The Role of Environmental Reservoirs in Human Campylobacteriosis
Abstract
:1. Introduction
2. Foodborne Pathogen
2.1. Poultry
2.2. Poultry Production
2.3. Poultry Processing
2.4. Other Foodborne Pathways
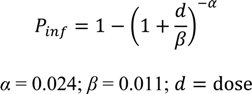
2.5. Public Perception and Food Safety
3. Animal Vectors
3.1. Domestic Animals
3.2. Wild Animal Faeces
4. Density and Fate in Solids
| Source | Density | Units | Reference |
|---|---|---|---|
| Poultry bird faeces | 105 to 108 | CFU g−1 of faeces | [31] |
| Domestic dogs | <103–106 | Copies g−1 of faeces | [52] |
| Wastewater | 1.9–3.2 | Log10 100 mL−1 | [64] |
| Sewage sludge | 1 × 105 | L−1 | [12] |
| Infected humans | 106–108 | g−1 of faeces | [65] |
| Cattle | <105 | Copies g−1 of faeces | [66] |
4.1. Storage of Biosolids and Manure
4.2. Anaerobic: Mesophilic Anaerobic Digestion
5. Sewage
6. Water Sources/Surface Waters
6.1. Groundwater
6.2. Drinking Water
6.3. Campylobacter spp. and Water Disinfection
7. Detection Methods
7.1. Molecular Typing
7.2. Comparison to Faecal Indicators
| Barrier | Source | Conditions | Inactivation | Units | Comparative inactivation | Reference |
|---|---|---|---|---|---|---|
| Solar inactivation | River water + STP effluent | Natural sunlight conditions | 1.65–1.68 | S90 (MJ·m−2) | Higher than E. coli and S. enterica | [62] |
| Sea water + STP effluent | Natural sunlight conditions | 1.28–1.38 | ||||
| Transparent water bottles | Optimal sunlight conditions | 7 (±3) | S90 (kJ·m−2) | Higher than S. epidermidis, E. coli and Y. enterocolitica and B. subtilis | [90] | |
| UV treatment | Potable water | UV fluence of 3 mJ/cm2 | 1 | Log10 | Higher than E. coli | [118] |
| UV fluence of 7 mJ/cm2 | 2 | |||||
| UV fluence of 10 mJ/cm2 | 3 | |||||
| Free chlorine | Potable water | 0.1 mg·L−1 after 5 min contact | 2 | Log10 | Higher than E. coli | [104] |
| Monochloramine | Potable water | 1.0 mg·L−1 after 15 min contact | 2 | |||
| Primary sedimentation | Sewage | 78 | % | [71] | ||
| Trickling filters | Sewage | 0.6 | [64] | |||
| Activated sludge + settling | Sewage | 1–2.5 | Log10 | Lower than E. coli | [61] | |
| 1 | [64] | |||||
| Dark inactivation | Unfiltered lake water | 14 days 4 °C | 100 | % | Higher than E. coli | [92] |
| 8 days at 25 °C | 100 | % | ||||
| 0.2 µm filter lake water | 27 days at 4 °C | 100 | % | |||
| 4 days at 25 °C | 100 | % | ||||
| River water + STP effluent | 120 L chambers | 82.6 | T90 (hours) | Higher than E. coli and S. enterica | [62] | |
| Seawater + STP effluent | 120 L chambers | 35 | T90 (hours) | |||
| Storage | Human biosolids | 49.5 °C for 1 day | 4.6–>6 | log10 | Higher than S. Typhimurium | [68] |
| 38 °C for 6 days | >6 | |||||
| 22 °C for 11 days | >6 | |||||
| 5 °C for 62 days | 2 | |||||
| Farmyard manure | 4 days at 50 °C | 3 | log10 | Higher than Salmonella, Listeria and E. coli | [63] | |
| 32 days 15–20 °C | 3 | |||||
| 4 and 17 °C for 112 days | 0 | log10 | Lower than E. coli, L. monocytogenes, Yersinia enterocolitica and S. typhimurium | [70] | ||
| Land application | Farmyard manure | Sandy arable soils. 4–8 days at 11–20 °C | >3 | log10 | Higher than Salmonella, Listeria and E. coli | [63] |
| Clay loam grassland soils. 8–32 days at 15–20 °C | 2 | |||||
| Bovine manure applied to pasture | Winter | 16 | T90 (days) | Higher than E. coli, fecal streptococci, enterococci and S. enterica | [62] | |
| Spring | 2.7 | |||||
| Summer | 1.2 | |||||
| Autumn | 4.7 | |||||
| Anaerobic digestion | Human biosolids | 22 days at 35 °C | 0 | log10 | Lower than E. coli, L. monocytogenes and S. senftenberg | [69] |
| 25 days at 15 °C | 0.36 | |||||
| Cattle slurry | 793 days at 35 °C | 1 | log10 | Lower than E. coli, L. monocytogenes, Yersinia enterocolitica and S. typhimurium | [70] |
8. Conclusions
Conflicts of Interest
References
