Vector Contact Rates on Eastern Bluebird Nestlings Do Not Indicate West Nile Virus Transmission in Henrico County, Virginia, USA
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Site
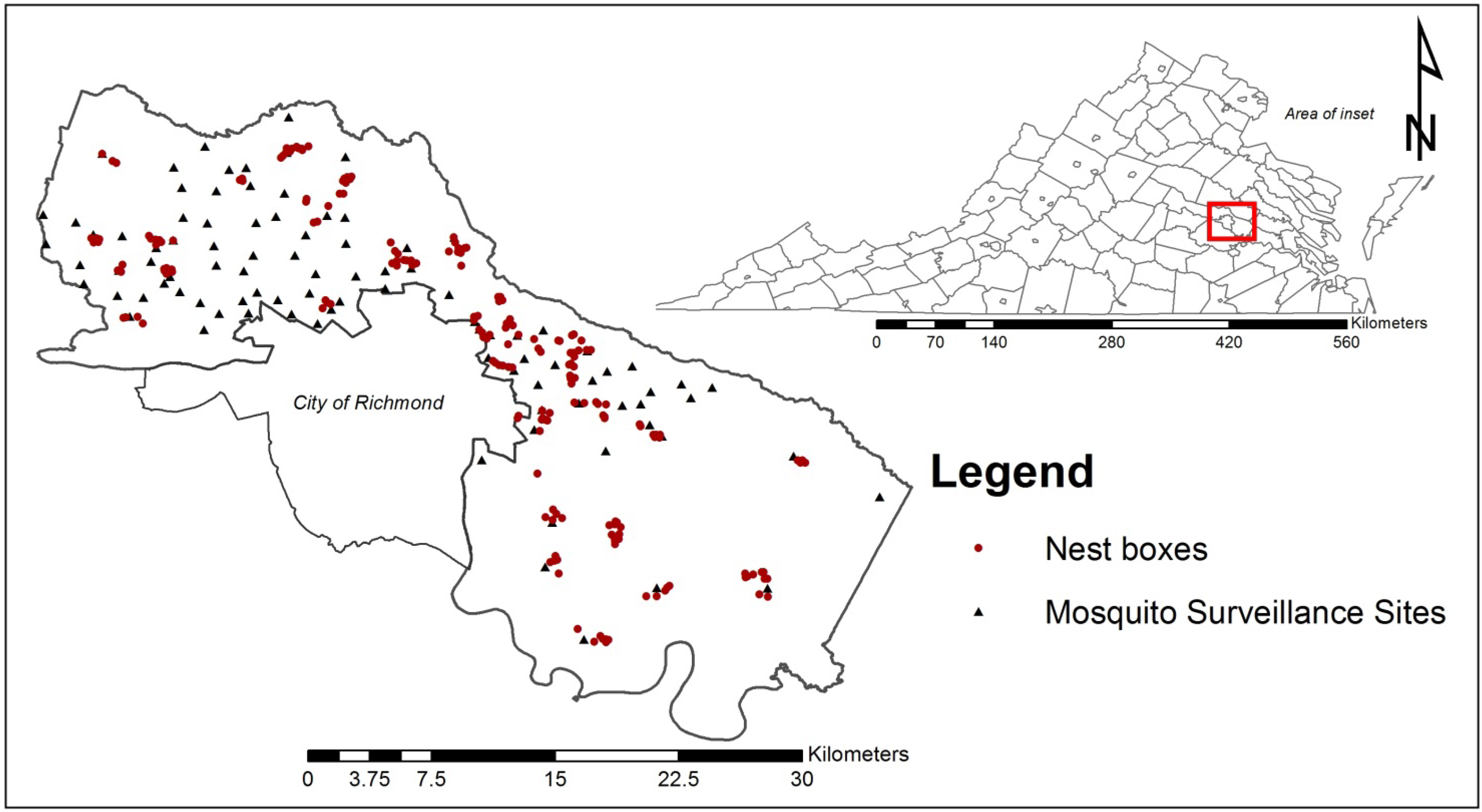
2.2. Nest Box Construction and Placement
2.3. Nest Box Monitoring
2.4. Nestling Swabbing and Virus Testing
2.5. Nest Mosquito Trapping
2.6. Calculation of Estimated Host-Seeking Rate
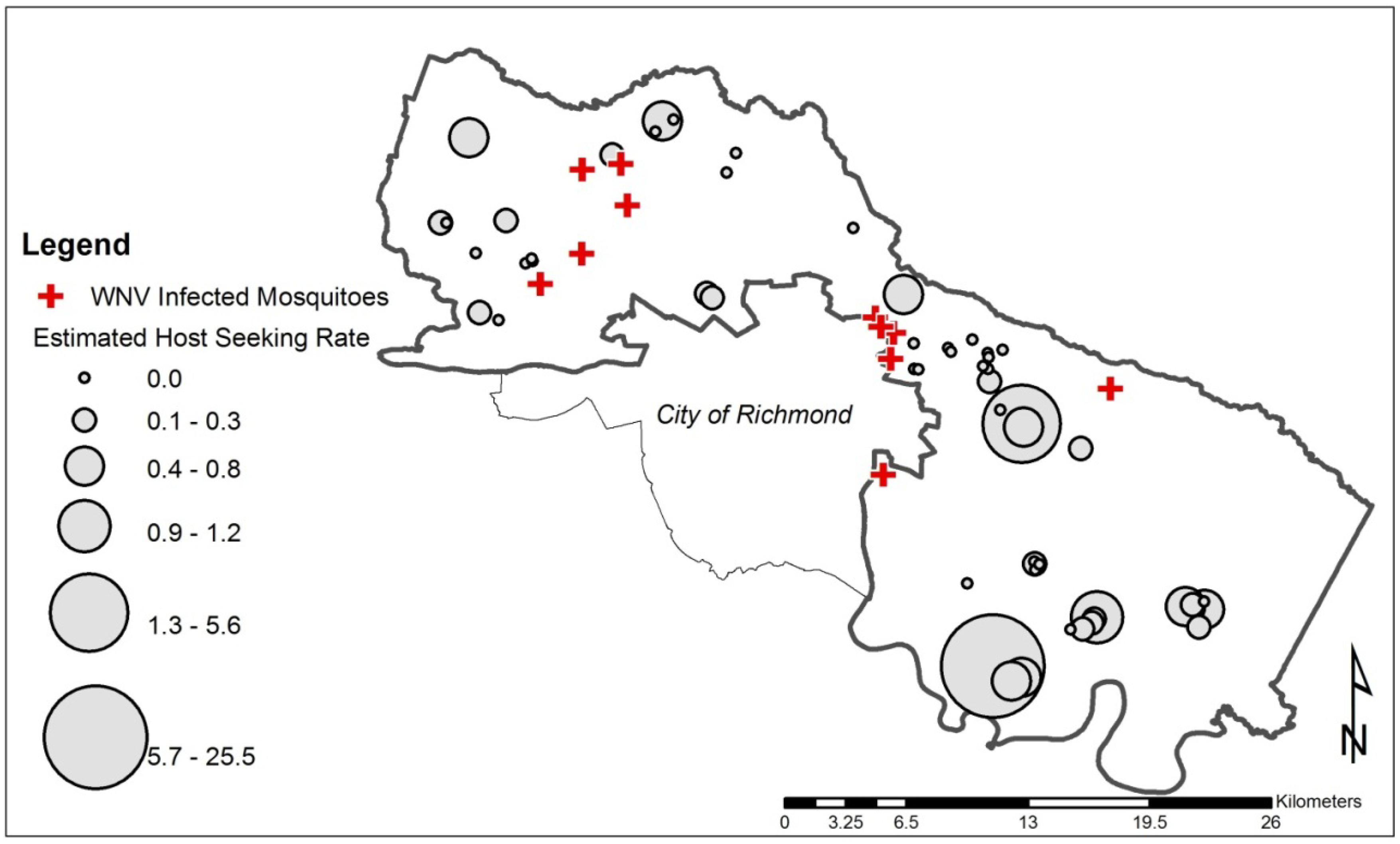
2.7. Ambient Mosquito Abundance
2.8. Statistical Analysis
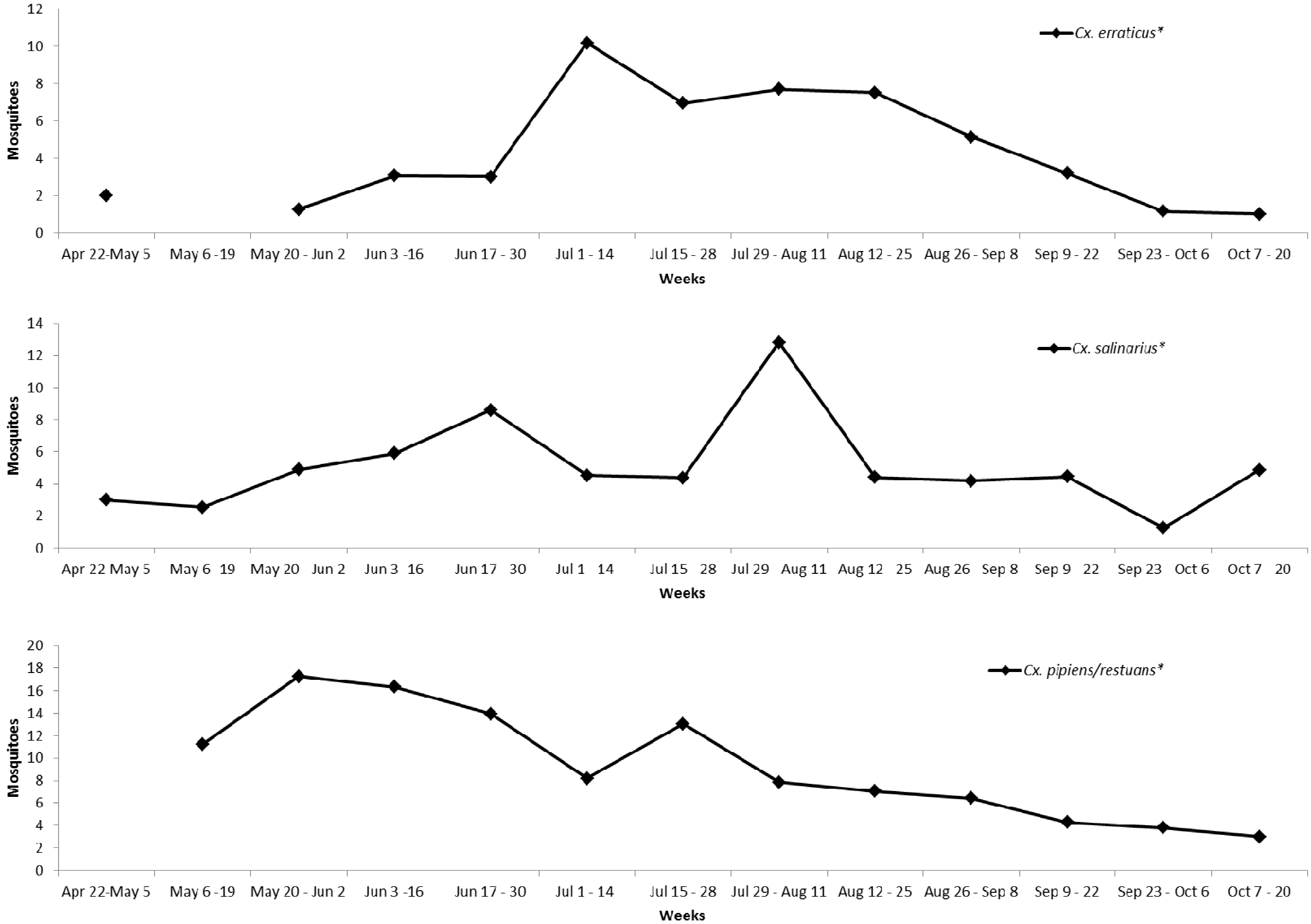
3. Results and Discussion
| Species | Non-Engorged | Gravid | Blood Engorged | Half Gravid/Engorged | ||||
|---|---|---|---|---|---|---|---|---|
| # | % | # | % | # | % | # | % | |
| Aedes albopictus | 1 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aedes hendersoni | 1 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aedes vexans | 4 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anopheles punctipennis | 1 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coquillettidia perturbans | 3 | 75.0 | 0 | 0 | 1 | 25.0 | 0 | 0 |
| Culex erraticus | 36 | 94.7 | 1 | 2.6 | 0 | 0 | 1 | 2.6 |
| Culex pipiens | 3 | 30.0 | 0 | 0 | 7 | 70.0 | 0 | 0 |
| Culex pipiens/restuans | 1 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Culex salinarius | 48 | 90.6 | 0 | 0 | 1 | 1.9 | 4 | 7.5 |
| Culex species | 3 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Culex territans | 1 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bird Species | n (Trap Nights) | Mean Culex spp. eHSR † ± SE |
|---|---|---|
| Eastern Bluebird | 217 | 1.4 ± 0.34 |
| House Sparrow | 4 | 1.8 ± 0.63 |
