West Nile Virus State of the Art Report of MALWEST Project
Abstract
:1. Introduction
2. Methods
3. WNV in Humans
3.1. General Aspects—Molecular Epidemiology
3.1.1. Taxonomy
3.1.2. The Virion
3.1.3. Molecular Epidemiology

3.2. Epidemiology of WNV in Humans
3.2.1. Worldwide WNV Epidemiology
| Year | Country/State | Cases | Deaths | Reference |
|---|---|---|---|---|
| 1951 | Israel | 123 | 0 | [54] |
| 1962 | France | Several encephalitis cases | [55] | |
| 1964 | France | 15 | 1 | [55] |
| 1974 | South Africa | 6 | [56] | |
| 1983–1984 | South Africa | 5 | [57] | |
| 1994 | Algeria | 50 | 8 | [58] |
| 1996 | Morocco | 1 | 1 | [59] |
| 1996 | Romania | 393 | 17 | [5,72] |
| 1997 | Tunisia | 173 | 8 | [55] |
| 1999 | Russia | 183 | 40 | [60] |
| 1999 | New York State | 62 | 7 | [62] |
| 2000 | Israel | 428 | 42 | [66] |
| 2000–2001 | Russia | 120 | [59] | |
| 2002 | Canada | 414 | [65] | |
| 2004 | California | 778 | 28 | [64] |
| 2008 | Hungary | 22 | [67] | |
| 2009 | Hungary | 9 | [67] | |
| 2008–2009 | Italy | 26 | [68] | |
| 2010 | Greece | 262 | 35 | [7] |
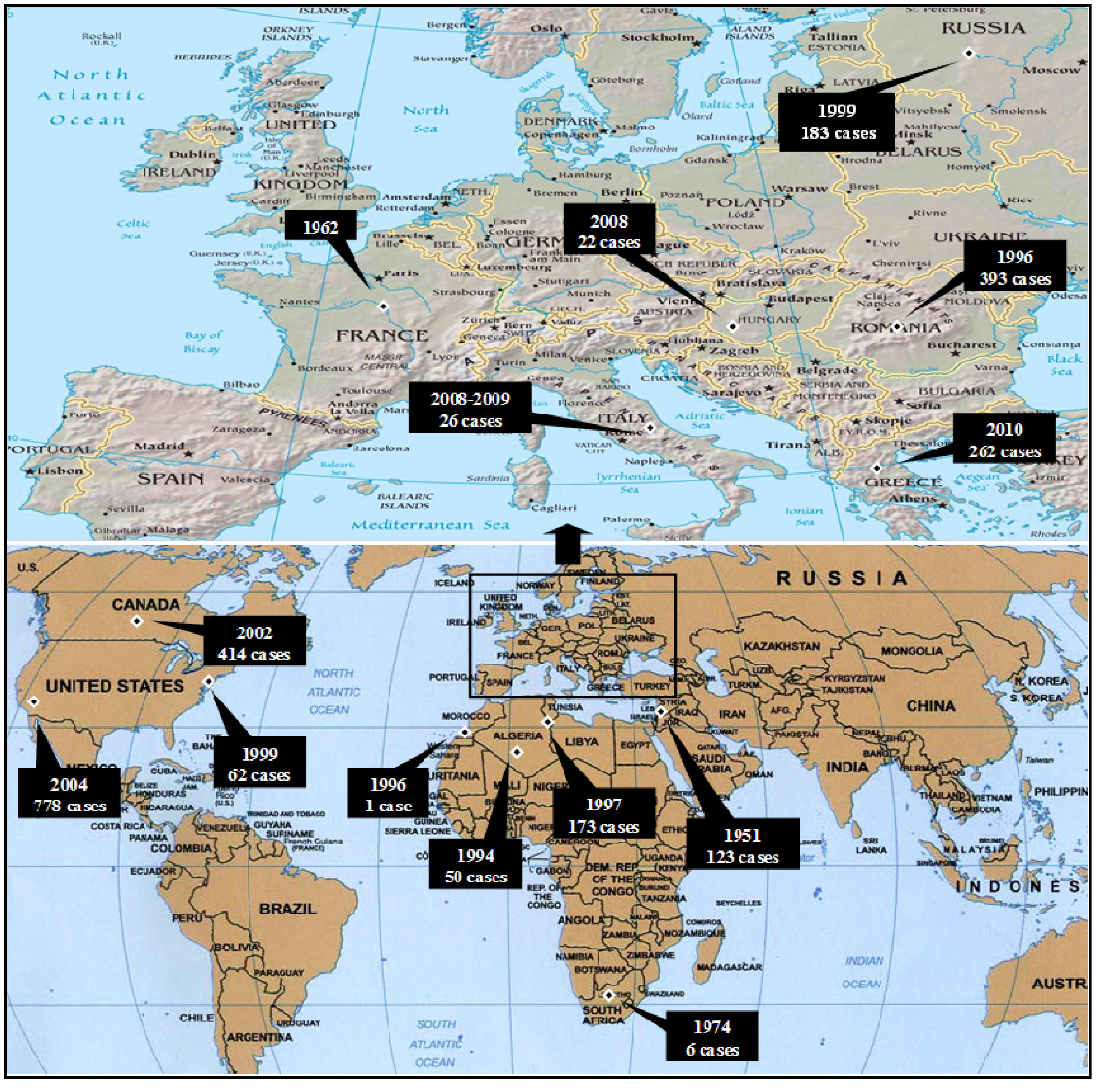
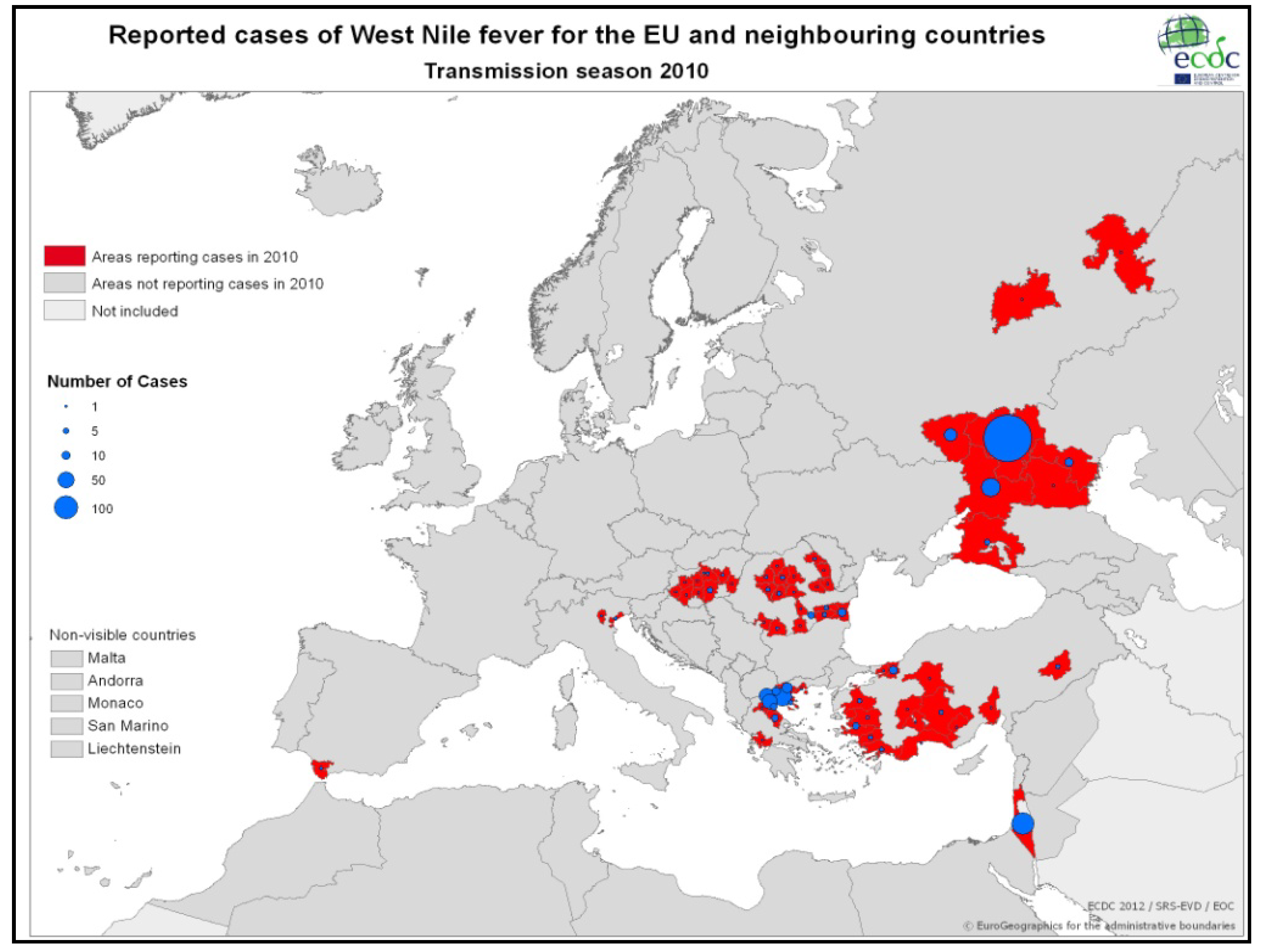
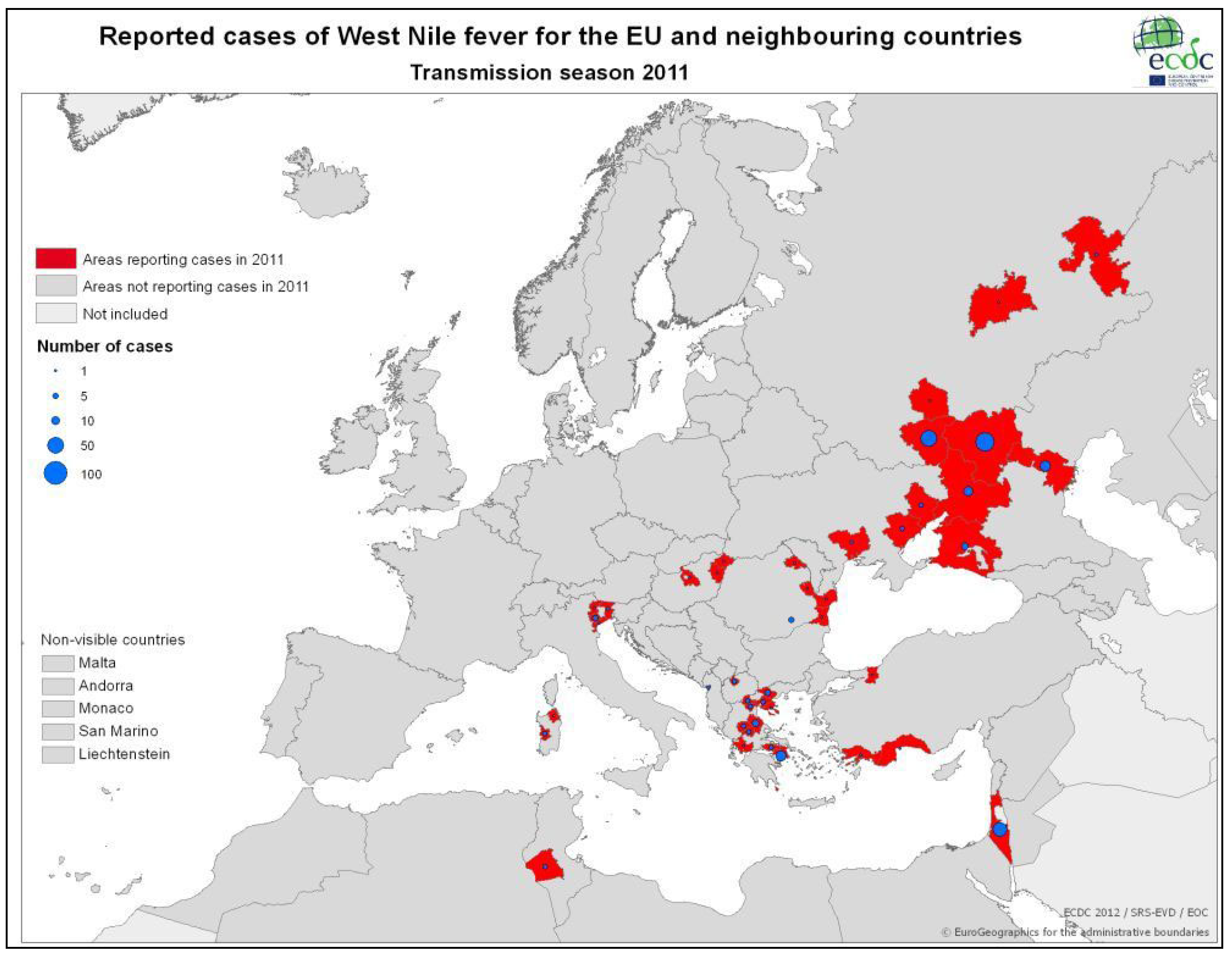
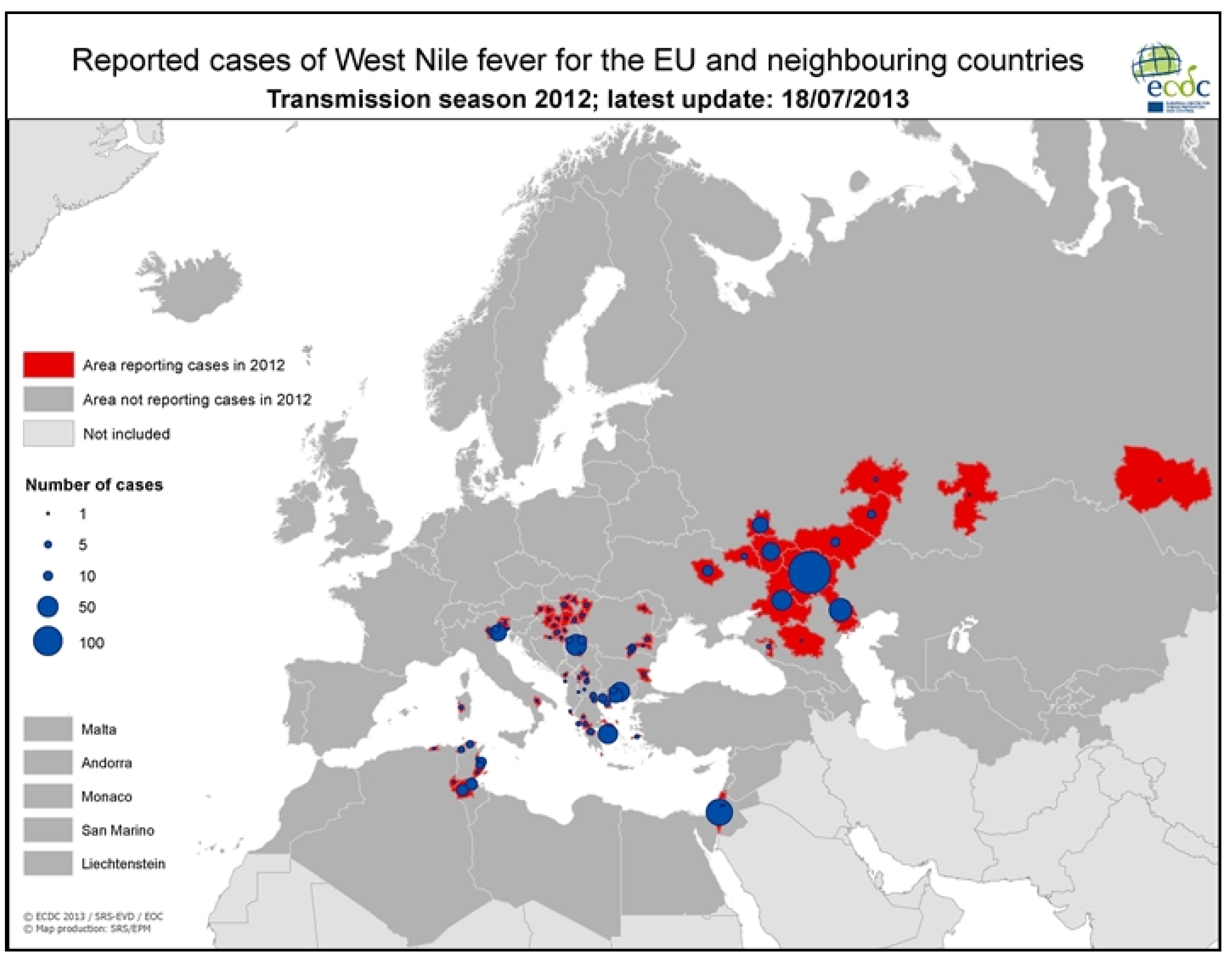
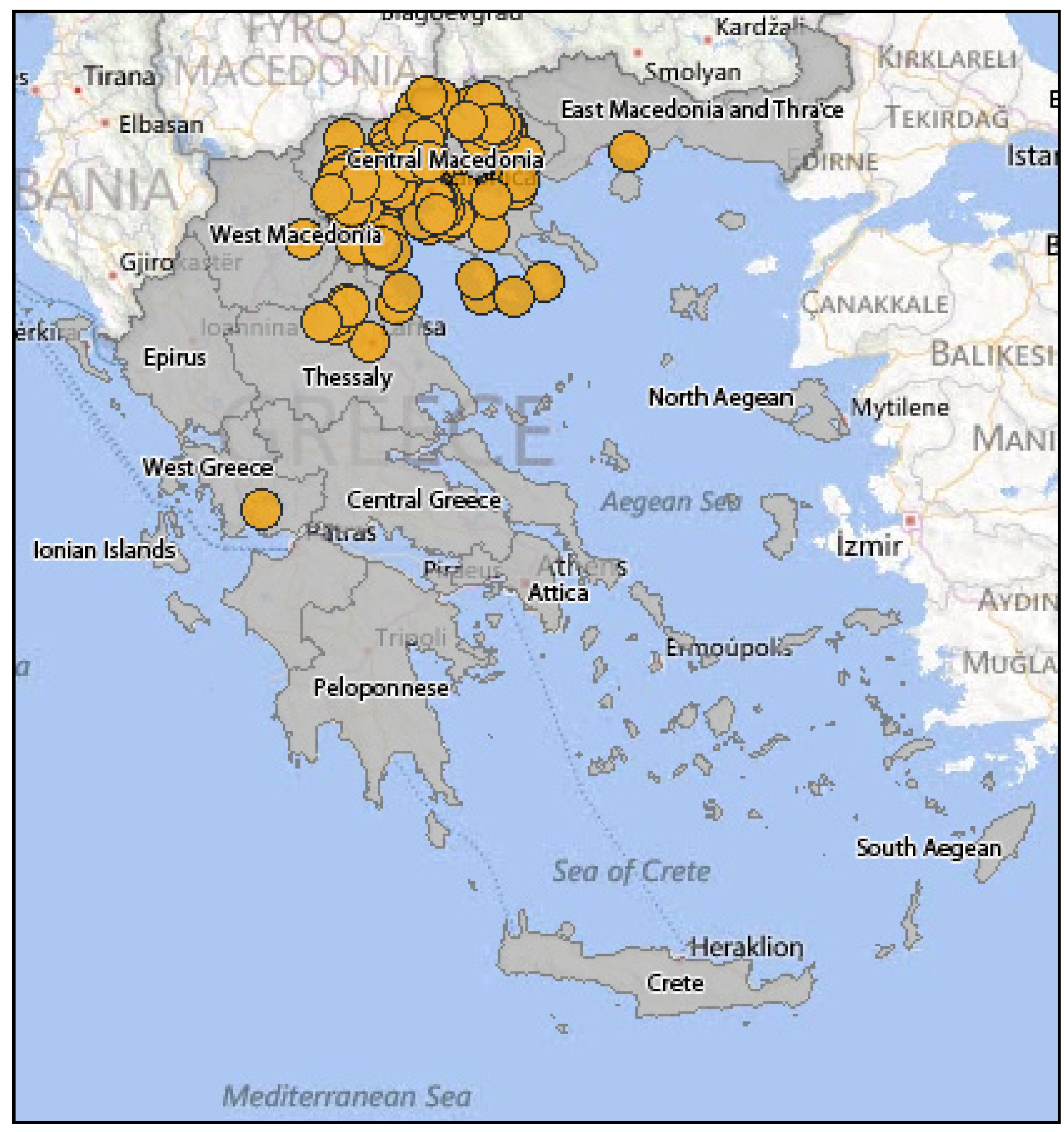
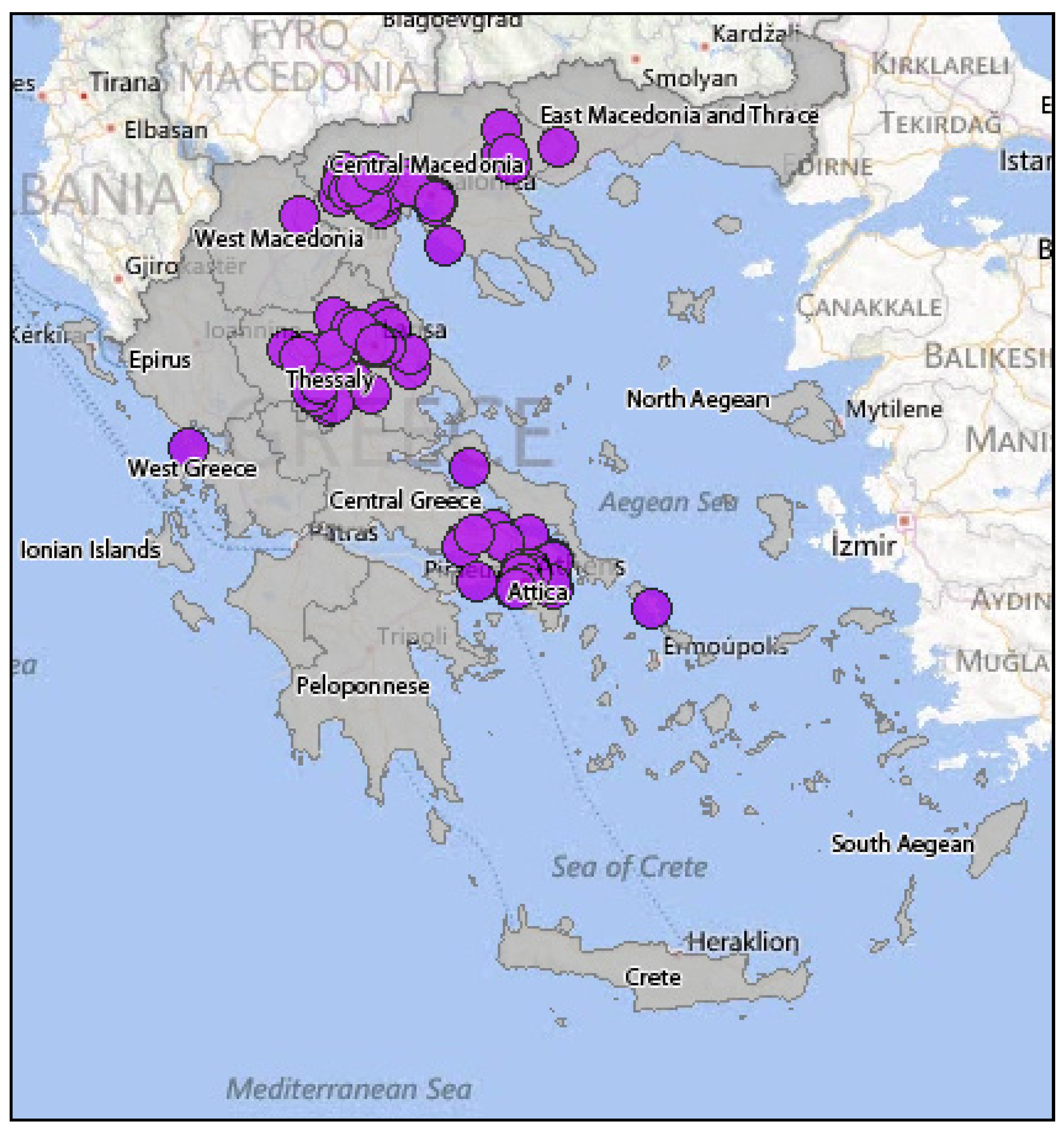
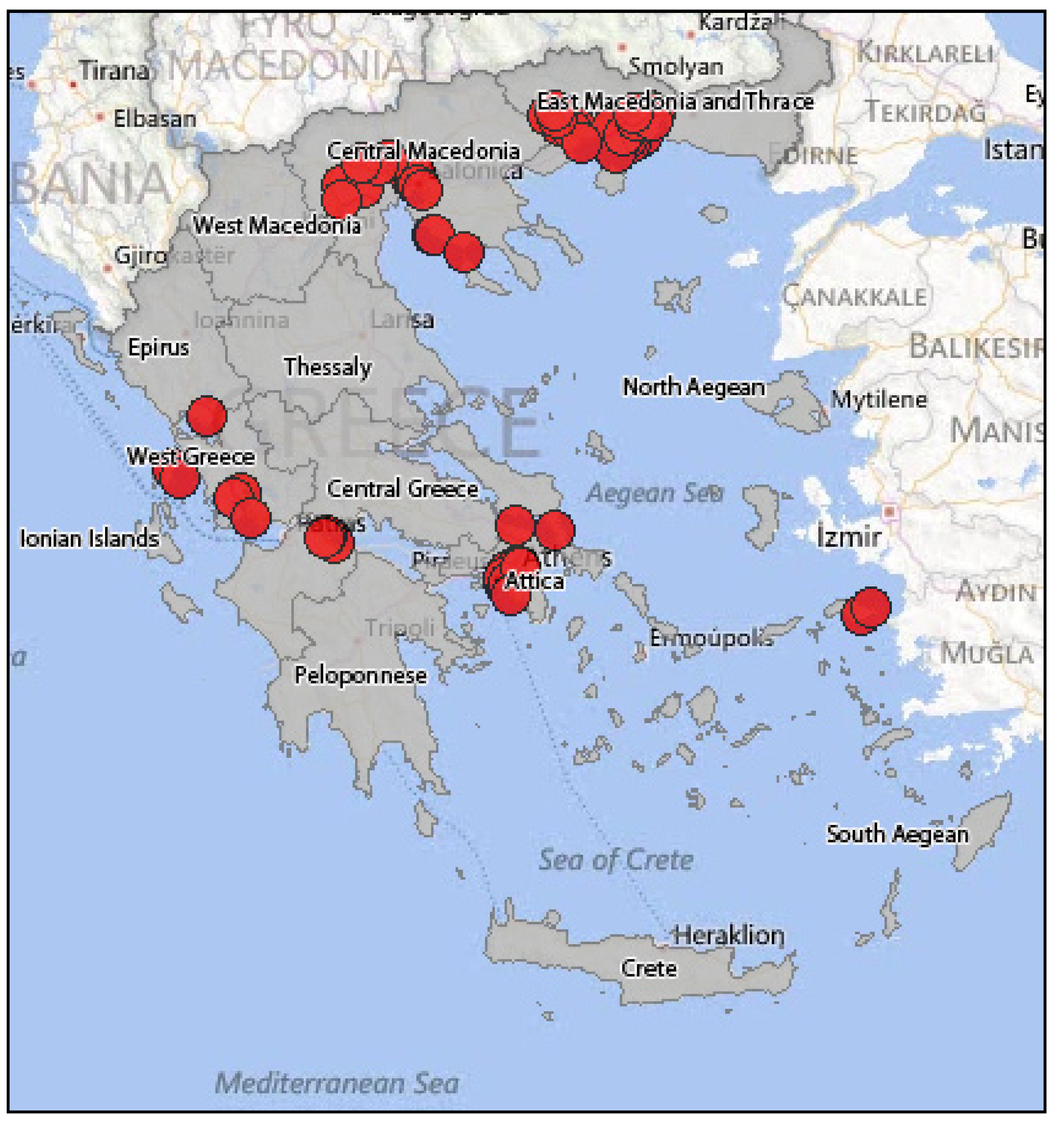
3.2.2. Epidemiology of WNV in Greece
3.3. Clinical Course
3.4. Laboratory Diagnosis
3.5. Treatment
3.6. Human Vaccines
| Vaccine Cadidate | Antigen | Clinical Trial Phase |
|---|---|---|
| ChimeriVax-WN02 | Yellow Fever 17D expressing WNV PrM/E | II |
| WN/DEN4-3’delta30 | Dengue virus 4 expressing WNV PrM/E | I |
| VRC | DNA expressing PrM/E | I |
| HBV-002 | Soluble E protein (no membrane domain) | I |
3.7. Non-Vector-Borne Transmission
3.8. Preventive Measures for Humans
| Mode of Transmission | Protective Measures |
|---|---|
| Mosquito bite | Personal protective measures |
| Avoiding being outdoors during mosquito peak activity hours | |
| Use of insect repellents | |
| Use of protecting clothes | |
| Domestic protective measures | |
| Use of screens | |
| Use of bed nets | |
| Use of air-conditioner/fan | |
| Elimination of backwater | |
| Animal-to-human | Use of gloves |
| Use of protective clothes | |
| Laboratory-acquired infection | Standard contact and droplet precautions |
| Minimizing aerosol production | |
| Intrauterine | Risk infection reduction by taking protective measures throughout pregnancy |
| Diagnostic testing when clinically appropriate | |
| Breastfeeding | Breastfeeding recommendations have not changed |
| Transfusion | NAT technology for blood units testing |
| Transplantation | There is no policy for organ donors screening |
3.9. Human Epidemiological Surveillance
| Type of Criteria | Analysis of Criteria |
|---|---|
| Clinical |
|
| Laboratory |
|
| Procedure | Details |
|---|---|
| Case reporting form | The fields that should be filled are: demographics, risk factors (occupation, recent trip abroad, recent move to another part of the country, blood transfusion, etc.), clinical features (symptoms, date of onset, symptoms from the nervous system etc.), laboratory results and the personal details of the attending physician |
| Clinical specimens | Specimens of serum (minimum quantity: 2 mL) and CSF (minimum quantity: 0.5 mL) should be transported to the Reference Center in special vials in a special container for biological samples. All samples should be labeled with patient’s personal details, sampling date and the hospital responsible for sampling. The accompanying consignment note is prerequisite |
| Consignment note | Information that should be included is: clinical features of the patient, sample type (serum, CSF) and personal details of the attending physician |
| Surveillance Type | Directions |
|---|---|
| Enhanced passive surveillance |
|
| Active surveillance |
|
3.10. WNV Risk Assessment
3.10.1. Introduction
3.10.2. The Use of Geographic Information Systems (GIS) in the Risk Assessment of WNV
- Where cases of disease A are located?
- Where spread of WNV to humans is located in relation to vectors of the virus?
- How is the spread of the virus correlated with environmental factors such as altitude, land use, distance from water surfaces?
- How is the spread of the virus correlated with demographic or socio-economic factors such as age, sex, occupation, living conditions, income?
- Will spread of the virus be different in the future if environmental conditions remain the same?
- Which is the number of cases in each area of interest in proportion to the population over the age of 60 years?
