How Do Grass Species, Season and Ensiling Influence Mycotoxin Content in Forage?
Abstract
:1. Introduction
2. Experimental Section
2.1. Plant Material and Cultivation
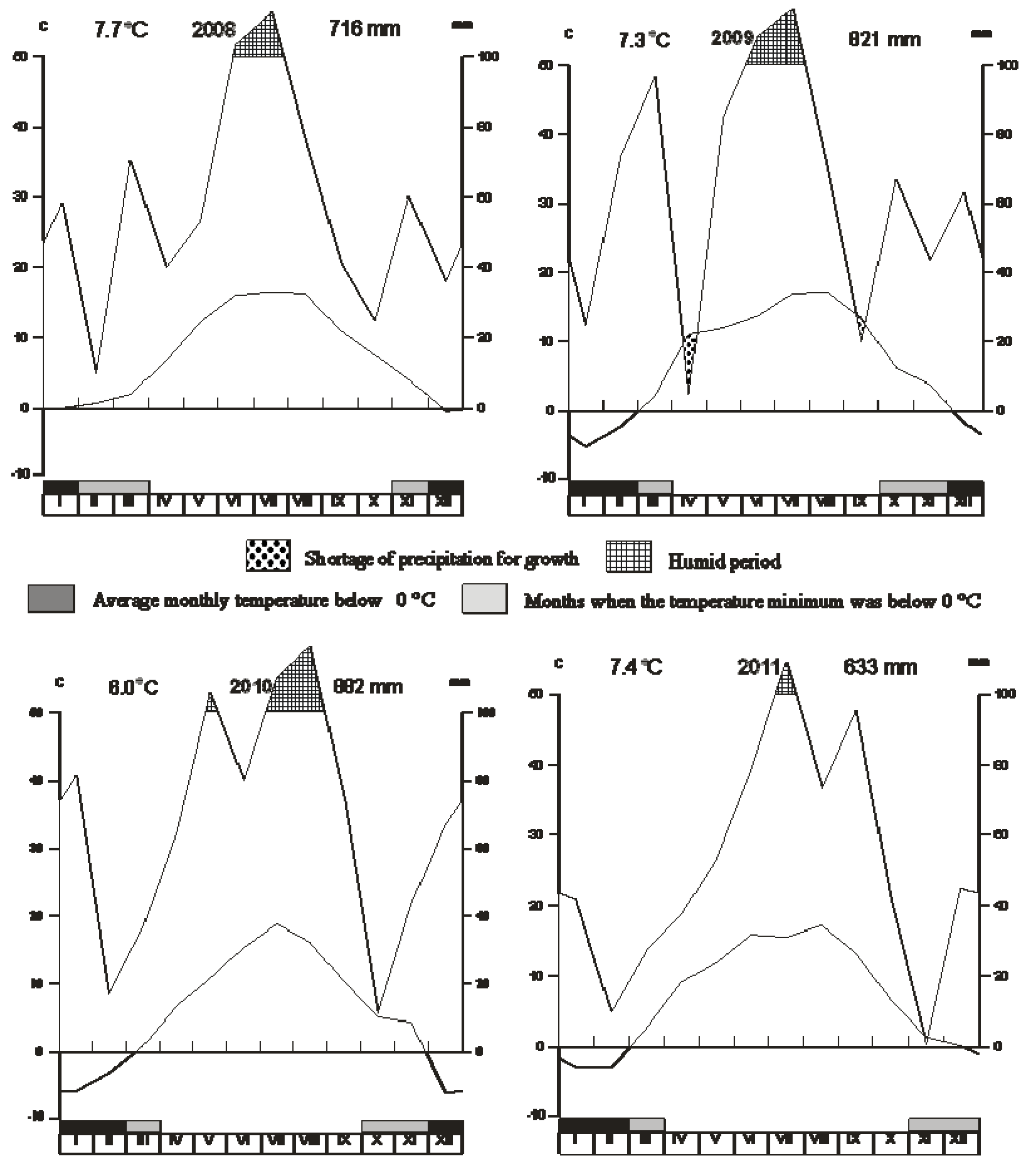
2.2. Fresh-Cut Material and Silages Preparation
2.3. Mycotoxin Determination
3. Results and Discussion
3.1. Fresh-Cut Material
| Factor | DON | FUM | AFL | ZEA | T-2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | S.D. | x | S.D. | x | S.D. | x | S.D. | x | S.D. | |
| Species | ||||||||||
| Lolium perenne | 41.03 | 6.12 | <LOQ | - | <LOQ | 0,01 | 17.06 | 15,52 | 24.80 | 5,76 |
| Festulolium pabulare | 31.02 | 6.27 | <LOQ | - | 0.07 | 0,05 | 4.95 | 2,70 | 24.19 | 6,27 |
| Festulolium braunii | 36.98 | 5.49 | <LOQ | - | <LOQ | 0,01 | 36.45 | 25,05 | 24.94 | 5,33 |
| Mixture with Festuca rubra | 42.15 | 6.72 | <LOQ | - | <LOQ | 0,01 | 47.37 | 31,50 | 30.40 | 6,45 |
| Mixture with Poa pratensis | 40.19 | 7.56 | <LOQ | - | <LOQ | 0,01 | 48.15 | 30,99 | 29.98 | 6,34 |
| p | 0.6347 | - | 0.5288 | 0.4581 | 0.7976 | |||||
| Season (time of cutting) | ||||||||||
| Beginning June | 16.09 a | 3,76 | <LOQ | - | <LOQ | 0,01 | 1.46 | 1,43 | 24.70 | 6,35 |
| End July | 51.90 b | 6,55 | <LOQ | - | 0.09 | 0,05 | 61.18 a | 33,51 | 28.49 | 6,70 |
| Beginning October | 41.94 b | 5,90 | <LOQ | - | <LOQ | 0,01 | 86.55 a | 37,83 | 36.49 | 5,80 |
| Beginning November | 41.58 b | 6,99 | <LOQ | - | <LOQ | 0,01 | 1.88 | 1,80 | 18.25 | 5,72 |
| Beginning December | 39.86 a,b | 5,97 | <LOQ | - | <LOQ | 0,01 | 2.91 | 1,97 | 26.39 | 4,90 |
| p | 0.0004 | - | 0.0176 | 0.0045 | 0.1112 | |||||
| Year | ||||||||||
| 2008 | 37.63 a,b | 7,65 | <LOQ | - | <LOQ | 0.01 | 115.76 a | 37,82 | 34.89 a,b | 5,26 |
| 2009 | 46.28 a | 5,67 | <LOQ | - | 0.08 | 0.04 | 6.15 b | 2,50 | 48.37 b | 3,99 |
| 2010 | 47.13 a | 3,64 | <LOQ | - | <LOQ | 0.01 | <LOQ b | 0,01 | 5.34 c | 3,03 |
| 2011 | 22.06 b | 3,78 | <LOQ | - | <LOQ | 0.01 | 1.23 b | 1,14 | 18.87 a,c | 4,51 |
| p | 0.0019 | - | 0.0138 | 0.0000 | 0.0000 | |||||
| Species × Season | 0.7797 | - | 0.6986 | 0.9950 | 0.9766 | |||||
| Species × Year | 0.9552 | - | 0.3676 | 0.6122 | 0.9906 | |||||
| Season × Year | 0.0000 | - | 0.0518 | 0.0000 | 0.0001 | |||||

3.2. Silages
| Factor | DON | FUM | AFL | ZEA | T-2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | S.D. | x | S.D. | x | S.D. | x | S.D. | x | S.D. | |
| Species | ||||||||||