| House Wren | 4 | 0 ± 0 |
| Tree Swallow | 5 | 9.3 ± 7.81 |
| Collection Round | eHSR Trap Nights 1 | Culex spp. Ehsr 1 | Culex spp. Abundance Trap Nights 2 | Culex spp. Abundance 2 | Nestlings | Culex: Nestlings | IR 3 |
|---|---|---|---|---|---|---|---|
| 1–15 June | 43 | 0.4 ± 0.26 | 161 | 9.9 ± 1.09 | 107.2 ± 2.06 | 0.09 | 1.9 |
| 16–30 June | 88 | 1.4 ± 1.13 | 172 | 10.2 ± 1.35 | 102.3 ± 3.75 | 0.1 | 0 |
| 1–13 July | 34 | 0.1 ± 0.08 | 137 | 7.5 ± 1.21 | 33.9 ± 2.59 | 0.22 | 0 |
| 14–28 July | 39 | 0.2 ± 0.08 | 187 | 9.7 ± 1.26 | 36.1 ± 1.27 | 0.27 | 1.7 |
| 29 July–11 August | 24 | 0.1 ± 0.10 | 171 | 9.0 ± 1.95 | 19.4 ± 3.35 | 0.47 | 7.9 |
| 12–26 August | 2 | 0 ± 0 | 206 | 5.9 ± 0.83 | 1.4 ± 0.53 | 4.15 | 2.1 |
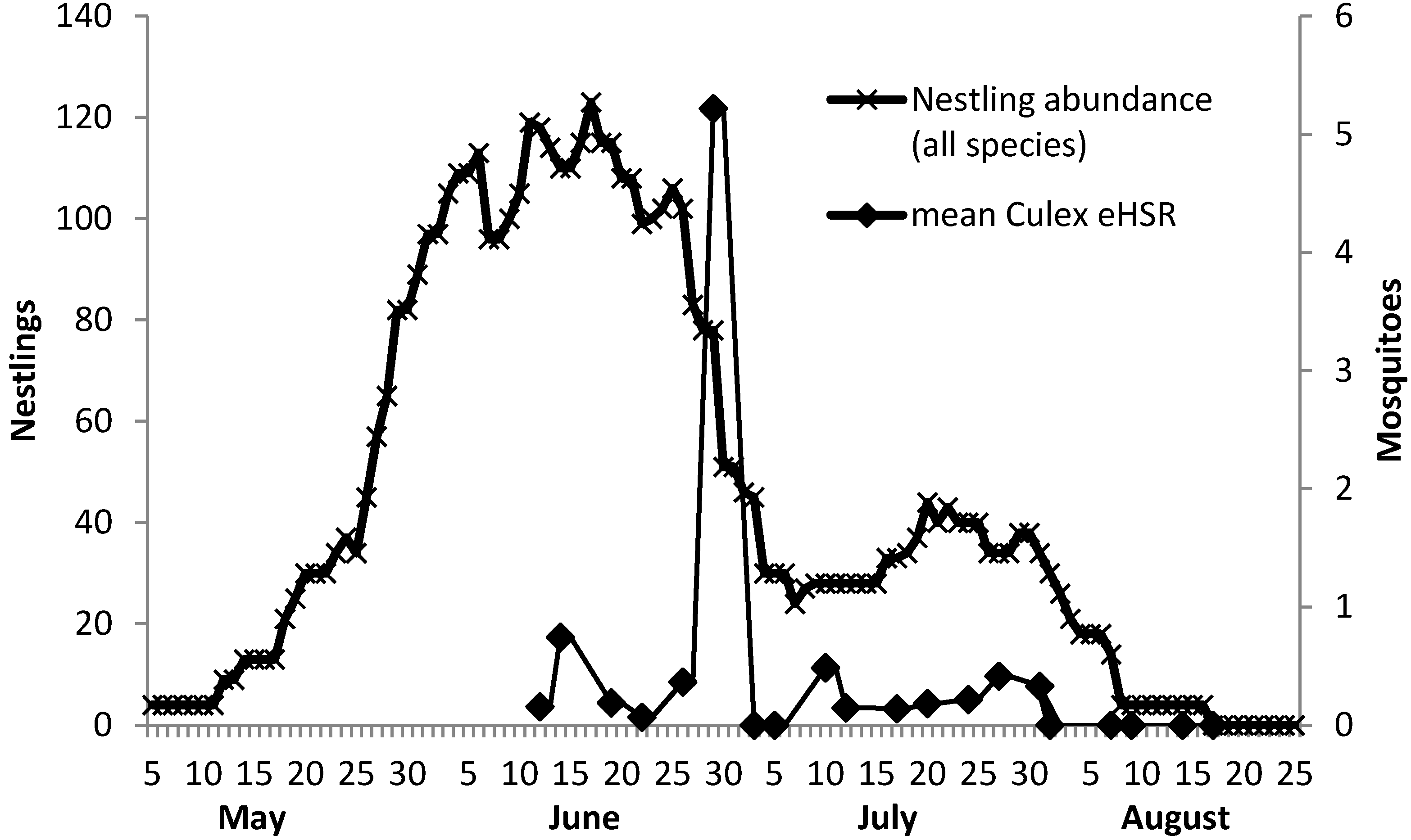
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kilpatrick, A.M.; Daszak, P.; Jones, M.J.; Marra, P.P.; Kramer, L.D. Host heterogeneity dominates West Nile virus transmission. Proc. R. Soc. B 2006, 273, 2327–2333. [Google Scholar] [CrossRef]
- Hamer, G.L.; Kitron, U.D.; Goldberg, T.L.; Brawn, J.D.; Loss, S.R.; Ruiz, M.O.; Hayes, D.B.; Walker, E.D. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am. J. Trop. Med. Hyg. 2009, 80, 268–278. [Google Scholar]
- Hamer, G.L.; Chaves, L.F.; Anderson, T.K.; Kitron, U.D.; Brawn, J.D.; Ruiz, M.O.; Loss, S.R.; Walker, E.D.; Goldberg, T.L. Fine-scale variation in vector host use and force of infection drive localized patterns of West Nile virus transmission. PLoS One 2011, 6, e23767. [Google Scholar] [CrossRef]