- Wilson, D.J.; Gabriel, E.; Leatherbarrow, A.J.H.; Cheesbrough, J.; Gee, S.; Bolton, E.; Fox, A.; Fearnhead, P.; Hart, C.A.; Diggle, P.J. Tracing the source of campylobacteriosis. PLoS Genet. 2008, 4, 1–9. [Google Scholar] [CrossRef]
- Strachan, N.J.; Gormley, F.J.; Rotariu, O.; Ogden, I.D.; Miller, G.; Dunn, G.M.; Sheppard, S.K.; Dallas, J.F.; Reid, T.M.; Howie, H.; et al. Attribution of Campylobacter infections in northeast Scotland to specific sources by use of multilocus sequence typing. J. Infect. Dis. 2009, 199, 1205–1208. [Google Scholar] [CrossRef]
- Sheppard, S.K.; Dallas, J.F.; Strachan, N.J.C.; MacRae, M.; McCarthy, N.D.; Wilson, D.J.; Gormley, F.J.; Falush, D.; Ogden, I.D.; Maiden, M.C.J.; et al. Campylobacter genotyping to determine the source of human infection. Clin. Infect. Dis. 2009, 48, 1072–1078. [Google Scholar] [CrossRef]
- European Food Safety Authority. The community summary report on trends and sources of zoonoses, zoonotic agents, antimicrobial resistance and foodborne outbreaks in the European Union in 2005. EFSA J. 2006, 94, 4–288. [Google Scholar]
- Altekruse, S.F.; Stern, N.J.; Fields, P.I.; Swerdlow, D.L. Campylobacter jejuni—An emerging foodbourne pathogen. Emerg. Infect. Dis. 1999, 5, 28–35. [Google Scholar] [CrossRef]
- Nguyen, H. Acanthamoeba-Campylobacter Interactions; University of Ottawa: Ottowa, ON, Canada, 2001. [Google Scholar]
- Australian National Notifiable Diseases Surveillance System. Number of Notifications for All Diseases by Year, Australia, 1991 to 2009 and Year-to-Date Notifications for 2010; Australian Department of Health and Aging: Adelaide, Australia, 2010.
- Patrick, M.E.; Gilbert, M.J.; Blaser, M.J.; Tauxe, R.V.; Wagenaar, J.A.; Fitzgerald, C. Human infections with new subspecies of Campylobacter fetus. Emerg. Infect. Dis. 2013, 19, 1679–1680. [Google Scholar]
- Ternhag, A.; Törner, A.; Svensson, Å.; Giesecke, J.; Ekdahl, K. Mortality following Campylobacter infection: A registry-based linkage study. BMC Infect. Dis. 2005, 5, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Gillespie, I.A.; O’Brien, S.J.; Bolton, F.J. Age patterns of persons with campylobacteriosis, England and Wales, 1990–2007. Emerg. Infect. Dis. 2009, 15, 2046–2048. [Google Scholar] [CrossRef]
- Mughini Gras, L.; Smid, J.H.; Wagenaar, J.A.; de Boer, A.G.; Havelaar, A.H.; Friesema, I.H.; French, N.P.; Busani, L.; van Pelt, W. Risk factors for campylobacteriosis of chicken, ruminant, and environmental origin: A combined case-control and source attribution analysis. PLoS One 2012, 7, e42599. [Google Scholar] [CrossRef]
- Jones, K. Campylobacters in water, sewage and the environment. Appl. Microbiol. 2001, 90, 68–79. [Google Scholar] [CrossRef]
- Newell, D.G.; Shreeve, J.E.; Toszeghy, M.; Domingue, G.; Bill, S.; Humphrey, T.; Mead, G. Changes in the carriage of Campylobacter strains by poultry carcasses during processing in abattoirs. Appl. Environ. Microbiol. 2001, 67, 2636–2640. [Google Scholar]
- Newell, D.G.; Fearnley, C. Sources of Camplobacter colonization in broiler chickens. Appl. Environ. Microbiol. 2003, 69, 4343–4351. [Google Scholar] [CrossRef]
- Stafford, R.J.; Schluter, P.J.; Wilson, A.J.; Kirk, M.D.; Hall, G.; Unicomb, L. Population-attributable risk estimates for risk factors associated with campylobacter infection, australia. Emerg. Infect. Dis. 2008, 14, 895–901. [Google Scholar] [CrossRef] [Green Version]
- Meldrum, R.J.; Griffiths, J.K.; Smith, R.M.M.; Evans, M.R. The seasonality of human Campylobacter infection and Campylobacter isolates from fresh, retail chicken in Wales. Epidemiol. Infect. 2005, 133, 49–52. [Google Scholar] [CrossRef]
- Mullner, P.; Spencer, S.E.F.; Wilson, D.J.; Jones, G.; Noble, A.D.; Midwinter, A.C.; Collins-Emerson, J.M.; Carter, P.; Hathaway, S.; French, N.P. Assigning the source of human campylobacteriosis in New Zealand: A comparative genetic and epidemiological approach. Infect. Genet. Evol. 2009, 9, 1311–1319. [Google Scholar] [CrossRef]