| Lolium perenne | 141.39 | 6,29 | <LOQ | 0,02 | <LOQ | 0,02 | 66.07 | 10,80 | 20.37 | 11,29 |
| Festulolium pabulare | 156.73 | 19,04 | <LOQ | 0,02 | <LOQ | 0,02 | 47.92 | 7,99 | 45.19 | 25,39 |
| Festulolium braunii | 143.60 | 13,24 | 6.07 | 6,04 | <LOQ | 0,01 | 46.34 | 8,96 | 43.04 | 26,10 |
| Mixture with Festuca rubra | 161.97 | 13,86 | <LOQ | 0,02 | <LOQ | 0.01 | 66.89 | 6,89 | 38.58 | 23,88 |
| Mixture with Poa pratensis | 167.70 | 15,82 | <LOQ | 0,02 | 0.21 | 0,12 | 54.46 | 6,64 | 19.96 | 12,1 |
| p | 0.5142 | 0.4207 | 0.2551 | 0.1577 | 0.8363 | |||||
| Preservative or inoculant | ||||||||||
| Untreated | 139.19 a | 8,91 | <LOQ | 0,01 | <LOQ | 0,01 | 60.28 | 6,35 | 21.81 | 13,64 |
| Chemical | 182.71 b | 12,41 | <LOQ | 3,62 | <LOQ | 0,01 | 53.40 | 8,23 | 21.64 | 11,67 |
| Biological-enzymatic | 140.93 a | 7,22 | 3.66 | 0,01 | 0.14 | 0,08 | 55.33 | 5,23 | 56.83 | 20,14 |
| p | 0.0042 | 0.3765 | 0.4899 | 0.6803 | 0.2137 | |||||
| Year | ||||||||||
| 2008 | 164.61 | 8,70 | <LOQ | 0.01 | <LOQ | 0,01 | 53.95 a,b | 5,59 | 12.67 | 5,22 |
| 2009 | 156.49 | 15,19 | <LOQ | 0.01 | <LOQ | 0,08 | 73.24 a | 6,94 | 42.97 | 11,82 |
| 2010 | 141.73 | 6,81 | 3.72 | 3.62 | 0.15 | 0,01 | 41.81 b | 4,68 | 44.65 | 23,94 |
| p | 0.2553 | 0.3590 | 0.4037 | 0.0016 | 0.2929 | |||||
| Species × Preservative | 0.9502 | 0.4596 | 0.3666 | 0.5255 | 0.3641 | |||||
| Species × Year | 0.4784 | 0.4560 | 0.3753 | 0.9177 | 0.8801 | |||||
| Preservative × Year | 0.0004 | 0.4212 | 0.3174 | 0.0362 | 0.2586 | |||||
| Factor | DON | ZEA | T-2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FCM | S | Rel.% | FCM | S | Rel.% | FCM | S | Rel.% | |
| Lolium perenne | 41.03 | 141.39 | 344.6 | 17.06 | 66.07 | 387.3 | 24.80 | 20.37 | 82.1 |
| Festulolium pabulare | 31.02 | 156.73 | 505.2 | 4.95 | 47.92 | 968.0 | 24.19 | 45.19 | 186.8 |
| Festulolium braunii | 36.98 | 143.60 | 388.3 | 36.45 | 46.34 | 127.1 | 24.94 | 43.04 | 172.6 |
| Mixture with Festuca rubra | 42.15 | 161.97 | 384.3 | 47.37 | 66.89 | 141.2 | 30.40 | 38.58 | 126.9 |
| Mixture with Poa pratensis | 40.19 | 167.70 | 417.3 | 48.15 | 54.46 | 113.1 | 29.98 | 19.96 | 66.6 |
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kreft, H.; Jetz, W.; Mutke, J.; Barthlott, W. Contrasting environmental and regional effects on global pteridophyte and seed plant diversity. Ecography 2010, 33, 408–419. [Google Scholar] [CrossRef]