- Kent, R.; Juliusson, L.; Weissmann, M.; Evans, S.; Komar, N. Seasonal blood-feeding behavior of Culex tarsalis (Diptera: Culicidae) in Weld County, Colorado, 2007. J. Med. Entomol. 2009, 46, 380–390. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Ladeau, S.L.; Marra, P.P. Ecology of West Nile virus transmission and its impact on birds in the Western Hemisphere. Auk 2007, 124, 1121–1136. [Google Scholar] [CrossRef]
- Hill, G.E.; Siefferman, L.; Liu, M.; Hassan, H.; Unnasch, T.R. The effects of West Nile virus on the reproductive success and overwinter survival of eastern bluebirds in Alabama. Vector-Borne Zoonotic Dis. 2010, 10, 159–163. [Google Scholar] [CrossRef]
- Carleton, R.E.; Mertins, J.W.; Yabsley, M.J. Parasites and pathogens of eastern bluebirds (Sialia sialis): A field survey of a population nesting within a grass-dominated agricultural habitat in Georgia, U.S.A., with a review of previous records. Comp. Parasitol. 2012, 79, 30–43. [Google Scholar] [CrossRef]
- Gibbs, S.E.J.; Allison, A.B.; Yabsley, M.J.; Mead, D.G.; Wilcox, B.R.; Stallknecht, D.E. West Nile virus antibodies in avian species of Georgia, USA: 2000–2004. Vector-Borne Zoonotic Dis. 2006, 6, 57–72. [Google Scholar] [CrossRef]
- Shelite, T.R.; Rogers, C.M.; Litzner, B.R.; Johnson, R.R.; Schneegurt, M.A. West Nile virus antibodies in permanent resident and overwintering migrant birds in south-central Kansas. Vector-Borne Zoonotic Dis. 2008, 8, 321–330. [Google Scholar] [CrossRef]
- Roberts, J.F.; Vickers, M.L.; Sells, S.F.; Watson, D.L.; Billings, S.K.; Jackson, C.B.; Palmer-Ball, B.L., Jr.; Harrison, L.R.; Bolin, D.C.; Giles, R.C.; Sebastian, M. Passive surveillance of West Nile virus from wild birds of Kentucky, 2002. Ky. Warbler 2003, 79, 53–59. [Google Scholar]