- Pitkänen, T. Review of Campylobacter spp. in drinking and environmental waters. J. Microbiol. Methods 2013, 95, 39–47. [Google Scholar] [CrossRef]
- French, N.P.; Midwinter, A.; Holland, B.; Collins-Emerson, J.; Pattison, R.; Colles, F.; Carter, P. Molecular epidemiology of Campylobacter jejuni isolates from wild-bird fecal material in children’s playgrounds. Appl. Environ. Microbiol. 2009, 75, 779–783. [Google Scholar]
- Brown, P.E.; Christensen, O.F.; Clough, H.E.; Diggle, P.J.; Hart, C.A.; Hazel, S.; Kemp, R.; Leatherbarrow, A.J.H.; Moore, A.; Sutherst, J.; et al. Frequency and spatial distribution of environmental Campylobacter spp. Appl. Environ. Microbiol. 2004, 70, 6501–6511. [Google Scholar] [CrossRef]
- Jones, A.B.; O’Donohue, M.J.; Udy, J.; Dennison, W.C. Assessing ecological impacts of shrimp and sewage effluent: Biological indicators with standard water quality analyses. Estuar. Coast. Shelf Sci. 2001, 52, 91–109. [Google Scholar] [CrossRef]
- Black, A.P.; Kirk, M.D.; Millard, G. Campylobacter outbreak due to chicken consumption at an australian capital territory restaurant. CDI 2006, 30, 373–377. [Google Scholar]
- Gillespie, I. Population-attributable risk estimates for Campylobacter infection, Australia. Emerg. Infect. Dis. 2009, 15, 850–851. [Google Scholar] [CrossRef]
- Meinersmann, R.J.; Phillips, R.W.; Hiett, K.L.; Fedorka-Cray, P. Differentiation of Campylobacter populations as demonstrated by flagellin short variable region sequences. Appl. Environ. Microbiol. 2005, 71, 6368–6374. [Google Scholar] [CrossRef]
- Stern, N.J.; Fedorka-Cray, P.; Bailey, J.S.; Cox, N.A.; Craven, S.E.; Hiett, K.L.; Musgrove, M.T.; Ladley, S.; Cosby, D.; Mead, G.C. Distribution of Campylobacter spp. In selected U.S. Poultry production and processing operations. J. Food Prot. 2001, 64, 1705–1710. [Google Scholar]
- European Food Safety Authority. Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses in the eu, 2008—Part A: Campylobacter and Salmonella prevalence estimates. EFSA J. 2010, 8, 1503. [Google Scholar] [CrossRef]
- Shanker, S.; Lee, A.; Sorrell, T.C. Horizontal transmission of Campylobacter jejuni amongst broiler chicks: Experimental studies. Epidemiol. Infect. 1990, 104, 101–110. [Google Scholar] [CrossRef]
- Pearson, A.D.; Greenwood, M.H.; Feltham, R.K.A.; Healing, T.D.; Donaldson, J.; Jones, D.M.; Colwell, R.R. Microbial ecology of Campylobacter jejuni in a united kingdom chicken supply chain: Intermittent common source, vertical transmission, and amplification by flock propagation. Appl. Environ. Microbiol. 1996, 62, 4614–4620. [Google Scholar]
- Nichols, G.L. Fly transmission of Campylobacter. Emerg. Infect. Dis. 2005, 11, 361–364. [Google Scholar] [CrossRef]
- Templeton, J.M.; Jong, A.J.D.; Blackall, P.J.; Miflin, J.K. Survival of Campylobacter spp. in darkling beetles (Alphitobius diaperinus) and their larvae in australia. Appl. Environ. Microbiol. 2006, 72, 7909–7911. [Google Scholar] [CrossRef] [Green Version]
- Keener, K.M.; Bashor, M.P.; Curtis, P.A.; Sheldon, B.W.; Kathariou, S. Comprehensive review of Campylobacter and poultry processing. Compr. Rev. Food Sci. Food Saf. 2004, 4, 105–116. [Google Scholar]
- Peyrat, M.B.; Soumet, C.; Maris, P.; Sanders, P. Recovery of Campylobacter jejuni from surfaces of poultry slaughterhouses after cleaning and disinfection procedures: Analysis of a potential source of carcass contamination. Int. J. Food Microbiol. 2008, 124, 188–194. [Google Scholar] [CrossRef]
- Luber, P.; Brynestad, S.; Topsch, D.; Scherer, K.; Bartelt, E. Quantification of Campylobacter species cross-contamination during handling of contaminated fresh chicken parts in kitchens. Appl. Environ. Microbiol. 2006, 72, 66–70. [Google Scholar] [CrossRef]
- Vereen, E., Jr.; Lowrance, R.R.; Cole, D.J.; Lipp, E.K. Distribution and ecology of Campylobacters in coastal plain streams (Georgia, United States of America). Appl. Environ. Microbiol. 2007, 73, 1395–1403. [Google Scholar] [CrossRef]