- May the spatial distribution of the disease A be due to a similar distribution of population sizes?
- Which is the range of antibody levels for disease A in people’s blood? Are they randomly distributed in the area of the city or have distinct geographical patterns?
- How are any patterns correlated with the characteristics of living conditions?
3.10.3. Recent Risk Assessment Models
| Risk Level | Rain and Temperature Levels | Mosquito Abundance | Mosquito MIR*/1,000 | Chicken Sero- Conversions | Equine Cases | Human Cases | Proximity of WNV Activity to Residential Areas |
|---|---|---|---|---|---|---|---|
| 1 | Significantly below average | <50% | 0 | 0 | 0 throughout state | 0 throughout state | Remote area |
| 2 | Below average | 50%–90% | 0.1–1.0 | 1 | Rural area | ||
| 3 | Average | 91%–150% | 1.1–2.0 | PF* > 1 C/PF < 1 | >1 throughout state 0 local | <1 throughout state 0 local | Small town |
| 4 | Above Average | 151%–300% | 2.1–5.0 | PF > 1 1 < C/PF * < 3 | 1–2 local | Suburban area | |
| 5 | Significantly above average | >300% | >5.0 | PF > 1 C/PF > 3 | >2 local | >1 local | Urban area |
4. WNV in Animals
4.1. WNV in Birds
4.1.1. WNV Transmission Cycle and Birds
4.1.2. The Disease in Birds
4.1.3. Surveillance of the WNV Circulation in Greece Using Domestic Birds
4.1.4. Surveillance of the WNV Circulation in Greece Using Wild Birds
4.2. WNV in Equids
4.2.1. Introduction
4.2.2. Pathology
Pathogenesis
Clinical Signs
Gross Lesions
Histopathological Lesions
Distribution of WNV in the Tissues—Immunophenotyping of Lesions
4.2.3. Diagnosis
Ante Mortem Diagnosis
Post Mortem Diagnosis
4.2.4. Treatment and Prevention
Therapeutic Care
Prevention—Vaccines
| Vaccine | Antigen | Status |
|---|---|---|
| West Nile Innovator®, Fort Dodge Animal Health/Pfizer-Zoetis | Whole viral particles inactivated in formalin | Approved and marketed |
| Vetera West Nile vaccine®, Boehringer Ingelheim | Inactivated virus | Approved and marketed |
| RecombiTek®, Merial | prM and E proteins in chimeric Canarypox virus | Approved and marketed |
| West Nile Innovator DNA®, Fort Dodge Animal Health/Pfizer-Zoetis | Plasmid DNA | Recalled from the company |
| PreveNile®, Intervet | prM and E proteins in chimeric Yellow Fever virus | Recalled because of side-effects |
| Chang et al., 2008 [233] | Plasmid DNA for the formation of SRIPs | Under evaluation |
4.2.5. Surveillance of WNF in Horses in Greece
WNV Infection in Horses Prior to 2010
Diagnosis of WNV Infections in Horses with Neurological Signs during the 2010 Epidemic
Clinical and Serological Surveillance of WNV in Horses in Greece (2010–2011)
4.3. WNV in Reptiles
4.4. WNV in Other Species
4.4.1. Mammals with Clinical Disease after Natural Infection with WNV
4.4.2. Mammals with only Detectable Antibodies against WNV but without Clinical Disease
4.4.3. Mammals Experimentally Infected with WNV
4.4.4. Amphibians
5. WNV in Mosquitoes
5.1. Transmission Cycle of WNV and the Role of Mosquitoes
5.1.1. Introduction
5.1.2. Role of Culex Species in the Epidemiology of WNV
5.2. Mosquito Management for Prevention and Control of WNV
5.2.1. Integrated Mosquito Management Plan
Surveillance
Reduction of Larval Habitats
Chemical Control
Resistance Management
Biological Control
Evaluation of Adult Mosquito Control Methods
5.3. Surveillance of WNV in Relation to Mosquitoes
5.3.1. Surveillance of Mosquito Species Infected with WNV
| Species | Countries |
|---|---|
| Culex modestus | France, Russia |
| Culex pipiens | Romania, Czechland, Bulgaria |
| Coquillettidia richiardii | South Russia, Bulgaria |
| Aedes cantans | Slovakia, Ukraine, Bulgaria |
| Aedes caspius | Ukraine |
| Aedes excrucians | Ukraine |
| Aedes vexans | Russia |
| Anopheles maculipennis | Portugal, Ukraine |
5.3.2. Operational Control Programmes against WNV Mosquito Vectors
5.4. Use of GIS for the Prediction, Prevention, and Control of WNV Mosquito Vectors
5.4.1. Introduction
5.4.2. Spatial Risk Models
6. Integrated Surveillance Systems for WNV
6.1. Introduction
6.2. The Example of California
- (1)
- Human surveillance: a network of 27 laboratories was established in order to enhance the surveillance efforts. In case of a positive result, an epidemiological investigation of the case is initiated by the local agencies, completing the proper form and then carrying it forward to the central health service of the state [178]. The human cases are not a sensitive indicator for the estimation of virus activity, since the greatest proportion of the patients does not have clinical symptoms, and even if they do, these sympotms usually appear two weeks after infection.