- Scott, T.W.; Edman, J.D. Effects of Avian Host Age and Arbovirus Infection on Mosquito Attraction and Blood-Feeding Success; Oxford University Press: New York, NY, USA, 1991. [Google Scholar]
- Day, J.F.; Stark, L.M. Avian serology in a St. Louis encephalitis epicenter before, during, and after a widespread epidemic in South Florida, USA. J. Med. Entomol. 1999, 36, 614–624. [Google Scholar]
- Shaman, J.; Day, J.F.; Stieglitz, M. Drought-induced amplification of Saint Louis encephalitis virus, Florida. Emerg. Infect. Dis. 2002, 8, 575–580. [Google Scholar] [CrossRef]
- Diaz, L.A.; Flores, F.S.; Quaglia, A.; Contigiani, M.S. Intertwined arbovirus transmission activity: Reassessing the transmission cycle paradigm. Front. Physiol. 2013, 3, 493. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Kramer, L.D.; Jones, M.J.; Marra, P.P.; Daszak, P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006, 4, e82. [Google Scholar] [CrossRef]
- Griffing, S.M.; Kilpatrick, A.M.; Clark, L.; Marra, P.P. Mosquito landing rates on nesting American robins (Turdus migratorius). Vector-Borne Zoonotic Dis. 2007, 7, 437–443. [Google Scholar] [CrossRef]
- Caillouët, K.A.; Riggan, A.E.; Rider, M.; Bulluck, L.P. Nest Mosquito Trap quantifies contact rates between nesting birds and mosquitoes. J. Vector Ecol. 2012, 37, 210–215. [Google Scholar] [CrossRef]
- Buchanan, R.; Carr, L. Henrico Standing Water Initiative, Henrico, VA, USA. Henrico County, Virginia mosquito surveillance from 2003–2013. Unpublished work. 2013. [Google Scholar]