- Inglis, G.D.; Kalischuk, L.D.; Busz, H.W. Chronic shedding of Campylobacter species in beef cattle. J. Appl. Microbiol. 2004, 97, 410–420. [Google Scholar] [CrossRef]
- Verhoeff-Bakkenes, L.; Jansen, H.A.P.M.; in ’t Veld, P.H.; Beumer, R.R.; Zwietering, M.H.; van Leusden, F.M. Consumption of raw vegetables and fruits: A risk factor for Campylobacter infections. Int. J. Food Microbiol. 2011, 144, 406–412. [Google Scholar] [CrossRef]
- Rapp, D.; Ross, C.M.; Pleydell, E.J.; Muirhead, R.W. Differences in the fecal concentrations and genetic diversities of Campylobacter jejuni populations among individual cows in two dairy herds. Appl. Environ. Microbiol. 2012, 78, 7564–7571. [Google Scholar] [CrossRef]
- Taylor, P.R.; Weinstein, W.M.; Bryner, J.H. Campylobacter fetus infection in human subjects: Association with raw milk. Am. J. Med. 1979, 66, 779–783. [Google Scholar] [CrossRef]
- Evans, M.R. A milk-borne Campylobacter outbreak following an educational farm visit. Epidemiol. Infect. 1996, 117, 457. [Google Scholar] [CrossRef]
- Van den Brandhof, W.; Wagenaar, J.A.; van den Kerkhof, H. An outbreak of campylobacteriosis after drinking unpasteurized milk, 2002, the Netherlands. Int. J. Med. Microbiol. 2003, 293, 548–549. [Google Scholar]
- Teunis, P.; van den Brandhof, W.; Nauta, M.; Wagenaar, J.; van den Kerkhof, H.; van Pelt, W. A reconsideration of the Campylobacter dose-response relation. Epidemiol. Infect. 2005, 133, 583. [Google Scholar] [CrossRef]
- Taylor, E.V.; Herman, K.M.; Ailes, E.C.; Fitzgerald, C.; Yoder, J.S.; Mahon, B.E.; Tauxe, R.V. Common source outbreaks of Campylobacter infection in the USA, 1997–2008. Epidemiol. Infect. 2013, 141, 987–996. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Stanley, K.; Andrew, S.; Jones, K. Use of pulsed-field gel electrophoresis and flagellin gene typing in identifying clonal groups of Campylobacter jejuni and Campylobacter coli in farm and clinical environments. Appl. Environ. Microbiol. 2001, 67, 1429–1436. [Google Scholar] [CrossRef]
- Schouls, L.M.; Reulen, S.; Duim, B.; Wagenaar, J.A.; Willems, R.J.L.; Dingle, K.E.; Colles, F.M.; Embden, J.D.A.V. Comparative genotyping of Campylobacter jejuni by amplified fragment length polymorphism, multilocus sequence typing, and short repeat sequencing: Strain diversity, host range, and recombination. J. Clin. Microbiol. 2003, 41, 15–26. [Google Scholar] [CrossRef]
- Itoh, T.; Saito, K.; Maruyama, T.; Sakai, S.; Ohashi, M.; Oka, A. An outbreak of acute enteritis due to Campylobacter fetus subspecies jejuni at a nursery school of tokyo. Microb. Immunol. 1980, 24, 371–379. [Google Scholar]
- Doorduyn, Y.; van Den Brandhof, W.E.; van Duynhoven, Y.T.H.P.; Breukink, B.J.; Wagenaar, J.A.; van Pelt, W. Risk factors for indigenous Campylobacter jejuni and Campylobacter coli infections in the Netherlands: A case-control study. Epidemiol. Infect. 2010, 138, 1391–1404. [Google Scholar] [CrossRef]
- Miles, S.; Braxton, D.S.; Frewer, L.J. Public perceptions about microbiological hazards in food. Br. Food J. 1999, 101, 744–753. [Google Scholar] [CrossRef]
- Worsfold, D.; Griffith, C.J. Food safety behaviour in the home. Br. Food J. 1997, 99, 97–104. [Google Scholar] [CrossRef]
- Todd, E.C.D. Microbiological safety standards and public health goals to reduce foodborne disease. Meat Sci. 2004, 66, 33–43. [Google Scholar] [CrossRef]
- Scott, E. Food safety and foodborne disease in 21st century homes. Can. J. Infect. Dis. 2003, 14, 277–280. [Google Scholar]
- Lenz, J.; Joffe, D.; Kauffman, M.; Zhang, Y.; LeJeune, J. Perceptions, practices, and consequences associated with foodborne pathogens and the feeding of raw meat to dogs. CVJ 2009, 50, 637–643. [Google Scholar]
- Chaban, B.; Ngeleka, M.; Hill, J. Detection and quantification of 14 Campylobacter species in pet dogs reveals an increase in species richness in feces of diarrheic animals. BMC Microbiol. 2010, 10, 73. [Google Scholar] [CrossRef]