- (2)
- Equine surveillance: due to systematic vaccination of equines, their surveillance does not serve anymore as a sensitive indicator for the epizootic activity of the virus [178]. Nevertheless, equines have been maintained in the surveillance system as the confirmed cases indicate increased likelihood of WNV transmission in the human population of the region, where the infected equine is found [178].
- (3)
- Bird surveillance: there are three ways that birds are utilized in the surveillance system: sentinel chicken trapping and regular blood examination for seroconversion testing, blood collection of wild bird for the estimation of seroprevalence and examination of dead birds (reported by the public).
- (4)
- Squirrel surveillance: based on indications that squirrels are vulnerable to WNV, the California Public Health Department introduced this parameter in the surveillance system in 2004. This indicator can provide information about a localized virus activity [178].
- (5)
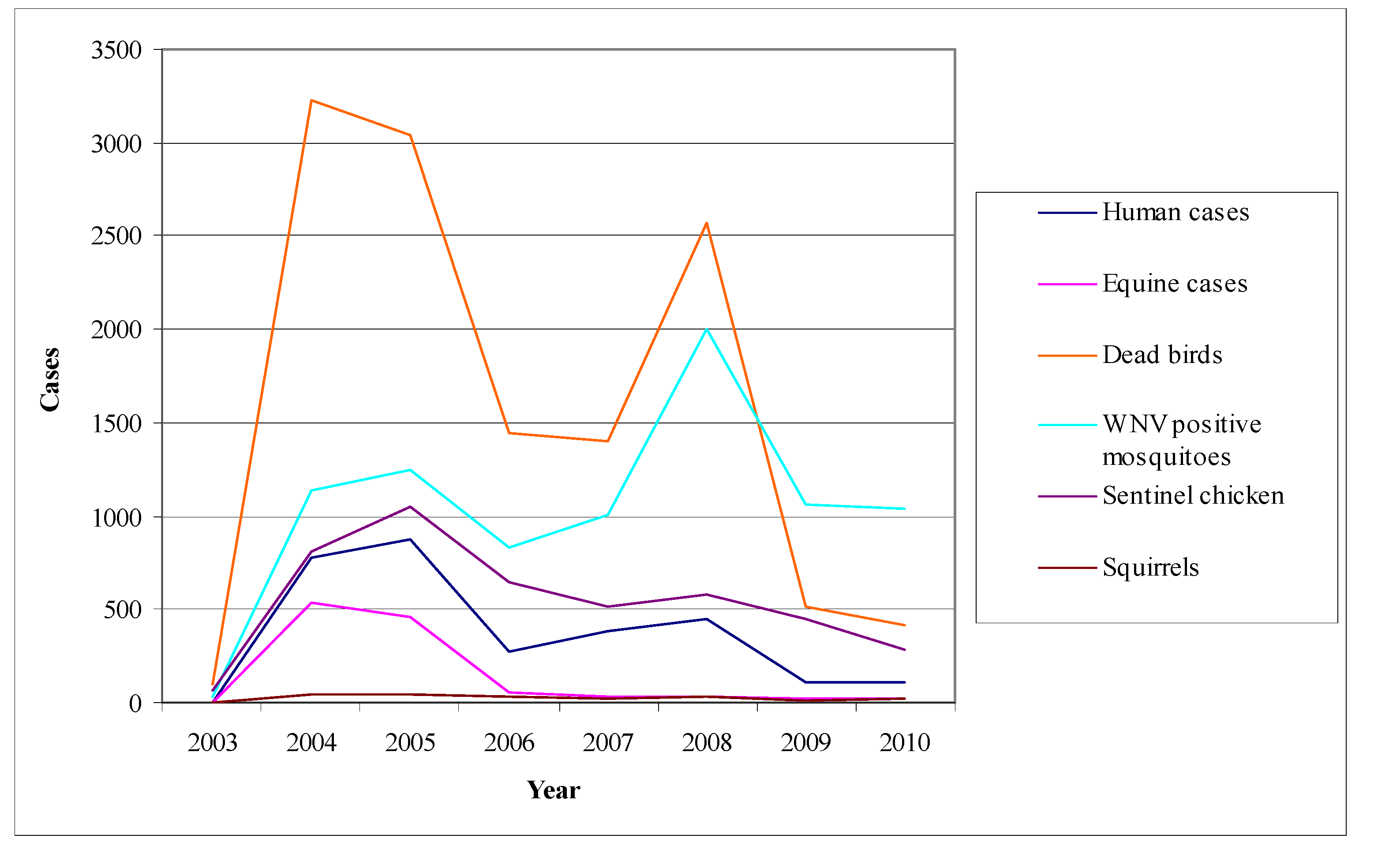
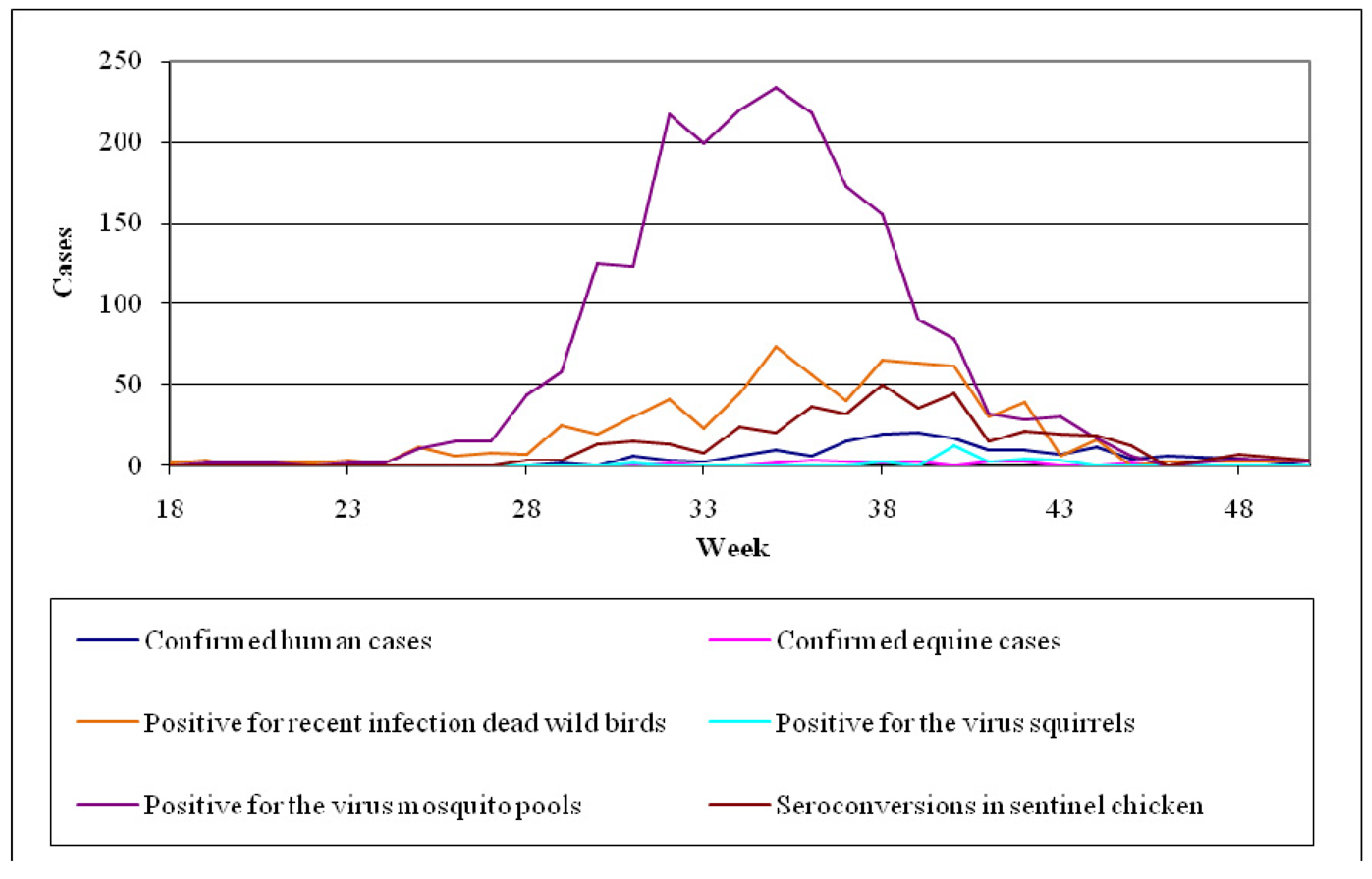
- Mosquito surveillance: although the virus activity detection in mosquitoes is considered to be less sensitive than in sentinel chicken, the mosquito pools examination for the virus presence offers the opportunity of a timely warning system concerning its activity [178]. Even though mosquitoes are not such a sensitive indicator as chicken, their infections may be detected sooner than the seroconversions of chicken. Figure 9 shows the fluctuation of the surveillance system numeric data in California during the period 2003–2010 highlighting two large outbreaks in 2004 and 2008.
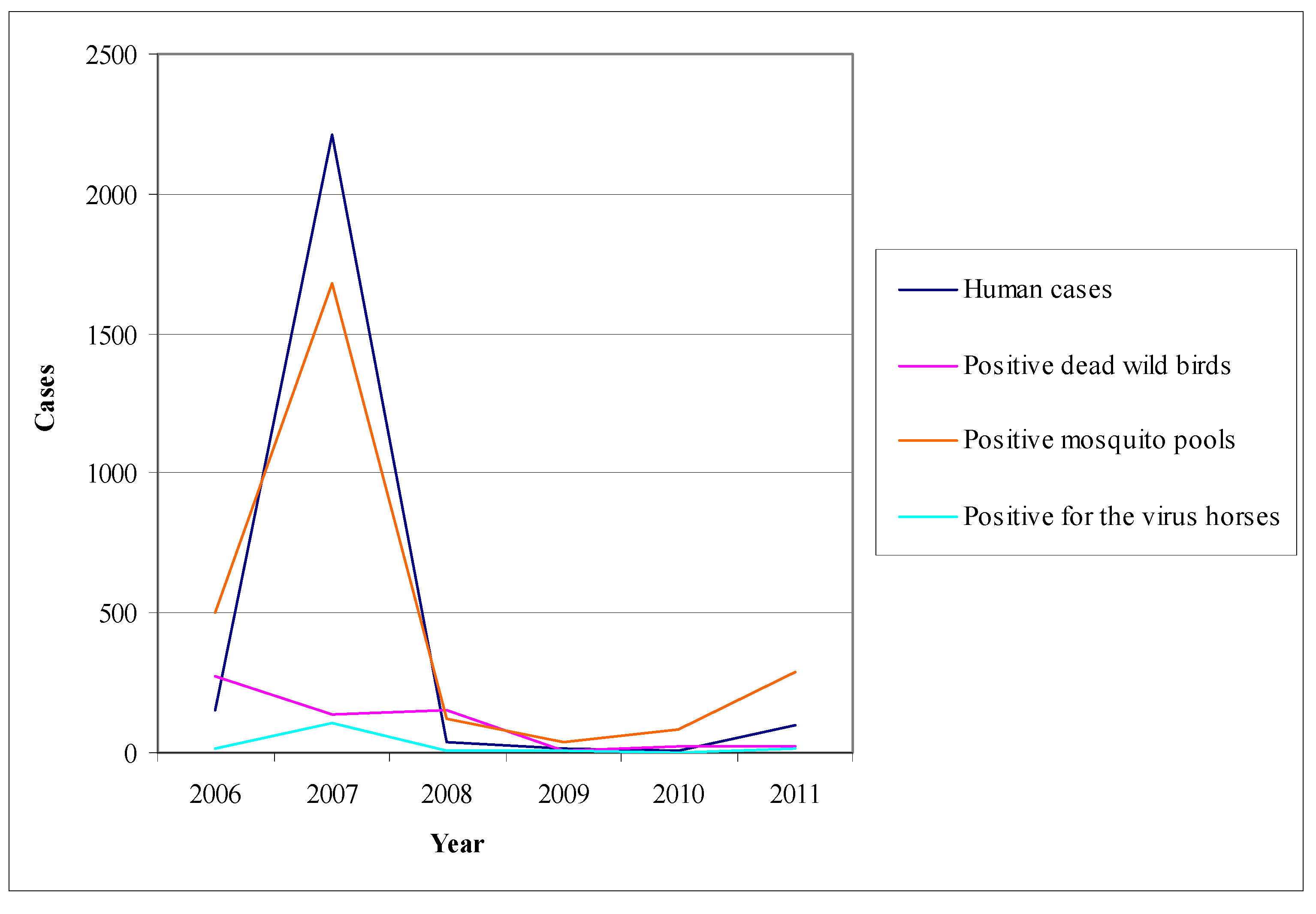
6.3. The Example of Canada
- (1)
- Human surveillance: physicians are alerted to report every suspected and confirmed case; they are supported by a laboratory network in which the samples of any suspected case are sent. In addition, the surveillance system is organized in such a way to ensure the safety of blood units.
- (2)
- Equine surveillance: equine surveillance is under the Canadian Food Inspection Agency in collaboration with Provincial Veterinary Laboratories [65].
- (3)
- Bird surveillance: the Cooperative Wildlife Health Centre of Canada, in collaboration with the Provincial Veterinary Laboratories and the National Microbiology Laboratory, undertake the examination of dead birds from April to the advent of winter. Based on past experience, indicators used are crows, jays and ravens that have been proved vulnerable to the virus and particularly sensitive in determining the extent of its activity [65].
- (4)
- Mosquito surveillance: mosquito surveillance is based on the predictable or existing virus activity, along with the planning of the appropriate interventions. Specifically, in areas with no virus activity, recording and quantification of mosquito species is being conducted, while in regions where virus activity has been ascertained, virological testing of mosquito pools takes place.