- Beaty, B.J.; Calisher, C.H.; Shope, R.E. Arboviruses. In Diagnostic Procedures for Viral, Rickettsial, and Chlamydial Infections, 7th ed.; Lennette, E.H., Lennette, D.A., Lennette, E.T., Eds.; American Public Health Association: Washington, DC, USA, 1995; pp. 189–212. [Google Scholar]
- Slaff, M.E.; Apperson, C.S.; Rogers, M.L. A Key to the Mosquitoes of North Carolina and the Mid-Atlantic States; North Carolina State University: Raleigh, NC, USA, 1989. [Google Scholar]
- Mosquito Surveillance Software. Available online: http://www.cdc.gov/westnile/resourcepages/mosqSurvSoft.html (accessed on 18 November 2013).
- The R Project for Statistiacl Computing. Available online: http://www.r-project.org. (accessed on 18 November 2013).
- Winters, A.M.; Eisen, R.J.; Lozano-Fuentes, S.; Moore, C.G.; Pape, W.J.; Eisen, L. Predictive spatial models for risk of West Nile virus exposure in eastern and western Colorado. Am. J. Trop. Med. Hyg. 2008, 79, 581–590. [Google Scholar]
- Patnaik, J.L.; Juliusson, L.; Vogt, R.L. Environmental predictors of human West Nile virus infections, Colorado. Emerg. Infect. Dis. 2007, 13, 1788–1790. [Google Scholar] [CrossRef]
- Brownstein, J.S.; Holford, T.R.; Fish, D. Enhancing West Nile virus surveillance, United States. Emerg. Infect. Dis. 2004, 10. [Google Scholar] [CrossRef]
- Chuang, T.-W.; Wimberly, M.C. Remote sensing of climatic anomalies and West Nile virus incidence in the northern great plains of the United States. PLoS One 2012, 7, e46882. [Google Scholar] [CrossRef]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef]
- Bunning, M.L.; Bowen, R.A.; Cropp, C.B.; Sullivan, K.G.; Davis, B.S.; Komar, N.; Godsey, M.S.; Baker, D.; Hettler, D.L.; Holmes, D.A.; Biggerstaff, B.J.; Mitchell, C.J. Experimental infection of horses with West Nile virus. Emerg. Infect. Dis. 2002, 8, 380–386. [Google Scholar] [CrossRef]