- Wolf, J.H.D.; Alejandro, F. Patterns in species richness and distribution of vascular epiphytes in Chiapas, Mexico. J. Biogeogr. 2003, 30, 1689–1707. [Google Scholar] [CrossRef]
- Von Boberfeld, W.O.; Banzhaf, K. Yield and forage quality of different xFestulolium cultivars in winter. J. Agron. Crop Sci. 2006, 192, 239–247. [Google Scholar] [CrossRef]
- Rodrigues, I.; Naehrer, K. Prevalence of mycotoxins in feedstuffs and feed surveyed worldwide in 2009 and 2010. Phytopathol. Mediterr. 2012, 51, 175–192. [Google Scholar]
- Yiannikouris, A.; Jouany, J.P. Mycotoxins in feeds and their fate in animals: A review. Anim. Res. 2002, 51, 81–99. [Google Scholar] [CrossRef]
- Cheli, F.; Campagnoli, A.; Dell’Orto, V. Fungal populations and mycotoxins in silages: From occurrence to analysis. Anim. Feed Sci. Technol. 2013, 183, 1–16. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, F. Global burden of aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef]
- Charmley, E.; Trenholm, H.L.; Thompson, B.K.; Vudathala, D.; Nicholson, J.W.G.; Prelusky, D.B.; Charmley, L.L. Influence of levels of deoxynivalenol in the diet of dairy cows on feed intake, milk production, and its composition. J. Dairy Sci. 1993, 76, 3580–3587. [Google Scholar] [CrossRef]
- Afshar, P.; Shokrzadeh, M.; Kalhori, S.; Babaee, Z.; Saravi, S.S.S. Occurrence of Ochratoxin A and Aflatoxin M1 in human breast milk in Sari, Iran. Food Control 2013, 31, 525–529. [Google Scholar] [CrossRef]
- Duarte, S.C.; Almeida, A.M.; Teixeira, A.S.; Pereira, A.L.; Falcao, A.C.; Pena, A.; Lino, C.M. Aflatoxin M-1 in marketed milk in Portugal: Assessment of human and animal exposure. Food Control 2013, 30, 411–417. [Google Scholar] [CrossRef]
- Signorini, M.L.; Gaggiotti, M.; Molineri, A.; Chiericatti, C.A.; de Basilico, M.L.Z.; Basilico, J.C.; Pisani, M. Exposure assessment of mycotoxins in cow’s milk in Argentina. Food Chem. Toxicol. 2012, 50, 250–257. [Google Scholar] [CrossRef]
- Edwards, S.G. Influence of agricultural practices on fusarium infection of cereals and subsequent contamination of grain by trichothecene mycotoxins. Toxicol. Lett. 2004, 153, 29–35. [Google Scholar] [CrossRef]
- Barnes, R.F.; Nelson, J.C.; Moore, K.J.; Collins, M. Forages, 6th ed.; Blackwell Publishing: Ames, IA, USA, 2007; Volume II. [Google Scholar]
- Unger, J. Comparisons of urban and rural bioclimatological conditions in the case of a Central-European city. Int. J. Biometeorol. 1999, 43, 139–144. [Google Scholar] [CrossRef]