- Baker, J.; Barton, M.; Lanser, J. Campylobacter species in cats and dogs in south Australia. Aust. Vet. J. 1999, 77, 662–666. [Google Scholar] [CrossRef]
- Kothary, M.H.; Babu, U.S. Infective dose of foodborne pathogens in volunteers: A review. J. Food Saf. 2001, 21, 49–68. [Google Scholar] [CrossRef]
- Gras, L.M.; Smid, J.H.; Wagenaar, J.A.; Koene, M.G.J.; Havelaar, A.H.; Friesema, I.H.M.; French, N.P.; Flemming, C.; Galson, J.D.; Graziani, C.; et al. Increased risk for Campylobacter jejuni and C. Coli infection of pet origin in dog owners and evidence for genetic association between strains causing infection in humans and their pets. Epidemiol. Infect. 2013, 141(12), 2526–2535. [Google Scholar] [CrossRef]
- Waldenstrom, J.; Axelsson-Olsson, D.; Olsen, B.; Hasselquist, D.; Griekspoor, P.; Jansson, L.; Teneberg, S.; Svensson, L.; Ellstrom, P. Campylobacter jejuni colonization in wild birds: Results from an infection experiment. PLoS One 2010, 5, 1–8. [Google Scholar]
- Tulve, N.S.; SUGGS, J.C.; McCURDY, T.; HUBAL, E.A.C.; MOYA, J. Frequency of mouthing behavior in young children. J. Expo. Anal. Environ. Epidemiol. 2002, 12, 259–264. [Google Scholar] [CrossRef]
- Wang, C.-M.; Shia, W.-Y.; Jhou, Y.-J.; Shyu, C.-L. Occurrence and molecular characterization of reptilian Campylobacter fetus strains isolated in Taiwan. Vet. Microbiol. 2013, 164, 67–76. [Google Scholar] [CrossRef]
- Stampi, S.; de Luca, G.; Varoli, O.; Zanetti, F. Occurrence, removal and seasonal variation of thermophilic Campylobacters and Arcobacter in sewage sludge. Zentralblatt für Hygiene und Umweltmedizin 1999, 202, 19–27. [Google Scholar]
- Stampi, S.; Varol, O.; Zanetti, F.; Luca, G.D. Arcobacter cryaerophilus and thermophilic Campylobacters in a sewage treatment plant in Italy: Two secondary treatments compared. Epidemiol. Infect. 1993, 110, 633–639. [Google Scholar] [CrossRef]
- Wéry, N.; Lhoutellier, C.; Ducray, F.; Delgenès, J.-P.; Godon, J.-J. Behaviour of pathogenic and indicator bacteria during urban wastewater treatment and sludge composting, as revealed by quantitative PCR. Water Res. 2008, 42, 53–62. [Google Scholar] [CrossRef]
- Sinton, L.W.; Braithwaite, R.R.; Hall, C.H.; Mackenzie, M.L. Survival of indicator and pathogenic bacteria in bovine feces on pasture. Appl. Environ. Microbiol. 2007, 73, 7917–7925. [Google Scholar] [CrossRef]
- Nicholson, F.A.; Groves, S.J.; Chambers, B.J. Pathogen survival during livestock manure storage and following land application. Bioresour. Technol. 2005, 96, 135–143. [Google Scholar] [CrossRef]
- Koenraad, P.M.F.J.; Hazeleger, W.C.; van der Laan, T.; Beumer, R.R.; Rombouts, F.M. Survey of Campylobacter spp. in sewage plants in the Netherlands. Food Microbiol. 1994, 11, 65–73. [Google Scholar] [CrossRef]
- Blaser, M.J.; Hardesty, H.L.; Powers, B.; Wang, W.L. Survival of Campylobacter fetus subsp. jejuni in biological milieus. J. Clin. Microbiol. 1980, 11, 309–313. [Google Scholar]
- Inglis, G.D.; Kalischuk, L.D. Direct quantification of Campylobacter jejuni and Campylobacter lanienae in feces of cattle by real-time quantitative PCR. Appl. Environ. Microbiol. 2004, 70, 2296–2306. [Google Scholar] [CrossRef]
- Nocker, A.; Sossa, P.; Burr, M.; Camper, A. Use of propidium monoazide for live-dead distinction in microbial ecology. Appl. Environ. Microbiol. 2007, 73, 5111–5117. [Google Scholar] [CrossRef]
- Ahmed, A.U.; Sorensen, D.L. Kinetics of pathogen destruction during storage of dewatered biosolids. Water Environ. Res. 1995, 67, 143–150. [Google Scholar] [CrossRef]
- Horan, N.J.; Fletcher, L.; Betmal, S.M.; Wilks, S.A.; Keevil, C.W. Die-off of enteric bacterial pathogens during mesophilic anaerobic digestion. Water Res. 2004, 38, 1113–1120. [Google Scholar] [CrossRef]