- Austgen, L.E.; Bowen, R.A.; Bunning, M.L.; Davis, B.S.; Mitchell, C.J.; Chang, G.-J.J. Experimental infection of cats and dogs with West Nile virus. Emerg. Infect. Dis. 2004, 10. [Google Scholar] [CrossRef]
- Bowen, R.A.; Nemeth, N.M. Experimental infections with West Nile virus. Curr. Opin. Infect. Dis. 2007, 20, 293–297. [Google Scholar] [CrossRef]
- Nemeth, N.M.; Bowen, R.A. Dynamics of passive immunity to West Nile virus in domestic chickens (Gallus gallus domesticus). Am. J. Trop. Med. Hyg. 2007, 76, 310–317. [Google Scholar]
- Hamer, G.L.; Walker, E.D.; Brawn, J.D.; Loss, S.R.; Ruiz, M.O.; Goldberg, T.L.; Schotthoefer, A.M.; Brown, W.M.; Wheeler, E.; Kitron, U.D. Rapid amplification of West Nile virus: The role of hatch-year birds. Vector-Borne Zoonotic Dis. 2008, 8, 57–68. [Google Scholar] [CrossRef]
- Loss, S.R.; Hamer, G.L.; Goldberg, T.L.; Ruiz, M.O.; Kitron, U.D.; Walker, E.D.; Brawn, J.D. Nestling passerines are not important hosts for amplification of West Nile Virus in Chicago, Illinois. Vector-Borne Zoonotic Dis. 2009, 9, 13–18. [Google Scholar] [CrossRef]
- Austin, R.J.; Whiting, T.L.; Anderson, R.A.; Drebot, M.A. An outbreak of West Nile virus-associated disease in domestic geese (Anser anser domesticus) upon initial introduction to a geographic region, with evidence of bird to bird transmission. Can. Vet. J. 2004, 45, 117–123. [Google Scholar]
- Millins, C.; Reid, A.; Curry, P.; Drebot, M.A.; Andonova, M.; Buck, P.; Leighton, F.A. Evaluating the use of house sparrow nestlings as sentinels for West Nile virus in Saskatchewan. Vector-Borne Zoonotic Dis. 2011, 11, 53–58. [Google Scholar]
