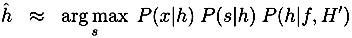
4. Discussion and Conclusion
Acknowledgements
Conflicts of Interest
References
- Kummervold, P.E.; Chronaki, C.E.; Lausen, B.; Prokosch, H.U.; Rasmussen, J.; Santana, S.; Staniszewski, A.; Wangberg, S.C. eHealth trends in Europe 2005–2007: A population-based survey. J. Med. Int. Res. 2008, 10, e42. [Google Scholar] [CrossRef]
- Powell, J.; Inglis, N.; Ronnie, J.; Large, S. The characteristics and motivations of online health information seekers: Cross-sectional survey and qualitative interview study. J. Med. Int. Res. 2011, 13, e20. [Google Scholar] [CrossRef]
- Hughes, B.; Joshi, I.; Lemonde, H.; Wareham, J. Junior physician's use of Web 2.0 for information seeking and medical education: A qualitative study. Int. J. Med. Inf. 2009, 78, 645–655. [Google Scholar] [CrossRef]
- Nickelson, D.W. Telehealth and the evolving health care system: Strategic opportunities for professional psychology. Prof. Psychol. Res. Pract. 1998, 29, 527–535. [Google Scholar] [CrossRef]
- Straus, S.E.; Richardson, W.S.; Glasziou, P.; Haynes, R.B. Evidence-Based Medicine: How to Practice and Teach EBM, 3rd ed.; Churchill Livingstone: London, UK, 2005. [Google Scholar]
- Britton, B.P. First home telehealth clinical guidelines developed by the American Telemedicine Association. Home Healthc. Nurse 2003, 21, 703–706. [Google Scholar] [CrossRef]
- Sackett, D.L.; Rosenberg, W.M.C.; Gray, M.J.A.; Haynes, B.R.; Richardson, S.W. Evidence based medicine: What it is and what it isn't. BMJ 1996, 312, 71–72. [Google Scholar] [CrossRef]
- Goldberger, J.J.; Buxton, A.E. Personalized medicine vs. guideline-based medicine. JAMA J. Am. Med. Assoc. 2013, 309, 2559–2560. [Google Scholar] [CrossRef]
- Romana, H.W. Is evidence-based medicine patient-centered and is patient-centered care evidence-based? Health Serv. Res. 2006, 41, 1–8. [Google Scholar] [CrossRef]
- Elstein, A.S. On the origins and development of evidence-based medicine and medical decision making. Springer 2004, 2004, 184–189. [Google Scholar]
- Field, M.; Lohr, K. Clinical Practice Guidelines: Directions for a New Program, 3rd ed.; National Academy Press: Washington, DC, USA, 1990. [Google Scholar]
- Graham, R.; Mancher, M.; Wolman, D.M.; Greenfield, S.; Steinberg, E. Clinical Practice Guidelines We Can Trust; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Grol, R.; Grimshaw, J. From best evidence to best practice: Effective implementation of change in patients' care. Lancet 2003, 362, 1225–1230. [Google Scholar] [CrossRef]
- Cosby, J.L. Improving patient care: The implementation of change in clinical practice. Qual. Saf. Health Care 2006, 15, 447. [Google Scholar] [CrossRef]
- PubMed Library. National Library of Medicine and The National Institutes of Health PubMed Library. Available online: http://www.pubmed.gov (accessed on 24 October 2013).
- Fisterra. Available online: http://www.fisterra.com/fisterrae/ (accessed on 24 October 2013).
- The Cochrane Collaboration. COCHRANE Library. Available online: http://www.cochrane.org/index.htm (accessed on 24 October 2013).
- Eden, J.; Wheatley, B.; McNeil, B.; Sox, H. Knowing What Works in Health Care: A Roadmap for the Nation; The National Academies Press: Washington, DC, USA, 2008. [Google Scholar]
- Buchan, H.A.; Currie, K.C.; Lourey, E.J.; Duggan, G.R. Australian clinical practice guidelines a national study. Med. J. Aust. 2010, 192, 490–494. [Google Scholar]
- Shaneyfelt TM, C.R. Reassessment of clinical practice guidelines: Go gently into that good night. JAMA 2009, 301, 868–869. [Google Scholar] [CrossRef]
- Fernandez-Llatas, C.; Meneu, T.; Benedi, J.M.; Traver, V. Activity-Based Process Mining for Clinical Pathways Computer Aided Design. In Proceedings of the IEEE Engineering in the 32nd Annual International Conference of the Medicine and Biology Society, Buenos Aires, Argentina, 1–4 September 2010.
- Ashby, D.; Smith, A.F. Evidence-based medicine as Bayesian decision-making. Stat. Med. 2000, 19, 3291–3305. [Google Scholar] [CrossRef]
- Goodman, S.N. Toward evidence-based medical statistics 2: The bayes factor. Ann. Intern. Med. 1999, 130, 1005–1013. [Google Scholar] [CrossRef]
- Duda, R.O.; Hart, P.E.; Stork, D.G. Pattern Classification, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2001. [Google Scholar]
- Toselli, A.H.; Vidal, E.; Casacuberta, F. Multimodal Interactive Pattern Recognition and Applications; Springer-Verlag: Berlin, Germany, 2011. [Google Scholar]
- Sedlmayr, M.; Rose, T.; Rhrig, R.; Meister, M. A Workflow Approach towards GLIF Execution. In Proceedings of the European Conference on Artificial Intelligence (ECAI), Riva del Garda, Italy, 29 August–1 September 2006.
- Fernandez-Llatas, C.; Pileggi, S.F.; Traver, V.; Benedi, J.M. Timed Parallel Automaton: A Mathematical Tool for Defining Highly Expressive Formal Workflows. In Proceedings of the IEEE 2011 Fifth Asia Modelling Symposium AMS, Manila, Philippines, 24–26 May 2011; pp. 56–61.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fernández-Llatas, C.; Meneu, T.; Traver, V.; Benedi, J.-M. Applying Evidence-Based Medicine in Telehealth: An Interactive Pattern Recognition Approximation. Int. J. Environ. Res. Public Health 2013, 10, 5671-5682. https://doi.org/10.3390/ijerph10115671
Fernández-Llatas C, Meneu T, Traver V, Benedi J-M. Applying Evidence-Based Medicine in Telehealth: An Interactive Pattern Recognition Approximation. International Journal of Environmental Research and Public Health. 2013; 10(11):5671-5682. https://doi.org/10.3390/ijerph10115671
Chicago/Turabian StyleFernández-Llatas, Carlos, Teresa Meneu, Vicente Traver, and José-Miguel Benedi. 2013. "Applying Evidence-Based Medicine in Telehealth: An Interactive Pattern Recognition Approximation" International Journal of Environmental Research and Public Health 10, no. 11: 5671-5682. https://doi.org/10.3390/ijerph10115671