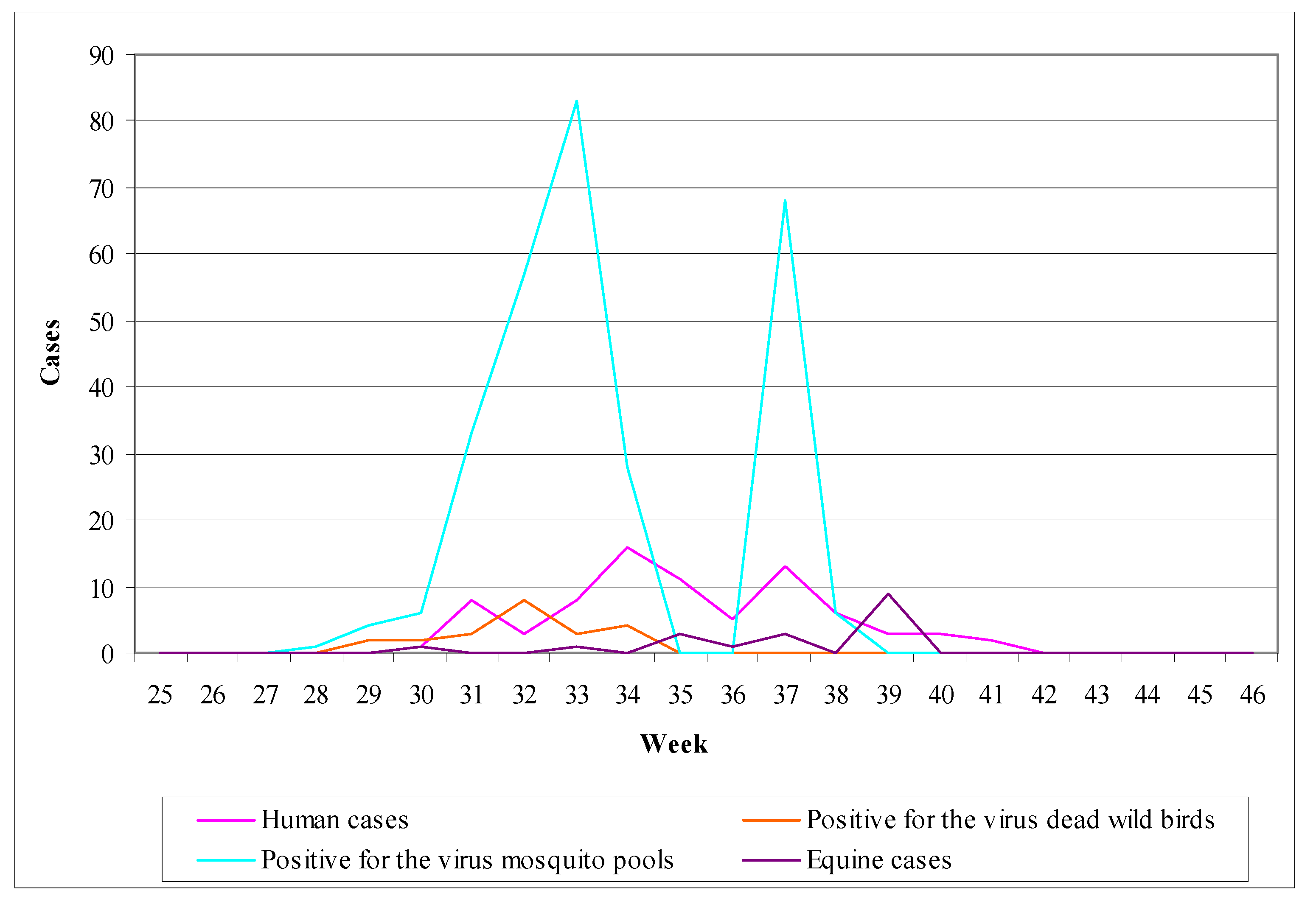
6.4. The Example of Italy (Emilia-Romagna)
- (1)
- Human surveillance: the main objective of human surveillance was the timely detection of human infections and planning of the appropriate interventions [293].
- (2)
- Animal surveillance: animal surveillance was applied from May to October implemented active and passive surveillance in equine and with active surveillance in non-migratory wild birds [293].
- (3)
- Mosquito surveillance: mosquito surveillance was based on a regular collection and examination of mosquitoes in regions where there were indications of virus activity in humans and animals [293].
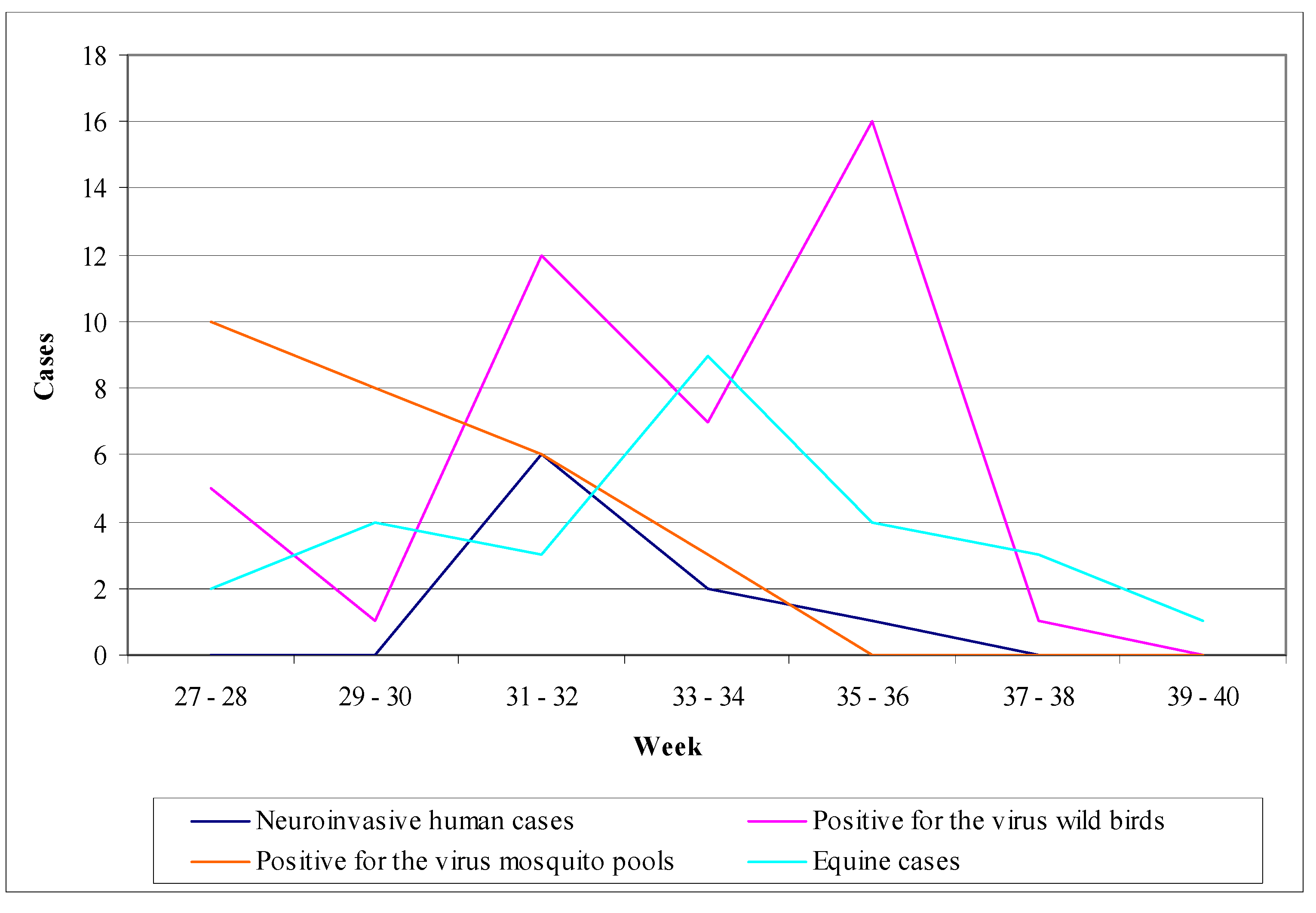
6.5. The example of Romania
- (1)
- Human surveillance: during the period 1997–2000, the implementation of enhanced passive surveillance resulted in recording 39 cases including 5 deaths [5].
- (2)
- Chicken surveillance: in 1997, sentinel chicken were used in Bucharest; every week a blood examination was performed in chickens giving an average seroconversion of 24%. During the period 1997–2000, domestic birds were used in Bucharest and in three additional regions in order to estimate virus seroprevalence in birds, giving a result of 8% [5].
- (3)
- Mosquito surveillance: mosquito collection and examination was implemented only in a small extent (16,000 mosquitoes in 1997 and 7,000 in 1999), which resulted negative detection of the virus [5].
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sips, G.J.; Wilschut, J.; Smit, J.M. Neuroinvasive flavivirus infections. Rev. Med. Virol. 2012, 22, 69–87. [Google Scholar] [CrossRef]
- Rossi, S.; Ross, T.; Evans, J. West Nile virus. Clin. Lab. Med. 2010, 30, 47–65. [Google Scholar] [CrossRef]
- Gyure, K.A. West Nile virus infections. J. Neuropath. Exp. Neurol. 2009, 68, 1053–1060. [Google Scholar] [CrossRef]
- Nash, D.; Mostashari, F.; Fine, A.; Miller, J.; O’Leary, D.; Murray, K.; Huang, A.; Rosenberg, A.; Greenberg, A.; Sherman, M.; et al. The outbreak of West Nile virus infection in the New York City area in 1999. N. Eng. J. Med. 2001, 344, 1807–1814. [Google Scholar] [CrossRef]
- Campbell, G.L.; Ceianu, C.S.; Savage, H.M. Epidemic West Nile encephalitis in Romania: Waiting for history to repeat itself. Ann. N. Y. Acad. Sci. 2001, 951, 94–101. [Google Scholar] [CrossRef]
- Ceianu, C.S.; Unqureanu, A.; Nikolescu, G.; Cernescu, C.; Nitescu, L.; Tardei, G.; Petrescu, A.; Pitigoi, D.; Martin, D.; Ciulacu-Purcarea, V.; et al. West Nile virus surveillance in Romania: 1997–2000. Viral. Immunol. 2001, 14, 251–262. [Google Scholar] [CrossRef]
- Papa, A. West Nile virus infections in humans-focus on Greece. J. Clin. Virol. 2013, 58, 351–353. [Google Scholar] [CrossRef]
- Gubler, D.J.; Kuno, G.; Markoff, L. Flaviviruses. In Fields virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Wolters Kluwer and Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 1153–1252. [Google Scholar]
- Mann, B.R.; McMullen, A.R.; Swetnam, D.M.; Barrett, A.D. Molecular epidemiology and evolution of West Nile virus in North America. Int. J. Environ. Res. Public Health 2013, 10, 5111–5129. [Google Scholar] [CrossRef]
- Sambri, V.; Capobianchi, M.; Charrel, R.; Fyodorova, M.; Gaibani, P.; Gould, E.; Niedrig, M.; Papa, A.; Pierro, A.; Rossini, G.; et al. West Nile virus in Europe: Emergence, epidemiology, diagnosis, treatment, and prevention. Clin. Microbiol. Infect. 2013, 19, 699–704. [Google Scholar] [CrossRef]
- Bakonyi, T.; Hubalek, Z.; Rudolf, I.; Nowotny, N. Novel flavivirus or new lineage of West Nile virus, Central Europe. Emerg. Infect. Dis. 2005, 11, 225–231. [Google Scholar] [CrossRef]
- Vazquez, A.; Sanchez-Seco, M.P.; Ruiz, S.; Molero, F.; Hernandez, L.; Moreno, J.; Magallanes, A.; Tejedor, C.G.; Tenorio, A. Putative new lineage of West Nile virus, Spain. Emerg. Infect. Dis. 2010, 16, 549–552. [Google Scholar] [CrossRef]
- King, A.M.Q.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses, 1st ed.; Elsevier: San Diego, CA, USA, 2011. [Google Scholar]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef]
- Nybakken, G.E.; Nelson, C.A.; Chen, B.R.; Diamond, M.S.; Fremont, D.H. Crystal structure of the West Nile virus envelope glycoprotein. J. Virol. 2006, 80, 11467–11474. [Google Scholar] [CrossRef]
- Sanchez, M.D.; Pierson, T.C.; McAllister, D.; Hanna, S.L.; Puffer, B.A.; Valentine, L.E.; Murtadha, M.M.; Hoxie, J.A.; Doms, R.W. Characterization of neutralizing antibodies to West Nile virus. Virology 2005, 336, 70–82. [Google Scholar] [CrossRef]
- Samuel, M.A.; Diamond, M.S. Pathogenesis of West Nile virus infection: A balance between virulence, innate and adaptive immunity, and viral evasion. J. Virol. 2006, 80, 9349–9360. [Google Scholar] [CrossRef]
- Guo, J.T.; Hayashi, J.; Seeger, C. West Nile virus inhibits the signal transduction pathway of alpha interferon. J. Virol. 2005, 79, 1343–1350. [Google Scholar] [CrossRef]
- Macdonald, J.; Tonry, J.; Hall, R.A.; Williams, B.; Palacios, G.; Ashok, M.S.; Jabado, O.; Clark, D.; Tesh, R.B.; Briese, T.; et al. NS1 protein secretion during the acute phase of West Nile virus infection. J. Virol. 2005, 79, 13924–13933. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Roehrig, J.T.; Deubel, V.; Smith, J.; Parker, M.; Steele, K.; Crise, B.; Volpe, K.E.; Crabtree, M.B.; Scherret, J.H.; et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 1999, 286, 2333–2337. [Google Scholar] [CrossRef]
- Jia, X.Y.; Briese, T.; Jordan, I.; Rambaut, A.; Chi, H.C.; Mackenzie, J.S.; Hall, R.A.; Scherret, J.; Lipkin, W.I. Genetic analysis of West Nile New York 1999 encephalitis virus. Lancet 1999, 354, 1971–1972. [Google Scholar] [CrossRef]
- Beasley, D.W.; Davis, C.T.; Guzman, H.; Vanlandingham, D.L.; Travassos da Rosa, A.P.; Parsons, R.E.; Higgs, S.; Tesh, R.B.; Barrett, A.D. Limited evolution of West Nile virus has occurred during its southwesterly spread in the United States. Virology 2003, 309, 190–195. [Google Scholar] [CrossRef]
- Ebel, G.D.; Carricaburu, J.; Young, D.; Bernard, K.A.; Kramer, L.D. Genetic and phenotypic variation of West Nile virus in New York, 2000–2003. Am. J. Trop. Med. Hyg. 2004, 71, 493–500. [Google Scholar]
- Moudy, R.M.; Meola, M.A.; Morin, L.L.; Ebel, G.D.; Kramer, L.D. A newly emergent genotype of West Nile virus is transmitted earlier and more efficiently by Culex mosquitoes. Amer. J. Trop. Med. Hyg. 2007, 77, 365–370. [Google Scholar]
- McMullen, A.R.; May, F.J.; Li, L.; Guzman, H.; Bueno, R.J.; Dennett, J.A.; Tesh, R.B.; Barrett, A.D. Evolution of new genotype of West Nile virus in North America. Emerg. Infect. Dis. 2011, 17, 785–793. [Google Scholar] [CrossRef]
- May, F.J.; Davis, C.T.; Tesh, R.B.; Barrett, A.D. Phylogeography of West Nile virus: From the cradle of evolution in Africa to Eurasia, Australia and the Americas. J. Virol. 2011, 85, 2964–2974. [Google Scholar] [CrossRef]
- Zehender, G.; Ebranati, E.; Bernini, F.; Presti, A.L.; Rezza, G.; Delogu, M.; Galli, M.; Ciccozzi, M. Phylogeography and epidemiological history of West Nile virus genotype 1a in Europe and the Mediterranean Basin. Infect. Genet. Evol. 2011, 11, 646–653. [Google Scholar] [CrossRef]
- Pesko, K.N.; Ebel, G.D. West Nile virus population genetics and evolution. Infect. Genet. Evol. 2012, 12, 181–190. [Google Scholar] [CrossRef]
- Botha, E.M.; Markotter, W.; Wolfaardt, M.; Paweska, J.T.; Swanepoel, R.; Palacios, G.; Nel, L.H.; Venter, M. Genetic determinants of virulence in pathogenic lineage 2 West Nile virus strains. Emerg. Infect. Dis. 2008, 14, 222–230. [Google Scholar]
- Venter, M.; Swanepoel, R. West Nile virus lineage 2 as a cause of zoonotic neurological disease in humans and horses in southern Africa. Vector Borne Zoonotic Dis. 2010, 10, 659–664. [Google Scholar] [CrossRef]
- Venter, M.; Human, S.; Zaayman, D.; Gerdes, G.H.; Williams, J.; Steyl, J.; Leman, P.A.; Paweska, J.T.; Setzkorn, H.; Rous, G.; et al. Lineage 2 West Nile virus as cause of fatal neurologic disease in horses, South Africa. Emerg. Infect. Dis. 2009, 15, 877–884. [Google Scholar] [CrossRef]
- Smithburn, K.C.; Haddow, A.J.; Mahaffy, A.F. A neurotropic virus isolated from Aedes mosquitoes caught in the Semliki forest. Amer. J. Trop. Med. Hyg. 1946, 26, 189–208. [Google Scholar]
- Beasley, D.W.; Davis, C.T.; Whiteman, M.; Granwehr, B.; Kinney, R.M.; Barrett, A.D. Molecular determinants of virulence of West Nile virus in North America. Arch. Vir. S. 2004, 18, 35–41. [Google Scholar]
- Bakonyi, T.; Ivanics, E.; Erdelyi, K.; Ursu, K.; Ferenczi, E.; Weissenbock, H.; Nowotny, N. Lineage 1 and 2 strains of encephalitic West Nile virus, Central Europe. Emerg. Infect. Dis. 2006, 12, 618–623. [Google Scholar] [CrossRef]
- Platonov, A.E.; Fedorova, M.V.; Karan, L.S.; Shopenskaya, T.A.; Platonova, O.V.; Zhuralev, V.I. Epidemiology of West Nile infection in Volgograd, Russia, in relation to climate change and mosquito (diptera: Culicidae) bionomics. Parasitol. Res. 2008, 103, S45–S53. [Google Scholar] [CrossRef]
- Kutasi, O.; Bakonyi, T.; Lecollinet, S.; Biksi, I.; Ferenczi, E.; Bahuon, C.; Sardi, S.; Zientara, S.; Szenci, O. Equine encephalomyelitis outbreak caused by a genetic lineage 2 West Nile virus in Hungary. J. Vet. Intern. Med. 2011, 25, 586–591. [Google Scholar] [CrossRef] [Green Version]
- Wodak, E.; Richter, S.; Bago, Z.; Revilla-Fernandez, S.; Weissenbock, H.; Nowothy, N.; Winter, P. Detection and molecular analysis of West Nile virus infections in birds of prey in the eastern part of Austria in 2008 and 2009. Vet. Microbiol. 2011, 149, 358–366. [Google Scholar] [CrossRef]
- Kecskemeti, S.; Balmocy, E.; Bacsadi, A.; Kiss, I.; Bakonyi, T. Encephalitis due to West Nile virus in a sheep. Vet. Rec. 2007, 161, 568–569. [Google Scholar] [CrossRef]
- Papa, A.; Xanthopoulou, K.; Gewehr, S.; Mourelatos, S. Detection of West Nile virus lineage 2 in mosquitoes during a human outbreak in Greece. Clin. Microbiol. Infect. 2011, 17, 1176–1180. [Google Scholar] [CrossRef]
- Papa, A.; Bakonyi, T.; Xanthopoulou, K.; Vazquez, A.; Tenorio, A.; Nowotny, N. Genetic characterization of West Nile virus lineage 2, Greece, 2010. Emerg. Infect. Dis. 2011, 17, 920–922. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Brault, A.C.; Huang, C.Y.; Langevin, S.A.; Kinney, R.M.; Bowen, R.A.; Ramey, W.N.; Panella, N.A.; Holmes, E.C.; Powers, A.M.; Miller, B.R. A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nat. Genet. 2007, 39, 1162–1166. [Google Scholar] [CrossRef]
- Barzon, L.; Papa, A.; Pacenti, M.; Franchin, E.; Lavezzo, E.; Squarzon, L.; Masi, G.; Martello, T.; Testa, T.; Cusinato, R.; et al. Genome sequencing of West Nile virus from human cases in Greece, 2012. Viruses 2013, 5, 2311–2319. [Google Scholar] [CrossRef]
- Papa, A.; Tsimitri, T.; Papadopoulou, E.; Testa, T.; Adamidis, A.; Gavana, E.; Aspragathou, S. Molecular detection and isolation of West Nile virus from human cases in Greece, 2013. New Microbe New Infect. 2013. accepted. [Google Scholar]
- Papa, A.; Politis, C.; Tsoukala, A.; Eglezou, A.; Bakaloudi, V.; Hatzitaki, M.; Tsergouli, K. West Nile virus lineage 2 from blood donor, Greece. Emerg. Infect. Dis. 2012, 18, 688–689. [Google Scholar] [CrossRef]
- Valiakos, G.; Touloudi, A.; Iacovakis, C.; Athanasiou, L.; Birtsas, P.; Spyrou, V.; Billinis, C. Molecular detection and phylogenetic analysis of West Nile virus lineage 2 in sedentary wild birds (Eurasian magpie), Greece, 2010. Eur. Surveill. 2011. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19862 (accessed on 29 July 2013).