- Kearney, T.E.; Larkin, M.J.; Levett, P.N. The effect of slurry storage and anaerobic digestion on survival of pathogenic bacteria. J. Appl. Microbiol. 1993, 74, 86–93. [Google Scholar] [CrossRef]
- Arimi, S.M.; Fricker, C.R.; Park, R.W.A. Occurrence of “thermophilic” campylobacters in sewage and their removal by treatment processes. Epidemiol. Infect. 1988, 101, 279–286. [Google Scholar] [CrossRef]
- Koenraad, P.M.F.J.; Ayling, R.; Hazeleger, W.C.; Romboutst, F.M.; Newell, D.G. The speciation and subtyping of Campylobacter isolates from sewage plants and waste water from a connected poultry abattoir using molecular techniques. Epidemniol. Infect. 1995, 115, 485–494. [Google Scholar] [CrossRef]
- Waage, A.S.; Vardund, T.; Lund, V.; Kapperud, G. Detection of small numbers of Campylobacter jejuni and Campylobacter coli cells in environmental water, sewage, and food samples by a seminested pcr assay. Appl. Environ. Microbiol. 1999, 65, 1636–1643. [Google Scholar]
- Rechenburg, A.; Kistemann, T. Sewage effluent as a source of Campylobacter sp. In a surface water catchment. Int. J. Environ. Health Res. 2009, 19, 239–249. [Google Scholar] [CrossRef]
- Lauria-Filgueiras, A.; Hofer, E. Diversity of Campylobacter isolates from three activated sludge systems. Mem Inst Oswaldo Cruz Rio de Janeiro 1998, 93, 295–298. [Google Scholar] [CrossRef]
- Smith, C.J.; Hopmans, P.; Cook, F.J. Accumulation of Cr, Pb, Cu, Ni, Zn and Cd in soil following irrigation with treated urban effluent in Australia. Environ. Pollut. 1996, 94, 317–323. [Google Scholar] [CrossRef]
- Rattan, R.K.; Datta, S.P.; Chhonkar, P.K.; Suribabu, K.; Singh, A.K. Long-term impact of irrigation with sewage effluents on heavy metal content in soils, crops and groundwater—A case study. Agric. Ecosyst. Environ. 2005, 109, 310–322. [Google Scholar] [CrossRef]
- Betaie, M.; Jones, B.K. Thermophilic Campylobacters in two sewage treatment plants in Libya. Lett. Appl. Microbiol. 1990, 11, 93–95. [Google Scholar] [CrossRef]
- Murphy, C.; Carroll, C.; Jordan, K.N. Environmental survival mechanisms of the foodborne pathogen Campylobacter jejuni. J. Appl. Microbiol. 2006, 100, 623–632. [Google Scholar] [CrossRef]
- Buswell, C.M. Extended survival and persistence of Campylobacter spp. In water and aquatic biofilms and their detection by immunofluorescent-antibody and -rRNA staining. Appl. Environ. Microbiol. 1998, 64, 733–741. [Google Scholar]
- Rollins, D.M.; Colwell, R.R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl. Environ. Microbiol. 1986, 52, 531–538. [Google Scholar]
- Cools, I.; Uyttendaele, M.; Caro, C.; D’Haese, E.; Nelis, H.J.; Debevere, J. Survival of Campylobacter jejuni strains of different origin in drinking water. J. Appl. Microbiol. 2003, 94, 886–892. [Google Scholar] [CrossRef]
- Daczkowska-Kozon, E.; Brzostek-Nowakowska, J. Campylobacter spp. In waters of three main western pomerania water bodies. Int. J. Hyg. Environ. Health 2001, 203, 435–443. [Google Scholar] [CrossRef]
- Horman, A.; Rimhanen-Finne, R.; Maunula, L.; Bonsdorff, C.-H.V.; Torvela, N.; Heikinheimo, A.; Hanninen, M.-L. Campylobacter spp., Giardia spp., Cryptosporidium spp., Noroviruses, and indicator organisms in surface water in southwestern Finland, 2000–2001. Appl. Environ. Microbiol. 2004, 70, 87–95. [Google Scholar] [CrossRef]
- Carter, A.M.; Pacha, R.E.; Clark, G.W.; Williams, E.A. Seasonal occurrence of Campylobacter spp. In surface waters and their correlation with standard indicator bacteria. Appl. Environ. Microbiol. 1987, 53, 523–526. [Google Scholar]
- Obiri-Danso, K.; Jones, K. The effect of a new sewage treatment plant on faecal indicator numbers, Campylobacters and bathing water compliance in morecambe bay. J. Appl. Microbiol. 1999, 83, 603–614. [Google Scholar] [CrossRef]
- Sari Kovats, R. Climate variability and Campylobacter infection: An international study. Int. J. Biometeorol. 2005, 49, 207. [Google Scholar] [CrossRef]