- Vollmann, J.; Fritz, C.N.; Wagentristl, H.; Ruckenbauer, P. Environmental and genetic variation of soybean seed protein content under Central European growing conditions. J. Sci. Food Agric. 2000, 80, 1300–1306. [Google Scholar] [CrossRef]
- Skladanka, J.; Nedelnik, J.; Adam, V.; Dolezal, P.; Moravcova, H.; Dohnal, V. Forage as a primary source of mycotoxins in animal diets. Int. J. Environ. Res. Public Health 2011, 8, 37–50. [Google Scholar]
- Behrendt, U.; Stauber, T.; Muller, T. Microbial communities in the phyllosphere of grasses on fenland at different intensities of management. Grass Forage Sci. 2004, 59, 169–179. [Google Scholar] [CrossRef]
- Goertz, A.; Zuehlke, S.; Spiteller, M.; Steiner, U.; Dehne, H.W.; Waalwijk, C.; de Vries, I.; Oerke, E.C. Fusarium species and mycotoxin profiles on commercial maize hybrids in Germany. Eur. J. Plant Pathol. 2010, 128, 101–111. [Google Scholar] [CrossRef]
- Oviedo, M.S.; Ramirez, M.L.; Barros, G.G.; Chulze, S.N. Influence of water activity and temperature on growth and mycotoxin production by Alternaria alternata on irradiated soya beans. Int. J. Food Microbiol. 2011, 149, 127–132. [Google Scholar] [CrossRef]
- Pietri, A.; Battilani, P.; Gualla, A.; Bertuzzi, T. Mycotoxin levels in maize produced in northern Italy in 2008 as influenced by growing location and FAO class of hybrid. World Mycotoxin J. 2012, 5, 409–418. [Google Scholar] [CrossRef]
- DeNijs, M.; Soentoro, P.; Asch, E.D.V.; Kamphuis, H.; Rombouts, F.M.; Notermans, S.H.W. Fungal infection and presence of deoxynivalenol and zearalenone in cereals grown in the Netherlands. J. Food Prot. 1996, 59, 772–777. [Google Scholar]
- Engels, R.; Kramer, J. Incidence of fusaria and occurence of selected Fusarium mycotoxins on Lolium ssp. in Germany. Mycotoxin Res. 1996, 12, 31–40. [Google Scholar] [CrossRef]
- Golinski, P.; von Boberfeld, W.O.; Koztecki, M.; Kaczmarek, Z.; Golinski, P.K. Accumulation of secondary metabolites formed by field fungi in autumn-saved herbage. J. Agron. Crop Sci. 2006, 192, 344–351. [Google Scholar] [CrossRef]
- Driehuis, F. Silage and the safety and quality of dairy foods: A review. Agric. Food Sci. 2013, 22, 16–34. [Google Scholar]
- Holdren, G.C.; Armstrong, D.E. Factors affecting phosphorus release from intact lake sediment cores. Environ. Sci. Technol. 1980, 14, 79–87. [Google Scholar] [CrossRef]