- Chaskopoulou, A.; Dovas, C.; Chaintoutis, S.; Bouzalas, I.; Ara, G.; Papanastasopoulou, M. Evidence of enzootic circulation of West Nile virus (Nea Santa-Greece-2010, lineage 2), Greece, May to July 2011. Eur. Surveill. 2011, 16. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19933 (accessed on 29 July 2013).
- Bagnarelli, P.; Marinelli, K.; Trotta, D.; Monachetti, A.; Tavio, M.; Gobbo, R.D.; Capobianchi, M.; Menzo, S.; Nikoletti, L.; Maqurano, F.; et al. Human case of autochthonous West Nile virus lineage 2 infection in Italy, September 2011. Eur. Surveill. 2011, 16. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20002 (accessed on 29 July 2013).
- Barzon, L.; Pacenti, M.; Franchin, E.; Lavezzo, E.; Masi, G.; Squarzon, L.; Pagni, S.; Toppo, S.; Russo, F.; Cattai, M.; et al. Whole genome sequencing and phylogenetic analysis of West Nile virus lineage 1 and lineage 2 from human cases of infection, Italy, August 2013. Eur. Surveill. 2013, 18. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20591 (accessed on 29 July 2013).
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A neurotropic virus isolated from the blood of a native of Uganda. Amer. J. Trop. Med. 1940, 20, 471–472. [Google Scholar]
- Melnick, J.L.; Paul, J.R.; Riordan, J.T.; Barnett, V.H.; Goldblum, N.; Zabin, E. Isolation from human sera in Egypt of a virus apparently identical to West Nile virus. Proc. Soc. Exp. Biol. Med. 1951, 77, 661–665. [Google Scholar] [CrossRef]
- Hubálek, Z.; Halouzka, J. West Nile fever—A reemerging mosquito-borne viral disease in Europe. Emerg. Infect. Dis. 1999, 5, 643–650. [Google Scholar] [CrossRef]
- Hayes, C.G. West nile virus: Uganda, 1937, to New York City, 1999. Ann. N. Y Acad. Sci. 2001, 951, 25–37. [Google Scholar] [CrossRef]
- Bernkopf, H.; Levine, S.; Nerson, R. Isolation of West Nile virus in Israel. J. Infect. Dis. 1953, 93, 207–218. [Google Scholar] [CrossRef]
- Murgue, B.; Murri, S.; Triki, H.; Deubel, V.; Zeller, H.G. West Nile in the Mediterranean Basin: 1950–2000. Ann. N. Y. Acad. Sci. 2001, 951, 117–126. [Google Scholar]
- McIntosh, B.M.; Jupp, P.G.; Santos, I.D.; Meenehan, G.M. Epidemics of West Nile and Sindbis viruses in South Africa with Culex (culex) univittatus theobald as vector. S. Afr. J. Sci. 1976, 72, 295–300. [Google Scholar]
- Jupp, P.G.; Blackburn, N.K.; Thomson, D.L.; Meenehan, G.M. Sindbis and West Nile virus infections in the Witwatersrand-Pretoria region. S. Afr. Med. J. 1986, 70, 218–220. [Google Scholar]
- Le Guenno, B.; Bougermouh, A.; Azzam, T.; Bouakaz, R. West Nile: A deadly virus? Lancet 1996, 348, 1315. [Google Scholar]
- Zeller, H.G.; Schuffenecker, I. West Nile virus: An overview of its spread in Europe and the Mediterranean Basin in contrast to its spread in the Americas. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 147–156. [Google Scholar] [CrossRef]
- Platonov, A.E.; Shipulina, G.A.; Shipulina, O.Y.; Tyutyunnik, E.N.; Frolochkina, T.I.; Lanciotti, R.S.; Yazyshina, S.; Platonova, O.V.; Obukhov, I.L.; Zhukov, A.N.; et al. Outbreak of West Nile virus infection, Volgograd region, Russia, 1999. Emerg. Infect. Dis. 2001, 7, 128–132. [Google Scholar] [CrossRef]
- Hayes, E.B.; Komar, N.; Nasci, R.S.; Montgomery, S.P.; O’Leary, D.R.; Campbell, G.L. Epidemiology and transmission dynamics of West Nile virus disease. Emerg. Infect. Dis. 2005, 11, 1167–1173. [Google Scholar] [CrossRef]
- Murray, K.O.; Mertenas, E.; Despres, P. West Nile virus and its emergence in the United States of America. Vet. Res. 2010, 41, 67. [Google Scholar] [CrossRef]
- West Nile Virus Disease Cases Reported to CDC by State and Year, 1999–2012. Available online: http://www.cdc.gov/westnile/resources/pdfs/cummulative/99_2012_cummulativeHumanCases.pdf. (accessed on 30 October 2013).
- California West Nile Virus Website. Available online: http://westnile.ca.gov (accessed on 29 July 2013).
- Public Health Agency of Canada Website. Available online: http://www.phac-aspc.gc.ca/index-eng.php (accessed on 29 July 2013).
- Green, M.S.; Weinberger, M.; Ben-Ezer, J.; Bin, H.; Mendelson, E.; Gandacu, D.; Kaufman, Z.; Dichtiar, R.; Sobel, A.; Cohen, D.; et al. Long-term death rates, West Nile virus epidemic, Israel, 2000. Emerg. Infect. Dis. 2005, 11, 1754–1757. [Google Scholar] [CrossRef]
- Bakonyi, T.; Ferenczi, E.; Erdélyi, K.; Kutasi, O.; Csörgő, T.; Seidel, B.; Weissenböck, H.; Brugger, K.; Bán, E.; Nowotny, N. Explosive spread of a neuroinvasive lineage 2 West Nile virus in Central Europe, 2008/2009. Vet. Microbiol. 2013, 165, 61–70. [Google Scholar] [CrossRef]
- Rizzo, C.; Salcuni, P.; Nikoletti, L.; Ciufolini, M.G.; Russo, F.; Masala, R.; Frongia, O.; Finarelli, A.C.; Gramegna, M.; Gallo, L.; et al. Epidemiological surveillance of West Nile neuroinvasive diseases in Italy, 2008 to 2011. Eur. Surveill. 2012, 17. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20172 (accessed on 29 July 2013).
- Blitvich, B.J. Transmission dynamics and changing epidemiology of West Nile virus. Anim. Health Res. Rev. 2008, 9, 71–86. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Williams, D.T. The zoonotic flaviviruses of southern, south-eastern and eastern Asia, and Australasia: The potential for emergent viruse. Zoonoses Public Health 2009, 56, 338–356. [Google Scholar] [CrossRef]
- Gray, T.J.; Burrow, J.N.; Markey, P.G.; Whelan, P.I.; Jackson, J.; Smith, D.W.; Currie, B.J. West Nile virus (Kunjin subtype) disease in the northern territory of Australia—A case of encephalitis and review of all reported cases. Amer. J. Trop. Med. Hyg. 2011, 85, 952–956. [Google Scholar] [CrossRef]
- Tsai, T.F.; Popovici, F.; Cernescu, C.; Campbell, G.L.; Nedelcu, N.I. West Nile encephalitis epidemic in southeastern Romania. Lancet 1998, 352, 767–771. [Google Scholar] [CrossRef]
- Danis, K.; Papa, A.; Theocharopoulos, C.; Dougas, G.; Athanasiou, M.; Detsis, M.; Baka, A.; Lytras, T.; Mellou, K.; Bonovas, S.; et al. Outbreak of West Nile virus infection in Greece, 2010. Emerg. Infect. Dis. 2011, 17, 1868–1872. [Google Scholar] [CrossRef]
- Sirbu, A.; Ceianu, C.S.; Panculescu-Gatej, R.I.; Vazquez, A.; Tenorio, A.; Rebreanu, R.; Niedrig, M.; Nicolescu, G.; Pistol, A. Outbreak of West Nile virus infection in humans, Romania, July to October 2010. Eur. Surveill. 2011, 16. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19762 (accessed on 29 July 2013).
- Barzon, L.; Pacenti, M.; Cusinato, R.; Cattai, M.; Franchin, E.; Pagni, S.; Martello, T.; Bressan, S.; Squarzon, L.; Cattelan, A.; et al. Human cases of West Nile infection in north eastern Italy, 15 June to 15 November 2010. Eur. Surveill. 2011, 16. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19949 (accessed on 29 July 2013).
- Garcia-Bocanegra, I.; Busquets, N.; Napp, S.; Alba, A.; Zorrilla, I.; Villalba, R.; Arenas, A. Serosurvey of West Nile virus and other flaviviruses of the Japanese encephalitis antigenic complex in birds from Andalusia, southern Spain. Vector Borne Zoonotic Dis. 2011, 11, 1107–1113. [Google Scholar] [CrossRef]
- Kalaycioglu, H.; Korukluoglu, G.; Ozkul, A.; Oncul, O.; Tosun, S.; Karabay, O.; Gozalan, A.; Uyar, Y.; Caglayik, D.Y.; Atasoylu, G.; et al. Emergence of West Nile virus infections in humans in Turkey, 2010 to 2011. Eur. Surveill. 2012, 17. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20182 (accessed on 29 July 2013).
- Gratz, N.G. Vector- and Rodent-borne Diseases in Europe and North. America, 1st ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Papa, A.; Perperidou, P.; Tzouli, A.; Castilletti, C. West Nile virus—Neutralizing antibodies in humans in Greece. Vector Borne Zoonotic Dis. 2010, 10, 655–658. [Google Scholar] [CrossRef]
- Marinis, E. Serological Studies on Humans and Animals for Detection of Antibodies against Arboviruses in Greece; University of Athens: Athens, Greece, 1967. [Google Scholar]
- Pavlatos, M.; Smith, C.E. Antibodies to arthropod-borne viruses in Greece. Trans. R. Soc. Trop. Med. Hyg. 1964, 58, 422–424. [Google Scholar] [CrossRef]
- Antoniadis, A.; Alexiou, S.; Malissiovas, N.; Doutsos, J.; Polyzoni, T.; LeDue, J.W.; Peters, C.J.; Saviolakis, G. Seroepidemiological survey for antibodies to arboviruses in Greece. Arch. Vir. S. 1991, 1, 277–285. [Google Scholar]
- Dimou, V.; Gerou, S.; Papa, A. The epidemic West Nile virus strain in Greece was a recent introduction. Vector Borne Zoonotic Dis. 2013, 13, 719–722. [Google Scholar] [CrossRef]
- Kantzanou, M.N.; Moschidis, Z.M.; Kremastinou, G.; Levidiotou, S.; Karafoulidou, A.; Politis, C.; Marantidou, O.; Kavallierou, L.; Kaperoni, A.; Veneti, C.; et al. Searching for West Nile virus (WNV) in Greece. Transfus. Med. 2010, 20, 113–117. [Google Scholar] [CrossRef]
- Papa, A.; Danis, K.; Baka, A.; Bakas, A.; Dougas, G.; Lytras, T.; Theocharopoulos, G.; Chrysagis, D.; Vassiliadou, E.; Kamaria, F.; et al. Ongoing outbreak of West Nile virus infections in humans in Greece, July–August 2010. Eur. Surveill. 2010, 15. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19644 (accessed on 29 July 2013).
- HCDCP. Annual Epidemiological Report on West Nile Virus in Greece, 2010. Available online: http://www.keelpno.gr/ (accessed on 29 July 2013).
- Danis, K.; Papa, A.; Papanikolaou, E.; Dougas, G.; Terzaki, I.; Baka, A.; Vrioni, G.; Kapsimali, V.; Tsakris, A.; Kansouzidou, A.; et al. Ongoing outbreak of West Nile virus infection in humans, Greece, July to August 2011. Eur. Surveill. 2011, 16. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19951 (accessed on 29 July 2013).
- HCDCP. Annual epidemiological report on West Nile virus in Greece, 2011. Available online: http://www.keelpno.gr/ (accessed on 29 July 2013).
- HCDCP. Annual epidemiological report on West Nile virus in Greece, 2012. Available online: http://www.keelpno.gr/Portals/0/Files/English%20files/WNV%20reports%202012/ReportWNV_2012_EN_final.pdf (accessed on 29 July 2013).