- Davies-Colley, R.J.; Bell, R.G.; Donnison, A.M. Sunlight inactivation of Enterococci and fecal coliforms in sewage effluent diluted in seawater. Appl. Environ. Microbiol. 1994, 60, 2049–2058. [Google Scholar]
- Sinton, L.W.; Davies-Colley, R.J.; Bell, R.G. Inactivation of Enterococci and fecal coliforms from sewage and meatworks effluents in seawater chambers. Appl. Environ. Microbiol. 1994, 60, 2040–2048. [Google Scholar]
- Boyle, M.; Sichel, C.; Fernández-Ibñez, P.; Arias-Quiroz, G.B.; Iriarte-Puña, M.; Mercado, A.; Ubomba-Jaswa, E.; McGuigan, K.G. Bactericidal effect of solar water disinfection under real sunlight conditions. Appl. Environ. Microbiol. 2008, 74, 2997–3001. [Google Scholar] [CrossRef]
- Sinton, L.W.; Hall, C.H.; Lynch, P.A.; Davies-Colley, R.J. Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Appl. Environ. Microbiol. 2002, 68, 1122–1131. [Google Scholar] [CrossRef]
- Korhonen, L.K.; Martikalnon, P.J. Survival of Escherichia coli and Campylobacter jejuni in untreated and filtered lake water. J. Appl. Microbiol. 1991, 71, 379–382. [Google Scholar] [CrossRef]
- Stanley, K.; Cunningham, R.; Jones, K. Isolation of Campylobacter jejuni from groundwater. J. Appl. Microbiol. 1998, 85, 187–191. [Google Scholar] [CrossRef]
- Pearson, A.D.; Greenwood, M.; Healing, T.D.; Rollins, D.; Shahamat, M.; Donaldson, J.; Colwell, R.R. Colonization of broiler chickens by waterborne Campylobacter jejuni. Appl. Environ. Microbiol. 1993, 59, 987–996. [Google Scholar]
- Giessen, A.W.V.D.; Bloemberg, B.P.M.; Ritmeester, W.S.; Tilburg, J.J.H.C. Epidemiological study on risk factors and risk reducing measures for Campylobacter infections in Dutch broiler flocks. Epidemiol. Infect. 1996, 117, 245–250. [Google Scholar] [CrossRef]
- Moore, J.; Caldwell, P.; Millar, B. Molecular detection of Campylobacter spp. in drinking, recreational and environmental water supplies. Int. J. Hyg. Environ. Health 2001, 204, 185–189. [Google Scholar] [CrossRef]
- Kuusi, M.; Klemets, P.; Miettinen, I.; Laaksonen, I.; Sarkkinen, H.; Ha¨nninen, M.L.; Rautelin, H.; Kela, E.; Nuorti, J.P. An outbreak of gastroenteritis from a non-chlorinated community water supply. J. Epidemiol. Community Health 2004, 58, 273–277. [Google Scholar] [CrossRef]
- Smith, A.; Reacher, M.; Smerdon, W.; Adak, G.K.; Nichols, G.; Chalmers, R.M. Outbreaks of waterborne infectious intestinal disease in England and Wales, 1992–2003. Epidemiol. Infect. 2006, 134, 1141–1149. [Google Scholar] [CrossRef]
- Ahmed, W.; Huygens, F.; Goonetilleke, A.; Gardner, T. Real-time PCR detection of pathogenic microorganisms in roof-harvested rainwater in southeast Queensland, Australia. J. Appl. Environ. Microbiol. 2008, 74, 5490–5496. [Google Scholar] [CrossRef] [Green Version]
- Said, B.; Wright, F.; Nichols, G.L.; Reacher, M.; Rutter, M. Outbreaks of infectious disease associated with private drinking water supplies in England and Wales 1970–2000. Epidemiol. Infect. 2003, 130, 469–479. [Google Scholar]
- Daoud, A.K.; Swaileh, K.M.; Hussein, R.M.; Matani, M. Quality assessment of roof-harvested rainwater in the west bank, palestinian authority. J. Water Health 2011, 9, 525–533. [Google Scholar] [CrossRef]
- Savill, M.G.; Hudson, J.A.; Ball, A.; Klena, J.D.; Scholes, P.; Whyte, R.J.; McCormick, R.E.; Jankovic, D. Enumeration of Campylobacter in New Zealand recreational and drinking waters. Appl. Microbiol. 2001, 91, 38–46. [Google Scholar] [CrossRef]
- Merritt, A.; Miles, R.; Bates, J. An outbreak of Campylobacter enteritis on an island resort, north Queensland. CDI 1999, 23, 215–218. [Google Scholar]
- Blaser, M.J.; Smith, P.F.; Wang, W.L.; Hoff, J.C. Inactivation of Campylobacter jejuni by chlorine and monochloramine. Appl. Environ. Microbiol. 1986, 51, 307–311. [Google Scholar]
- Snelling, W.J.; McKenna, J.P.; Lecky, D.M.; Dooley, J.S.G. Survival of Campylobacter jejuni in waterborne protozoa. Appl. Environ. Microbiol. 2005, 71, 5560–5571. [Google Scholar] [CrossRef]