- Caillouët, K.A.; Riggan, A.E.; Bulluck, L.P.; Carlson, J.C.; Sabo, R.T. Nesting bird “host funnel” increases mosquito-bird contact rate. J. Med. Entomol. 2013, 50, 462–466. [Google Scholar] [CrossRef]
- Burkett-Cadena, N.D.; McClure, C.J.W.; Ligon, R.A.; Graham, S.P.; Guyer, C.; Hill, G.E.; Ditchkoff, S.S.; Eubanks, M.D.; Hassan, H.K.; Unnasch, T.R. Host reproductive phenology drives seasonal patterns of host use in mosquitoes. PLoS One 2011, 6, e17681. [Google Scholar] [CrossRef]
Appendix
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Caillouët, K.A.; Robertson, C.W.; Wheeler, D.C.; Komar, N.; Bulluck, L.P. Vector Contact Rates on Eastern Bluebird Nestlings Do Not Indicate West Nile Virus Transmission in Henrico County, Virginia, USA. Int. J. Environ. Res. Public Health 2013, 10, 6366-6379. https://doi.org/10.3390/ijerph10126366
Caillouët KA, Robertson CW, Wheeler DC, Komar N, Bulluck LP. Vector Contact Rates on Eastern Bluebird Nestlings Do Not Indicate West Nile Virus Transmission in Henrico County, Virginia, USA. International Journal of Environmental Research and Public Health. 2013; 10(12):6366-6379. https://doi.org/10.3390/ijerph10126366
Chicago/Turabian StyleCaillouët, Kevin A., Charles W. Robertson, David C. Wheeler, Nicholas Komar, and Lesley P. Bulluck. 2013. "Vector Contact Rates on Eastern Bluebird Nestlings Do Not Indicate West Nile Virus Transmission in Henrico County, Virginia, USA" International Journal of Environmental Research and Public Health 10, no. 12: 6366-6379. https://doi.org/10.3390/ijerph10126366