- Petersen, L.R.; Marfin, A.A. West Nile virus: A primer for the clinician. Ann. Intern. Med. 2002, 137, 173–179. [Google Scholar] [CrossRef]
- Watson, J.T.; Pertel, P.E.; Jones, R.C.; Siston, A.M.; Paul, W.S.; Austin, C.C.; Gerber, S.I. Clinical characteristics and functional outcomes of West Nile fever. Ann. Intern. Med. 2004, 141, 360–365. [Google Scholar] [CrossRef]
- Murray, K.O.; Walker, C.; Gould, E. The virology, epidemiology, and clinical impact of West Nile virus: A decade of advancements in research since its introduction into the western hemisphere. Epidemiol. Infect. 2011, 139, 807–817. [Google Scholar] [CrossRef]
- Anastasiadou, A.; Economopoulou, A.; Kakoulidis, I.; Zilidou, R.; Butel, D.; Zorpidou, D.; Ferentinos, G.; Markou, P.; Kougas, E.; Papa, A. Non-neuroinvasive West Nile virus infections during the outbreak in Greece. Clin. Microbiol. Infect. 2011, 17, 1681–1683. [Google Scholar] [CrossRef]
- Kramer, L.D.; Li, J.; Shi, P.Y. West Nile virus. Lancet Neurol. 2007, 6, 171–181. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Haddad, M.B.; Tierney, B.C.; Campbell, G.L.; Marfin, A.A.; Van Gerpen, J.A.; Fleischauer, A.; Leis, A.A.; Stokic, D.S.; Petersen, L.R. Neurologic manifestations and outcome of West Nile virus infection. JAMA 2003, 290, 511–515. [Google Scholar]
- Granwehr, B.P.; Lillibridge, K.M.; Higgs, S.; Mason, P.W.; Aronson, J.F.; Campbell, G.A.; Barrett, A.D. West Nile virus: Where are we now? Lancet Infect. Dis. 2004, 4, 547–556. [Google Scholar] [CrossRef]
- Hayes, E.B.; Sejvar, J.J.; Zaki, S.R.; Lanciotti, R.S.; Bode, A.V.; Campbell, G.L. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg. Infect. Dis. 2005, 11, 1174–1179. [Google Scholar] [CrossRef]
- Fratkin, J.D.; Leis, A.A.; Stokic, D.S.; Slavinski, S.A.; Geiss, R.W. Spinal cord neuropathology in human West Nile virus infection. Arch. Pathol. Lab. Med. 2004, 128, 533–537. [Google Scholar]
- Lim, S.M.; Koraka, P.; Osterhaus, A.D.; Martina, B.E. West Nile virus: Immunity and pathogenesis. Viruses 2011, 3, 811–828. [Google Scholar] [CrossRef]
- Suthar, M.S.; Diamond, M.S.; Gale, M., Jr. West Nile virus infection and immunity. Nat. Rev. Microbiol. 2013, 11, 115–128. [Google Scholar] [CrossRef]
- Busch, M.P.; Kleinman, S.H.; Tobler, L.H.; Kamel, H.T.; Norris, P.J.; Walsh, I.; Matud, J.L.; Prince, H.E.; Lanciotti, R.S.; Wright, D.J.; et al. Virus and antibody dynamics in acute West Nile virus infection. J. Infect. Dis. 2008, 198, 984–993. [Google Scholar]
- Cho, H.; Diamond, M.S. Immune responses to West Nile virus infection in the central nervous system. Viruses 2012, 4, 3812–3830. [Google Scholar] [CrossRef]
- Petropoulou, K.A.; Gordon, S.M.; Prayson, R.A.; Ruggierri, P.M. West Nile virus meningoencephalitis: MR imaging findings. AJNR Amer. J. Neuroradiol. 2005, 26, 1986–1995. [Google Scholar]
- Pepperell, C.; Rau, N.; Krajden, S.; Kern, R.; Humar, A.; Mederski, B.; Simor, A.; Low, D.E.; McGeer, A.; Mazzulli, T.; et al. West Nile virus infection in 2002: Morbidity and mortality among patients admitted to hospital in southcentral Ontario. CMAJ 2003, 168, 1399–1405. [Google Scholar]
- Anastasiadou, A.; Kakoulidis, I.; Butel, D.; Kehagia, E.; Papa, A. Follow-up study of Greek patients with West Nile virus neuroinvasive disease. Int. J. Infect. Dis. 2013, 17, 494–497. [Google Scholar] [CrossRef]
- Papa, A.; Danis, K.; Tsergouli, K.; Tsioka, K.; Gavana, E. Development time of IgG antibodies to West Nile virus. Arch. Virol. 2011, 156, 1661–1663. [Google Scholar] [CrossRef]
- Roehrig, J.T.; Nash, D.; Maldin, B.; Labowitz, A.; Martin, D.A.; Lanciotto, R.S.; Campbell, G.L. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg. Infect. Dis. 2003, 9, 376–379. [Google Scholar]
- Prince, H.E.; Tobler, L.H.; Yeh, C.; Gefter, N.; Custer, B.; Busch, M.P. Persistence of West Nile virus-specific antibodies in viremic blood donors. Clin. Vaccine Immunol. 2007, 14, 1228–1230. [Google Scholar] [CrossRef]
- Papa, A.; Danis, K.; Athanasiadou, A.; Delianidou, M.; Panagiotopoulos, T. Persistence of West Nile virus immunoglobulin M antibodies, Greece. J. Med. Virol. 2011, 83, 1857–1860. [Google Scholar] [CrossRef]
- Papa, A.; Karabaxoglou, D.; Kansouzidou, A. Acute West Nile virus neuroinvasive infections: Cross-reactivity with Dengue virus and tick-borne encephalitis virus. J. Med. Virol. 2011, 83, 1861–1865. [Google Scholar] [CrossRef]
- Tonry, J.H.; Xiao, S.Y.; Siirin, M.; Chen, H.; da Rosa, A.P.; Tesh, R.B. Persistent shedding of West Nile virus in urine of experimentally infected hamsters. Amer. J. Trop. Med. Hyg. 2005, 72, 320–324. [Google Scholar]
- Barzon, L.; Pacenti, M.; Franchin, E.; Pagni, S.; Martello, T.; Cattai, M.; Cusinato, R.; Palu, G. Excretion of West Nile virus in urine during acute infection. J. Infect. Dis. 2013, 208, 1086–1092. [Google Scholar] [CrossRef]
- Barzon, L.; Pacenti, M.; Franchin, E.; Martello, T.; Lavezzo, E.; Squarzon, L.; Toppo, S.; Fiorin, F.; Marchiori, G.; Scotton, G.P.; et al. Clinical and virological findings in the ongoing outbreak of West Nile virus livenza strain in northern Italy, July to September 2012. Eur. Surveill. 2012, 17. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20260 (accessed on 29 July 2013).
- Debiasi, R.L. West Nile virus neuroinvasive disease. Curr. Infect. Dis. Rep. 2011, 13, 350–359. [Google Scholar] [CrossRef]
- Day, C.W.; Smee, D.F.; Julander, J.G.; Yamshchikov, V.F.; Sidwell, R.W.; Morrey, J.D. Error-prone replication of West Nile virus caused by ribavirin. Antiviral Res. 2005, 67, 38–45. [Google Scholar] [CrossRef]
- Leis, A.A.; Stokic, D.S. Neuromuscular manifestations of West Nile Virus infection. Front. Neurol. 2012, 3. [Google Scholar] [CrossRef]
- Ben-Nathan, D.; Lustig, S.; Tam, G.; Robinzon, S.; Segal, S.; Rager-Zisman, B. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating West Nile virus infection in mice. J. Infect. Dis. 2003, 188, 5–12. [Google Scholar]
- Saquib, R.; Randall, H.; Chandrakantan, A.; Spak, C.W.; Barri, Y.M. West Nile virus encephalitis in a renal transplant recipient: The role of intravenous immunoglobulin. Amer. J. Kidney Dis. 2008, 52, 19–21. [Google Scholar] [CrossRef]
- Nath, A.; Tyler, K.L. Novel approaches and challenges to treatment of central nervous system viral infections. Ann. Neurol. 2013, 74, 412–422. [Google Scholar]
- Repik, P.M. West Nile virus. In The Jordan Report: Accelerated Development of Vaccines 2012; National Institute of Health: Bethesda, MD, USA, 2012; pp. 106–108. [Google Scholar]
- Brandler, S.; Tangy, F. Vaccines in development against West Nile virus. Viruses 2013, 5, 2384–2409. [Google Scholar] [CrossRef]
- Monath, T.P.; Liu, J.; kanesa-Thasan, N.; Myers, G.A.; Nichols, R.; Deary, A.; McCarthy, K.; Johnson, C.; Ermak, T.; Shin, S.; et al. A live, attenuated recombinant West Nile virus vaccine. Proc. Natl. Acad. Sci. U S A 2006, 103, 6694–6699. [Google Scholar] [CrossRef]
- Initiative for Vaccine Research Team. West Nile virus. In State of the Art of Vaccine Research and Development; WHO: Geneva, Switzerland, 2005; pp. 71–72. [Google Scholar]
- Biedenbender, R.; Bevilacqua, J.; Gregg, A.M.; Watson, M.; Dayan, G. Phase II, randomized, double- blind, placebo-controlled, multicenter study to investigate the immunogenicity and safety of a West Nile virus vaccine in healthy adults. J. Infect. Dis. 2011, 203, 75–84. [Google Scholar] [CrossRef]
- de Filette, M.; Ulbert, S.; Diamond, M.; Sanders, N.N. Recent progress in West Nile virus diagnosis and vaccination. Vet. Res. 2012, 43. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.E.; Pierson, T.C.; Hubka, S.; Rucker, S.; Gordon, I.J.; Enama, M.E.; Andrews, C.A.; Xu, Q.; Davis, B.S.; Nason, M.; et al. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J. Infect. Dis. 2007, 196, 1732–1740. [Google Scholar] [CrossRef]
- Ledgerwood, J.E.; Pierson, T.C.; Hubka, S.A.; Desai, N.; Rucker, S.; Gordon, I.J.; Enama, M.E.; Nelson, S.; Nason, M.; Gu, W.; et al. A West Nile virus DNA vaccine utilizing a modified prooter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J. Infect. Dis. 2011, 203, 1396–1404. [Google Scholar] [CrossRef]
- Ledizet, M.; Kar, K.; Foellmer, H.G.; Wang, T.; Bushmich, S.L.; Anderson, J.F.; Fikrig, E.; Koski, R.A. A recombinant envelope protein vaccine against West Nile virus. Vaccine 2005, 23, 3915–3924. [Google Scholar]
- Iyer, A.V.; Kousoulas, K.G. A review of vaccine approaches for West Nile virus. Int. J. Environ. Res. Public Health 2013, 10, 4200–4223. [Google Scholar] [CrossRef]
- Hall, R.A.; Nisbet, D.J.; Pham, K.B.; Pyke, A.T.; Smith, G.A.; Khromykh, A.A. DNA vaccine coding for the full-length infectious Kunjin virus RNA protects mice against the New York strain of West Nile virus. Proc. Natl. Acad. Sci. U S A 2003, 100, 10460–10464. [Google Scholar]
- Nelson, M.H.; Winkelmann, E.; Ma, Y.; Xia, J.; Mason, P.; Bourne, N.; Milligan, G.N. Immunogenicity of Replivax WN, a novel single-cycle West Nile virus vaccine. Vaccine 2010, 29, 174–182. [Google Scholar]
- Francis, R.O.; Strauss, D.; Williams, J.D.; Whaley, S.; Shaz, B.H. West Nile virus infection in blood donors in the New York City area during the 2010 seasonal epidemic. Transfusion 2012, 52, 2664–2670. [Google Scholar] [CrossRef]
- Pealer, L.N.; Marfin, A.A.; Petersen, L.R.; Lanciotti, R.S.; Page, P.L.; Stramer, S.L.; Stobierski, M.G.; Signs, K.; Newman, B.; Kapoor, H.; et al. Transmission of West Nile virus through blood transfusion in the United States in 2002. N. Engl. J. Med. 2003, 349, 1236–1245. [Google Scholar] [CrossRef]
- CDC. Update: West Nile virus screening of blood bonations and transfusion-associated transmission—United States, 2003. In MMWR; 2004; 53, pp. 281–284. [Google Scholar]
- Stramer, S.L.; Fang, C.T.; Foster, G.A.; Wagner, A.G.; Brodsky, J.P.; Dodd, R.Y. West Nile virus among blood donors in the United States, 2003 and 2004. N. Engl. J. Med. 2005, 353, 451–459. [Google Scholar] [CrossRef]
- de Oliveira, A.M.; Beecham, B.D.; Montgomery, S.P.; Lanciotti, R.S.; Linnen, J.M.; Giachetti, C.; Pietrelli, L.A.; Stramer, S.L.; Safranek, T.J. West Nile virus blood transfusion-related infection despite nucleic acid testing. Transfusion 2004, 44, 1695–1699. [Google Scholar] [CrossRef]
- Custer, B.; Tomasulo, P.A.; Murphy, E.L.; Caglioti, S.; Harpool, D.; McEvoy, P.; Busch, M.P. Triggers for switching from minipool testing by nucleic acid technology to individual-donation nucleic acid testing for West Nile virus: Analysis of 2003 data to inform 2004 decision making. Transfusion 2004, 44, 1547–1554. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, J.; Li, Y.; Li, F.; Njoo, H. Rapid and accurate in vitro assays for detection of West Nile virus in blood and tissues. Transfus. Med. Rev. 2009, 23, 146–154. [Google Scholar] [CrossRef]
- Biggerstaff, B.J.; Petersen, L.R. Estimated risk of transmission of the West Nile virus through blood transfusion in the US, 2002. Transfusion 2003, 43, 1007–1017. [Google Scholar] [CrossRef]
- Iwamoto, M.; Jernigan, D.B.; Guasch, A.; Trepka, M.J.; Blackmore, C.G.; Hellinger, W.C.; Pham, S.M.; Zaki, S.; Lanciotti, R.S.; Lance-Parker, S.E.; et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N. Eng. J. Med. 2003, 348, 2196–2203. [Google Scholar] [CrossRef]
- CDC. Update: Investigations of West Nile virus infections in recipients of organ transplantation and blood transfusion. In MMWR; 2002; 51, pp. 833–836. [Google Scholar]
- Nett, R.J.; Kuehnert, M.J.; Ison, M.G.; Orlowski, J.P.; Fischer, M.; Staples, J.E. Current practices and evaluation of screening solid organ donors for West Nile virus. Transpl. Infect. Dis. 2012, 14, 268–277. [Google Scholar] [CrossRef]
- Inojosa, W.O.; Scotton, P.G.; Fuser, R.; Giobbia, M.; Paolin, A.; Maresca, M.C.; Brunello, A.; Nascimben, E.; Sorbara, C.; Rigoli, R.; et al. West Nile virus transmission through organ transplantation in north-eastern Italy: A case report and implications for pre-procurement screening. Infection 2012, 40, 557–562. [Google Scholar] [CrossRef]
- CDC. Intrauterine West Nile virus infection—New York 2002. In MMWR; 2002; pp. 1135–1136. [Google Scholar]
- O’Leary, D.R.; Kuhn, S.; Kniss, K.L.; Hinckley, A.F.; Rasmussen, S.A.; Pape, W.J.; Kightlinger, L.K.; Beecham, B.D.; Miller, T.K.; Neitzel, D.F.; et al. Birth outcomes following West Nile virus infection of pregnant women in the United States: 2003–2004. Pediatrics 2006, 117, 537–545. [Google Scholar] [CrossRef]
- CDC. Possible West Nile virus transmission to an infant through breast-feeding—Michigan, 2002. MMWR 2002, 51, 877–878. [Google Scholar]
- Hinckley, A.F.; O'Leary, D.R.; Hayes, E.B. Transmission of West Nile virus through breast milk seems to be rare. Pediatrics 2007, 119, 666–671. [Google Scholar] [CrossRef]
- Hayes, E.B.; O’Leary, D.R. West Nile virus infection: A pediatric perspective. Pediatrics 2004, 113, 1375–1381. [Google Scholar] [CrossRef]
- Sampathkumar, P. West Nile virus: Epidemiology, clinical presentation, diagnosis, and prevention. Mayo Clinic Proc. 2003, 78, 1137–1143. [Google Scholar] [CrossRef]
- CDC. Laboratory-acquired West Nile virus infections—United States 2002. In MMWR; 2002; 51, pp. 1133–1135. [Google Scholar]
- Nir, Y.; Beemer, A.; Goldwasser, R.A. West Nile virus infection in mice following exposure to a viral aerosol. Br. J. Exp. Pathol. 1965, 46, 443–449. [Google Scholar]
- CDC. West Nile virus infection among turkey breeder farm workers, Wisconsin, 2002. In MMWR; 2003; 52, pp. 1017–1019. [Google Scholar]
- Hartemink, N.A.; Davis, S.A.; Reiter, P.; Hubalek, Z.; Heesterbeek, J.A. Importance of bird-to-bird transmission for the establishment of West Nile virus. Vector-Borne Zoonotic Dis. 2007, 7, 575–584. [Google Scholar] [CrossRef]
- Gubler, D.J.; Petersen, L.R.; Roehrig, J.T.; Campbell, G.L.; Komar, N.; Nasci, R.S.; Zielinski-Gutierrez, E.; Marfin, A.A.; Lanciotti, R.S.; Bunning, M.L.; et al. Epidemic/Epizootic West Nile Virus in the United States: Guidelines for Surveillance, Prevention, and Control; CDC: Fort Collins, CO, USA, 2003. [Google Scholar]
- Burrough, P.A. Principles of Geographical Information Systems for Land Resources Assessment; Clarendon Press: Oxford, UK, 1986. [Google Scholar]
- Koutsopoulos, K. Geographical Information Systems and Spatial Analysis, 2nd ed.; Papasotiriou Press: Athens, Greece, 2005. [Google Scholar]
- Griffith, D.A.; Doyle, P.G.; Wheeler, D.C.; Johnson, D.L. A tale of two swaths: Urban childhood blood-lead levels across Syracuse, New York. Ann. Assoc. Am. Geogr. 1998, 88, 640–665. [Google Scholar]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors on conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- James, F.C.; McCulloch, C.E. Predicting species presence and abundance. In Predicting Species Occurences: Issues of Accuracy and Scale; Scott, J.M., Heglund, P.J., Morrison, M.L., Eds.; Island Press: Washington, DC, USA, 2002. [Google Scholar]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Eckhoff, A.R.; Mitchell, J.T. GIS utilization in large scale health studies and surveys: Pennsylvania’s West Nile virus surveillance program. Middle States Geogr. 2000, 33, 63–73. [Google Scholar]
- Tachiiri, K.; Klinkenberg, B.; Mak, S.; Kazmi, J. Predicting outbreaks: A spatial risk assessment of West Nile virus in British Columbia. Int. J. Health Geogr. 2006, 5. [Google Scholar] [CrossRef]
- Cooke, W.H.; Grala, K.; Wallis, R.C. Avian GIS models signal human risk for West Nile virus in Mississippi. Int. J. Health Geogr. 2006, 5. [Google Scholar] [CrossRef]
- Brownstein, J.S.; Rosen, H.; Purdy, D.; Miller, J.R.; Merlino, M.; Mostashari, F.; Fish, D. Spatial analysis of West Nile virus: Rapid risk assessment of an introduced vector-borne zoonosis. Vector Borne Zoonotic Dis. 2002, 2, 157–164. [Google Scholar] [CrossRef]
- Gibbs, S.E.; Allison, A.B.; Yabsley, M.J.; Mead, D.G.; Wilcox, B.R.; Stallknecht, D.E. West Nile virus antibodies in avian species of Georgia, USA: 2000–2004. Vector-Borne Zoonotic Dis. 2006, 6, 57–72. [Google Scholar] [CrossRef]
- Gujral, I.B.; Zielinski-Gutierrez, E.C.; LeBailly, A.; Nasci, R. Behavioral risks for West Nile virus disease, northern Colorado, 2003. Emerg. Infect. Dis. 2007, 13, 419–425. [Google Scholar] [CrossRef]
- Liu, A.; Lee, V.; Galusha, D.; Slade, M.D.; Diuk-Wasser, M.; Andreadis, T.; Scotch, M.; Rabinowitz, P.M. Risk factors for human infection with West Nile virus in Connecticut: A multi-year analysis. Int. J. Health Geogr. 2009, 8. [Google Scholar] [CrossRef]
- Rochlin, I.; Turbow, D.; Gomez, F.; Ninivaggi, D.V.; Campbell, S.R. Predictive mapping of human risk for West Nile virus (WNV) based on environmental and socioeconomic factors. PloS One 2011, 6. [Google Scholar] [CrossRef]
- Hosmer, D.W.J.; Lemeshow, S. Applied logistic Regression, 2nd ed.; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Winters, A.M.; Eisen, R.J.; Lozano-Fuentes, S.; Moore, C.G.; Pape, W.J.; Eisen, L. Predictive spatial models for risk of West Nile virus exposure in eastern and western Colorado. Amer. J. Trop. Med. Hyg. 2008, 79, 581–590. [Google Scholar]
- Shuai, J.; Buck, P.; Sockett, P.; Aramini, J.; Pollari, F. A GIS-driven integrated real-time surveillance pilot system for national West Nile virus dead bird surveillance in Canada. Int. J. Health Geogr. 2006, 5. [Google Scholar] [CrossRef]
- LaBeaud, A.D.; Gorman, A.M.; Koonce, J.; Kippes, C.; McLeod, J.; Lynch, J.; Gallagher, T.; King, C.H.; Mandalakas, A.M. Rapid GIS-based profiling of West Nile virus transmission: Defining environmental factors associated with an urban-suburban outbreak in northeast Ohio, USA. Geospat. Health 2008, 2, 215–225. [Google Scholar]
- Valiakos, G.; Touloudi, A.; Athanasiou, L.V.; Giannakopoulos, A.; Iacovakis, C.; Birtsas, P.; Spyrou, V.; Dalabiras, Z.; Petrovska, L.; Billinis, C. Exposure of eurasian magpies and turtle doves to West Nile virus during a major human outbreak, Greece, 2011. Eur. J. Wildl. Res. 2012, 58, 749–753. [Google Scholar] [CrossRef]
- Giannakopoulos, A.; Valiakos, G.; Touloudi, A.; Athanasiou, L.V.; Spyrou, V.; Birtsas, P.; Papaspyropoulos, K.; Sokos, C.; Dalabiras, Z.; Billinis, C. Identifying factors associated with the spatial distribution of West Nile virus in wild birds in northern Greece. In Joint WDA EWDA Conference, Lyon, France, 22–27 July 2012.