- Sondergaard, M.; Kristensen, P.; Jeppesen, E. Phosphorus release from resuspended sediment in the shallow and wind-exposed lake arreso, Denmark. Hydrobiologia 1992, 228, 91–99. [Google Scholar] [CrossRef]
- D’Mello, J.P.F. Food Safety Contaminants and Toxins; CABI Publishing: Wallingford, CT, USA, 2003; p. 452. [Google Scholar]
- Marasas, W.F.O.; Vanrensburg, S.J.; Mirocha, C.J. Incidence of fusarium species and the mycotoxins, deoxynivalenol and zearalenone, in corn produced in esophageal cancer areas in Transkei. J. Agric. Food Chem. 1979, 27, 1108–1112. [Google Scholar] [CrossRef]
- Dogi, C.A.; Fochesato, A.; Armando, R.; Pribull, B.; de Souza, M.M.S.; Coelho, I.D.; de Melo, D.A.; Dalcero, A.; Cavaglieri, L. Selection of lactic acid bacteria to promote an efficient silage fermentation capable of inhibiting the activity of Aspergillus parasiticus and Fusarium gramineraum and mycotoxin production. J. Appl. Microbiol. 2013, 114, 1650–1660. [Google Scholar] [CrossRef]
- Murata, H.; Yamaguchi, D.; Nagai, A.; Shimada, N. Reduction of deoxynivalenol contaminating corn silage by short-term ultraviolet irradiation: A pilot study. J. Vet. Med. Sci. 2011, 73, 1059–1060. [Google Scholar] [CrossRef]
- Moon, N.J. Inhibition of the growth of acid tolerant yeast by acetate, lactate and propionate and their synergistic mixtures. J. Appl. Bacteriol. 1983, 55, 453–460. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Skladanka, J.; Adam, V.; Dolezal, P.; Nedelnik, J.; Kizek, R.; Linduskova, H.; Mejia, J.E.A.; Nawrath, A. How Do Grass Species, Season and Ensiling Influence Mycotoxin Content in Forage? Int. J. Environ. Res. Public Health 2013, 10, 6084-6095. https://doi.org/10.3390/ijerph10116084
Skladanka J, Adam V, Dolezal P, Nedelnik J, Kizek R, Linduskova H, Mejia JEA, Nawrath A. How Do Grass Species, Season and Ensiling Influence Mycotoxin Content in Forage? International Journal of Environmental Research and Public Health. 2013; 10(11):6084-6095. https://doi.org/10.3390/ijerph10116084
Chicago/Turabian StyleSkladanka, Jiri, Vojtech Adam, Petr Dolezal, Jan Nedelnik, Rene Kizek, Hana Linduskova, Jhonny Edison Alba Mejia, and Adam Nawrath. 2013. "How Do Grass Species, Season and Ensiling Influence Mycotoxin Content in Forage?" International Journal of Environmental Research and Public Health 10, no. 11: 6084-6095. https://doi.org/10.3390/ijerph10116084