- Brown, M.R.W.; Barker, J. Unexplored reservoirs of pathogenic bacteria: Protozoa and biofilms. Trends Microbiol. 1999, 7, 46–50. [Google Scholar] [CrossRef]
- Lawson, A.J.; Linton, D.; Stanley, J.; Owen, R.J. Polymerase chain reaction detection and speciation of Campylobacter upsaliensis and C. Helveticus in human faeces and comparison with culture techniques. J. Appl. Microbiol. 1997, 83, 375–380. [Google Scholar]
- Kulkarni, S.P.; Lever, S.; Logan, J.M.J. Detection of Campylobacter species: A comparison of culture and polymerase chain reaction based methods. J. Clin. Pathol. 2002, 55, 749–753. [Google Scholar] [CrossRef]
- Maher, M.; Finnegan, C.; Collins, E.; Ward, B.; Carroll, C.; Cormican, M. Evaluation of culture methods and a DNA probe-based pcr assay for detection of campylobacter species in clinical specimens of feces. J. Clin. Microbiol. 2003, 41, 2980–2986. [Google Scholar] [CrossRef]
- Baserisalehi, M.; Bahador, N.; Kapadnis, B.P. A novel method for isolation of Campylobacter spp. From environmental samples, involving sample processing, and blood- and antibiotic-free medium. J. Appl. Microbiol. 2004, 97, 853–860. [Google Scholar] [CrossRef]
- Novitsky, J.A. Degradation of dead microbial biomass in a marine sediment. Appl. Environ. Microbiol. 1986, 52, 504–509. [Google Scholar]
- Rudi, K.; Moen, B.; Dromtorp, S.M.; Holck, A.L. Use of ethidium monoazide and PCR in combination for quantification of viable and dead cells in complex samples. Appl. Environ. Microbiol. 2005, 71, 1018–1024. [Google Scholar] [CrossRef]
- Pan, Y.; Breidt, F. Enumeration of viable Listeria monocytogenes cells by real-time PCR with Propidium monoazide and Ethidium monoazide in the presence of dead cells. Appl. Environ. Microbiol. 2007, 73, 8028–8031. [Google Scholar] [CrossRef]
- Kärenlampi, R.; Rautelin, H.; Schönberg-Norio, D.; Paulin, L.; Hänninen, M.-L. Longitudinal study of finnish Campylobacter jejuni and C. Coli isolates from humans, using multilocus sequence typing, including comparison with epidemiological data and isolates from poultry and cattle. Appl. Environ. Microbiol. 2007, 73, 148–155. [Google Scholar] [CrossRef]
- Lévesque, S.; Frost, E.; Arbeit, R.D.; Michaud, S. Multilocus sequence typing of Campylobacter jejuni isolates from humans, chickens, raw milk, and environmental water in Quebec, Canada. J. Clin. Microbiol. 2008, 46, 3404–3411. [Google Scholar] [CrossRef]
- Sheppard, S.K.; Dallas, J.F.; MacRae, M.; McCarthy, N.D.; Sproston, E.L.; Gormley, F.J.; Strachan, N.J.C.; Ogden, I.D.; Maiden, M.C.J.; Forbes, K.J. Campylobacter genotypes from food animals, environmental sources and clinical disease in Scotland 2005/6. Int. J. Food Microbiol. 2009, 134, 96–103. [Google Scholar] [CrossRef]
- Taboada, E.N.; Clark, C.G.; Sproston, E.L.; Carrillo, C.D. Current methods for molecular typing of Campylobacter species. J. Microbiol. Methods 2013, 95, 24–31. [Google Scholar] [CrossRef]
- Hijnen, W.A.M.; Beerendonk, E.F.; Medema, G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: A review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Whiley, H.; Van den Akker, B.; Giglio, S.; Bentham, R. The Role of Environmental Reservoirs in Human Campylobacteriosis. Int. J. Environ. Res. Public Health 2013, 10, 5886-5907. https://doi.org/10.3390/ijerph10115886
Whiley H, Van den Akker B, Giglio S, Bentham R. The Role of Environmental Reservoirs in Human Campylobacteriosis. International Journal of Environmental Research and Public Health. 2013; 10(11):5886-5907. https://doi.org/10.3390/ijerph10115886
Chicago/Turabian StyleWhiley, Harriet, Ben Van den Akker, Steven Giglio, and Richard Bentham. 2013. "The Role of Environmental Reservoirs in Human Campylobacteriosis" International Journal of Environmental Research and Public Health 10, no. 11: 5886-5907. https://doi.org/10.3390/ijerph10115886