- Ozdenerol, E.; Taff, G.N.; Akkus, C. Exploring the spatio-temporal dynamics of reservoir hosts, vectors, and human hosts of West Nile virus: A review of the recent literature. Int. J. Environ. Res. Public Health 2013, 10, 5399–5432. [Google Scholar] [CrossRef]
- Edison, M.; Kramer, L.; Stone, W.; Haqiwara, Y.; Schmit, K. Dead bird surveillance as an early warning system for West Nile virus. Emerg. Infect. Dis. 2001, 7, 631–635. [Google Scholar]
- Wonham, M.J.; de-Camino-Beck, T.; Lewis, M.A. An epidemiological model for West Nile vitus: Invasion analysis and control applications. Proc. Biol. Sci. 2004, 271, 501–507. [Google Scholar] [CrossRef]
- California Mosquito-borne Virus Surveillance & Response Plan; California Department of Public Health, Mosquito & Vector control Association of California, University of California at Davis: Davis, CA, USA, 2012.
- Barker, C.M.; Reisen, W.K.; Kramer, V.L. California state mosquito-borne virus surveillance and response plan: A retrospective evaluation using conditional simulations. Amer. J. Trop. Med. Hyg. 2003, 68, 508–518. [Google Scholar]
- Kilpatrick, A.M.; Kramer, L.D.; Campbell, S.R.; Alleyne, E.O.; Dobson, A.P.; Daszak, P. West Nile virus risk assessment and the bridge vector paradigm. Emerg. Infect. Dis. 2005, 11, 425–429. [Google Scholar] [CrossRef]
- Theophilides, C.N.; Ahearn, S.C.; Grady, S.; Merlino, M. Identifying West Nile virus risk areas: The dynamic continuous-area space-time system. Amer. J. Epidemiol. 2003, 157, 843–854. [Google Scholar] [CrossRef]
- Watson, J.T.; Jones, R.C.; Gibbs, K.; Paul, W. Dead crow reports and location of human West Nile virus cases, Chicago, 2002. Emerg. Infect. Dis. 2004, 10, 938–940. [Google Scholar] [CrossRef]
- Carney, R.M.; Ahearn, S.C.; McConchie, A.; Glasner, C.; Jean, C.; Barker, C.; Park, B.; Padgett, K.; Parker, E.; Aquino, E.; et al. Early warning system for West Nile virus risk areas, California, USA. Emerg. Infect. Dis. 2011, 17, 1445–1454. [Google Scholar]
- Mostashari, F.; Kulldorff, M.; Hartman, J.J.; Miller, J.R.; Kulasekera, V. Dead bird clusters as an early warning system for West Nile virus activity. Emerg. Infect. Dis. 2003, 9, 641–646. [Google Scholar] [CrossRef]
- Zou, L.; Miller, S.N.; Schmidtmann, E.T. A GIS tool to estimate West Nile virus risk based on a degree-day model. Environ. Monit. Assess. 2007, 129, 413–420. [Google Scholar] [CrossRef]
- Winters, A.M.; Eisen, R.J.; Delorey, M.J.; Fischer, M.; Nasci, R.S.; Zielinski-Gutierrez, E.; Moore, C.G.; Pape, W.J.; Eisen, L. Spatial risk assessments based on vector-borne disease epidemiological data: Importance of scale for West Nile virus disease in Colorado. Amer. J. Trop. Med. Hyg. 2010, 82, 945–953. [Google Scholar]
- Peterson, R.K.; Macedo, P.A.; Davis, R.S. A human-health risk assessment for West Nile virus and insecticides used in mosquito management. Environ. Health Perspect. 2006, 114, 366–372. [Google Scholar] [CrossRef]
- Murphy, B.L. Spatial Risk Assessment of West Nile Virus in Montana based upon Temperature Effects on Cx. Tarsalis. Available online: http://www.carroll.edu/library/thesisArchive/MurphyB_2012final.pdf (accessed on 25 November 2013).
- Ezenwa, V.O.; Godsey, M.S.; King, R.J.; Guptill, S.C. Avian diversity and West Nile virus: Testing associations between biodiversity and infectious disease risk. Proc. Biol. Sci. 2006, 273, 109–117. [Google Scholar] [CrossRef]
- Komar, N. West Nile viral encephalitis. Rev. Sci. Tech. 2000, 19, 166–176. [Google Scholar]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental infection of north american birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef]
- McLean, R.G.; Ubico, S.R.; Docherty, D.E.; Hansen, W.R.; Sileo, L.; McNamara, T.S. West Nile virus transmission and ecology in birds. Ann. N. Y. Acad. Sci. 2001, 951, 54–57. [Google Scholar]
- Meece, J.K.; Henkel, J.S.; Glaser, L.; Reed, K.D. Mosquito surveillance for West Nile virus in southeastern Wisconsin—2002. Clin. Med. Res. 2003, 1, 37–42. [Google Scholar] [CrossRef]
- Reiter, P. West Nile virus in Europe: Understanding the present to gauge the future. Eur. Surveill. 2010, 15. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19508 (accessed on 29 July 2013).
- Rappole, J.H. Migratory birds and west nile virus. J. Appl. Microbiol. 2003, 94, S47–S58. [Google Scholar] [CrossRef]
- LaDeau, S.; Kilpatrick, A.M.; Marra, P.P. West Nile virus emergence and large-scale declines of north american bird populations. Nature 2007, 447, 710–713. [Google Scholar] [CrossRef]
- Weingartl, H.M.; Neufeld, J.L.; Copps, J.; Marszal, P. Experimental west nile virus infection in blue jays (cyanocitta cristata) and crows (corvus brachyrhynchos). Vet. Pathol. 2004, 41, 362–370. [Google Scholar] [CrossRef]
- Wünschmann, A.; Shivers, J.; Caroll, L.; Bender, J. Pathological and immunohistochemical findings in american crows (corvus brachyrhynchos) naturally infected with West Nile virus. J. Vet. Diagn. Invest. 2004, 16, 329–333. [Google Scholar] [CrossRef]
- Dauphin, G.; Zientara, S.; Zeller, H.; Murque, B. West Nile: Worldwide current situation in animals and humans. Comp. Immunol. Microbiol. Infect. 2004, 27, 343–355. [Google Scholar] [CrossRef]
- Jourdain, E.; Schuffenecker, I.; Korimbocus, J.; Reynard, S.; Murri, S.; Kayser, Y.; Gauthier-Clerc, M.; Sabatier, P.; Zeller, H.G. West Nile virus in wild resident birds, southern France, 2004. Vector-Borne Zoonotic Dis. 2007, 7, 448–452. [Google Scholar] [CrossRef]
- Malkinson, M.; Banet, C. The role of birds in the ecology of West Nile virus in Europe and Africa. Curr. Top. Microbiol. Immunol. 2002, 267. [Google Scholar]
- Erdélyi, K.; Ursu, K.; Frenczi, E.; Sceredi, L.; Rátz, F.; Skáre, J.; Bakonyi, T. Clinical and pathologic features of lineage 2 West Nile virus infections in birds of prey in Hungary. Vector-Borne Zoonotic Dis. 2007, 7, 181–188. [Google Scholar] [CrossRef]
- Sotelo, E.; Gutierrez-Guzmán, A.V.; Amo, J.D.; Llorente, F.; El-Harrak, M.; Pérez-Ramírez, E.; Blanco, J.M.; Höfle, U.; Jiménez-Clavero, M.A. Pathogenicity of two recent Western Mediterranean West Nile virus isolates in a wild bird species indigenous to southern Europe: The red-legged partridge. Vet. Res. 2011, 42. [Google Scholar] [CrossRef]
- Busquets, N.; Bertran, K.; Costa, T.P.; Rivas, R.; de la Fuerte, J.G.; Villabra, R.; Solanes, D.; Bensaid, A.; Majó, N.; Pagès, N. Experimental West Nile virus infection in gyr-saker hybrid falcons. Vector Borne Zoonotic Dis. 2012, 12, 482–489. [Google Scholar] [CrossRef]
- Rizzoli, A.; Rosà, R.; Rosso, F.; Buckley, A.; Gould, E. West Nile virus circulation detected in northern Italy in sentinel chickens. Vector-Borne Zoonotic Dis. 2007, 7, 411–417. [Google Scholar] [CrossRef]
- Cernescu, C.; Nedelcu, N.I.; Tardei, G.; Ruta, S.; Tsai, T.F. Continued transmission of West Nile virus to humans in southeastern Romania, 1997–1998. J. Infect. Dis. 2000, 181, 710–712. [Google Scholar] [CrossRef]
- Calistri, P.; Giovanni, A.; Savini, G.; Monaco, F.; Bonfanti, L.; Ceolin, C.; Terreqino, C.; Tamba, M.; Cordioli, P.; Lelli, R. West Nile virus transmission in 2008 in north-eastern Italy. Zoonoses Public Health 2010, 57, 211–219. [Google Scholar] [CrossRef]
- Dovas, C.I.; Chaintoutis, S.C.; Chaskopoulou, A. Comparison of Three Surveillance Systems for the Determination of West Nile Virus Enzootic Circulation during the 2010–2011 Epidemics in Greece. In Proceedings of 6th Annual Meeting of Epizone, Brighton, UK, 12–14 June 2012.
- Chaintoutis, S.C.; Chaskopoulou, A.; Chassalevris, T.; Koehler, P.G.; Papanastassopoulou, M.; Dovas, C.I. West Nile virus lineage 2 strain in Greece, 2012. Emerg. Infect. Dis. 2013, 19, 827–829. [Google Scholar]
- Valiakos, G.; Touloudi, A.; Athanasiou, L.V.; Giannakopoulos, A.; Iacovakis, C.; Birtsas, P.; Spyrou, V.; Dalabiras, Z.; Petrovska, L.; Billinis, C. Serological and molecular investigation into the role of wild birds in the epidemiology of West Nile virus in Greece. Virol. J. 2012, 9. [Google Scholar] [CrossRef]
- Maxie, M.G.; Youssef, S. Nervous system. In Kennedy and Palmer’s Pathology of Domestic Animals, 5th ed.; Maxie, M.G., Ed.; Saunders Elsevier: Edinburgh, UK, 2007. [Google Scholar]
- Sebastian, M.M.; Stewart, I.; Williams, N.M.; Poonacha, K.B.; Sells, S.F.; Vickers, M.L.; Harrison, L.R. Pathological, entomological, avian and meteorological investigation of a West Nile virus epidemic in a horse farm. Transbound. Emerg. Dis. 2008, 55, 134–139. [Google Scholar] [CrossRef]
- Abutarbush, S.M.; O’Connor, B.P.; Clark, C.; Sampieri, F.; Naylor, J.M. Clinical West Nile virus infection in 2 horses in western Canada. Can. Vet. J. 2004, 45, 315–317. [Google Scholar]
- Venter, M.; Human, S.; van Niekerk, S.; Williams, J.; Eeden, C.v.; Freeman, F. Fatal neurologic disease and abortion in mare infected with lineage 1 West Nile virus, south Africa. Emerg. Infect. Dis. 2011, 17, 1534–1536. [Google Scholar]
- Bunning, M.L.; Bowen, R.A.; Cropp, C.B.; Sullivan, K.G.; Davis, B.S.; Komar, N.; Godsey, M.S.; Baker, D.; Hettler, D.L.; Holmes, D.A.; et al. Experimental infection of horses with West Nile virus. Emerg. Infect. Dis. 2002, 8, 380–386. [Google Scholar] [CrossRef]
- Nielsen, C.F.; Reisen, W.K.; Armijos, M.V.; Maclachlan, N.J.; Scott, T.W. High subclinical west nile virus incidence among nonvaccinated horses in northern California associated with low vector abundance and infection. Amer. J. Trop. Med. Hyg. 2008, 78, 45–52. [Google Scholar]
- Castillo-Olivares, J.; Wood, J. West Nile virus infection of horses. Vet. Res. 2004, 35, 467–483. [Google Scholar] [CrossRef]
- Venter, M.; Steyl, J.; Human, S.; Weyer, J.; Zaayman, D.; Blumberg, L.; Leman, P.A.; Paweska, J.; Swanepoel, R. Transmission of West Nile virus during horse autopsy. Emerg. Infect. Dis. 2010, 16, 573–575. [Google Scholar] [CrossRef]
- Ward, M.P.; Schuermann, J.A.; Highfield, L.D.; Murray, K.O. Characteristics of an outbreak of West Nile virus encephalomyelitis in a previously uninfected population of horses. Vet. Microbiol. 2006, 118, 255–259. [Google Scholar]
- Epp, T.; Waldner, C.; West, K.; Townsend, H. Factors associated with West Nile virus disease fatalities in horses. Can. Vet. J. 2007, 48, 1137–1145. [Google Scholar]
- Cantile, C.; Piero, F.D.; di Guardo, G.; Arispici, M. Pathologic and immunohistochemical findings in naturally occurring West Nile virus infection in horses. Vet. Pathol. 2001, 38, 414–421. [Google Scholar] [CrossRef]
- Steinman, A.; Banet, C.; Sutton, G.A.; Yadin, H.; Hadar, S.; Brill, A. Clinical signs of West Nile virus encephalomyelitis in horses during the outbreak in Israel in 2000. Vet. Rec. 2002, 151, 47–49. [Google Scholar] [CrossRef]
- Weese, J.S.; Baird, J.D.; DeLay, J.; Kenney, D.G.; Staempfli, H.R.; Viel, L.; Parent, J.; Smith-Maxie, L.; Poma, R. West Nile virus encephalomyelitis in horses in Ontario: 28 cases. Can. Vet. J. 2003, 44, 469–473. [Google Scholar]
- Cantile, C.; di Guardo, G.; Eleni, C.; Arispici, M. Clinical and neuropathological features of West Nile virus equine encephalomyelitis in Italy. Equine Vet. J. 2000, 32, 31–35. [Google Scholar]
- Bourgeois, M.A.; Denslow, N.D.; Seino, K.S.; Barber, D.S.; Long, M.T. Gene expression analysis in the thalamus and cerebrum of horses experimentally infected with West Nile virus. PLoS One 2011, 6. [Google Scholar] [CrossRef]
- Zaayman, D.; Human, S.; Venter, M. A highly sensitive method for the detection and genotyping of West Nile virus by real-time PCR. J. Virol. Methods 2009, 157, 155–160. [Google Scholar] [CrossRef]
- Johnson, A.L. Update on infectious diseases affecting the equine nervous system. Vet. Clin. North. Amer. Equine Pract. 2011, 27, 573–587. [Google Scholar] [CrossRef]
- Kleinboeker, S.B.; Loiacono, C.M.; Rottinghaus, A.; Pue, H.L.; Johnson, G.C. Diagnosis of West Nile virus infection in horses. J. Vet. Diagn. Invest. 2004, 16, 2–10. [Google Scholar]
- Ng, T.; Hathaway, D.; Jennings, N.; Champ, D.; Chiang, Y.W.; Chu, H.J. Equine vaccine for West Nile virus. Dev. Biol. (Basel) 2003, 114, 221–227. [Google Scholar]
- Garch, H.E.; Minke, J.M.; Rehder, J.; Richard, S.; Toulemonde, C.E.; Dinic, S.; Andreoni, C.; Audonnet, J.C.; Nordgren, R.; Juillard, V. A West Nile virus (WNV) recombinant canarypox virus vaccine elicits WNV-specific neutralizing antibodies and cell-mediated immune responses in the horse. Vet. Immunol. Immunopathol. 2008, 123, 230–239. [Google Scholar] [CrossRef]
- Seino, K.K.; Long, M.T.; Gibbs, E.P.; Bowen, R.A.; Beachboard, S.E.; Humphrey, P.P.; Dixon, M.A.; Bourgeois, M.A. Comparative efficacies of three commercially available vaccines against West Nile virus (WNV) in a short-duration challenge trial involving an equine WNV encephalitis model. Clin. Vaccine Immunol. 2007, 14, 1465–1471. [Google Scholar]
- Minke, J.M.; Siger, L.; Cupillard, L.; Powers, B.; Bakonyi, T.; Boyum, S.; Nowotny, N.; Bowen, R. Protection provided by a recombinant alvac(®)-WNV vaccine expressing the prm/e genes of a lineage 1 strain of WNV against a virulent challenge with a lineage 2 strain. Vaccine 2011, 29, 4608–4612. [Google Scholar] [CrossRef]
- Chang, D.C.; Liu, W.J.; Anraku, I.; Clark, D.C.; Pollitt, C.C.; Suhrbier, A.; Hall, R.A.; Khromykh, A.A. Single-round infectious particles enhance immunogenicity of a DNA vaccine against West Nile virus. Nat. Biotechnol. 2008, 26, 571–577. [Google Scholar] [CrossRef]
- Koptopoulos, G.; Papadopoulos, O. A serological survey for tick-borne encephalitis and West Nile viruses in Greece. In Arboviruses in the Mediterranean Countries; Vesenjak-Hirjan, J., Porterfield, J., Eds.; Gustav Fischer: Stuttgart, Germany, 1980. [Google Scholar]
- Dovas, C.I.; Chaintoutis, S.C.; Chaskopoulou, A.; Diakakis, N.; Bouzalas, I.; Papanastasopoulou, M.; Danis, K.; Papa, A.; Papadopoulos, O. Detection of WNV enzootic circulation in horses with neurological signs and in captive sentinel chickens in the prefecture of thessaloniki, Greece. In 2nd Congress of the European Association of Veterinary Laboratory Diagnosticians; Kazimierz: Dolny, Poland, 2012. [Google Scholar]
- Immediate Notification on West Nile Fever, Greece. Available online: http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapFullEventReport&reportid=9642 (accessed on 25 November 2013).
- Ariel, E. Viruses in reptiles. Vet. Res. 2011, 42. [Google Scholar] [CrossRef]
- Jacobson, E.R.; Ginn, P.E.; Troutman, J.M.; Farina, L.; Stark, L.; Klenk, K.; Burkhalter, K.L.; Komar, N. West Nile virus infection in farmed american alligators (alligator mississippiensis) in Florida. J. Wildl. Dis. 2005, 41, 96–106. [Google Scholar]
- St Leger, J.; Wu, G.; Anderson, M.; Dalton, L.; Nilson, E.; Wang, D. West Nile virus infection in killer whale, Texas, USA, 2007. Emerg. Infect. Dis. 2011, 17, 1531–1533. [Google Scholar]
- Bowen, R.A.; Nemeth, N.M. Experimental infections with West Nile virus. Curr. Opin. Infect. Dis. 2007, 20, 293–297. [Google Scholar] [CrossRef]
- Austgen, L.E.; Bowen, R.A.; Bunning, M.L.; Davis, B.S.; Mitchell, C.J.; Chang, G.J. Experimental infection of cats and dogs with West Nile virus. Emerg. Infect. Dis. 2004, 10, 82–86. [Google Scholar]
- West Nile Virus Infection. Available online: http://www.cfsph.iastate.edu/Factsheets/pdfs/west_nile_fever.pdf (accessed on 30 October 2013).
- Kramer, L.D.; Styer, L.M.; Ebel, G.D. A global perspective on the epidemiology of West Nile virus. Annu. Rev. Entomol. 2008, 53, 61–81. [Google Scholar] [CrossRef]
- Ribeiro, J.M.; Charlab, R.; Pham, V.M.; Garfield, M.; Valenzuela, J.G. An insight into the salivary transcriptome and proteome of the adult female mosquito Culex pipiens quinquefasciatus. Insect Biochem. Mol. Biol. 2004, 34, 543–563. [Google Scholar] [CrossRef]
- Ribeiro, J.M.; Francischetti, I.M. Role of arthropod saliva in blood feeding: Sialome and post-sialome perspectives. Annu. Rev. Entomol. 2003, 48, 73–88. [Google Scholar] [CrossRef]
- Titus, R.G.; Bishop, J.V.; Mejia, J.S. The immunomodulatory factors of arthropod saliva and the potential for these factors to serve as vaccine targets to prevent pathogen transmission. Parasite Immunol. 2006, 28, 131–141. [Google Scholar]
- Diamond, M.S.; Blair, C.D. Vector biology and West Nile virus. In West Nile Encephalitis Virus Infection; Diamond, M.S., Ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Kramer, L.D.; Ebel, G.D. Dynamics of flavivirus infection in mosquitoes. Adv. Virus Res. 2003, 60, 187–232. [Google Scholar]
- Turell, M.J.; Dohm, D.J.; Sardelis, M.R.; Oquinn, M.L.; Andreadis, T.G.; Blow, J.A. An update on the potential of north american mosquitoes (diptera: Culicidae) to transmit West Nile virus. J. Med. Entomol. 2005, 42, 57–62. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Daszak, P.; Jones, M.J.; Marra, P.P.; Kramer, L.D. Host heterogeneity dominates West Nile virus transmission. Proc. Biol. Sci. 2006, 273, 2327–2333. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Kramer, L.D.; Jones, M.J.; Marra, P.P.; Daszak, P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006, 4. [Google Scholar] [CrossRef]
- Hamer, G.L.; Kitron, U.D.; Brawn, J.D.; Loss, S.R.; Ruiz, M.O.; Goldberg, T.L.; Walker, E.D. Culex pipiens (diptera: Culicidae): A bridge vector of West Nile virus to humans. J. Med. Entomol. 2008, 45, 125–128. [Google Scholar] [CrossRef]
- Fonseca, D.M.; Keyghobadi, N.; Malcom, C.A.; Mehmet, C.; Schaffner, F.; Mogi, M.; Fleischer, R.C.; Wilkerson, R.C. Emerging vectors in the Culex pipiens complex. Science 2004, 303, 1535–1538. [Google Scholar] [CrossRef]
- Savage, H.M.; Ceianu, C.; Nicolescu, G.; Karabatsos, N.; Lanciotti, R.; Vladimirescu, A.; Laiv, L.; Unqureanu, A.; Romanca, C.; Tsai, T.F. Entomologic and avian investigations of an epidemic of West Nile fever in Romania in 1996, with serologic and molecular characterization of a virus isolate from mosquitoes. Amer. J. Trop. Med. Hyg. 1999, 61, 600–611. [Google Scholar]
- Fyodorova, M.V.; Savage, H.M.; Lopatina, J.V.; Bulqakova, T.A.; Ivanitsky, A.V.; Platonova, O.V.; Platonov, A.E. Evaluation of potential West Nile virus vectors in Volgograd region, Russia, 2003 (diptera: Culicidae): Species composition, bloodmeal host utilization, and virus infection rates of mosquitoes. J. Med. Entomol. 2006, 43, 552–563. [Google Scholar] [CrossRef]
- Farajollahi, A.; Fonseca, D.M.; Kramer, L.D.; Kilpatrick, A.M. “Bird biting” mosquitoes and human disease: A review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 2011, 11, 1577–1585. [Google Scholar]
- Vinogradova, E.B. Culex Pipiens Pipiens Mosquitoes: Taxonomy, Distribution, Ecology, Physiology, Genetics, Applied Importance and Control; Pensoft: Moscow, Russia, 2000. [Google Scholar]
- Gomes, B.; Kioulos, E.; Papa, A.; Almeida, A.P.; Vontas, J.; Pinto, J. Distribution and hybridization of Culex pipiens forms in Greece during the West Nile virus outbreak of 2010. Infect. Genet. Evol. 2013, 16, 218–225. [Google Scholar] [CrossRef]
- Gomes, B.; Sousa, C.A.; Novo, M.T.; Freitas, F.B.; Alves, R.; Côrte-Real, A.R.; Salqueiro, P.; Donnelly, M.J.; Almeida, A.P.; Pinto, J. Asymmetric introgression between sympatric molestus and pipiens forms of Culex pipiens (diptera: Culicidae) in the Comporta region, Portugal. BMC Evol. Biol. 2009, 9. [Google Scholar] [CrossRef]
- Papa, A.; Xanthopoulou, K.; Tsioka, A.; Kalaitzopoulou, S.; Mourelatos, S. West Nile virus in mosquitoes in Greece. Parasitol. Res. 2013, 112, 1551–1555. [Google Scholar] [CrossRef]
- Andreadis, T.G.; Anderson, J.F.; Vossbrinck, C.R.; Main, A.J. Epidemiology of West Nile virus in connecticut: A five-year analysis of mosquito data 1999–2003. Vector-Borne Zoonotic Dis. 2004, 4, 360–378. [Google Scholar] [CrossRef]
- Sardelis, M.R.; Turell, M.J.; Dohm, D.J.; O'Guinn, M.L. Vector competence of selected North american Culex and Coquillettidia mosquitoes for West Nile virus. Emerg. Infect. Dis. 2001, 7, 1018–1022. [Google Scholar] [CrossRef]
- Savage, H.M.; Anderson, M.; Gordon, E.; McMillen, L.; Colton, L.; Charnetzky, D.; Delorey, M.; Aspen, S.; Burkhalter, K.; Biggerstaff, B.J.; et al. Oviposition activity patterns and West Nile virus infection rates for members of the Culex pipiens complex at different habitat types within the hybrid zone, Shelby county, TN, 2002 (diptera: Culicidae). J. Med. Entomol. 2006, 43, 1227–1238. [Google Scholar] [CrossRef]
- Anderson, J.F.; Main, A.J. Importance of vertical and horizontal transmission of West Nile virus by Culex pipiens in the northeastern United States. J. Infect. Dis. 2006, 194, 1577–1579. [Google Scholar] [CrossRef]
- Nasci, R.S.; Savage, H.M.; White, D.J.; Miller, J.R.; Cropp, B.C.; Godsey, M.S.; Kerst, A.J.; Bennett, P.; Gottfried, K.; Lanciotti, R.S. West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerg. Infect. Dis. 2001, 7, 742–744. [Google Scholar]
- Hubálek, Z. Mosquito-borne viruses in Europe. Parasitol. Res. 2008, 103, S29–S43. [Google Scholar] [CrossRef]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control; Springer: Berlin-Heidelberg, Germany, 2010. [Google Scholar]
- Silver, J.B. Mosquito ecology: Field Sampling Methods, 3rd ed.; Springer: New York, NY, USA, 2008. [Google Scholar]
- WHOPES. WHOPES-Recommended Compounds and Formulations for Control of Mosquito Larvae. Available online: http://www.who.int/whopes/Mosquito_Larvicides_25_Oct_2013.pdf (accessed on 18 November 2013).
- WHOPES. Who Recommended insectiCides for Space Spraying Against Mosquitoes. Available online: http://www.who.int/whopes/Insecticides_for_space_spraying_Jul_2012.pdf (accessed on 29 July 2013).
- Georghiou, G.P. Principles of insecticide resistance management. Phytoprotection 1994, 75, 51–59. [Google Scholar] [CrossRef]
- Savopoulou-Soultani, M.; Andreadis, S.; Soultani-Zouroulidi, C. Insects and Other Arthropods of Sanitary Importance: Biology, Ecology, Control; Copy City: Thessaloniki, Greece, 2011. [Google Scholar]
- CDC. Mosquito Species in Which West Nile Virus has been Detected, United States, 1999–2012. Available online: http://www.cdc.gov/westnile/resources/pdfs/Mosquito%20Species%201999-2012.pdf (accessed on 29 July 2013).
- Labuda, M.; Kozuch, O.; Gresíková, M. Isolation of West Nile virus from Aedes cantans mosquitoes in West Slovakia. Acta Virol. 1974, 18, 424–433. [Google Scholar]
- Lvov, D.K.; Butenko, A.M.; Gromashevsky, V.L.; Kovtunov, A.I.; Pripilov, A.G.; Kinney, R.; Aristova, V.A.; Dzharkenov, A.F.; Samokhvalov, E.I.; Savage, H.M.; et al. West Nile virus and other zoonotic viruses in Russia: Examples of emerging-reemerging situations. Arch. Virol. Suppl. 2004, 18, 85–96. [Google Scholar]
- Calistri, P.; Giovannini, A.; Hubalek, Z.; Ionescu, A.; Monaco, F.; Savini, G.; Lelli, R. Epidemiology of West Nile in Europe and in the Mediterranean Basin. Open Virol. J. 2010, 4, 29–37. [Google Scholar]
- Hannoun, C.; Panthier, R.; Mouchet, J.; Eouzan, J.P. Isolement en France du virus West Nile à partir de malades et du vecteur Culex molestus ficalbi. C. R. Hebd. Seances Acad. Sci. 1964, 259, 4170–4172. [Google Scholar]
- Filipe, A.R. Isolation in Portugal of West Nile virus from Anopheles maculipennis mosquitoes. Acta Virol. 1972, 16, 361. [Google Scholar]
- Almeida, A.P.; Galão, R.P.; Sousa, C.A.; Novo, M.T.; Parreira, R.; Pinto, J.; Piedade, J.; Esteves, A. Potential mosquito vectors of arboviruses in portugal: Species, distribution, abundance and West Nile infection. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 823–832. [Google Scholar] [CrossRef]
- Vazquez, A.; Ruiz, S.; Herrero, L.; Moreno, J.; Molero, F.; Magallanes, A.; Sánchez-Seco, M.P.; Fiquerola, J.; Tenorio, A. West Nile and Usutu viruses in mosquitoes in Spain, 2008–2009. Amer. J. Trop. Med. Hyg. 2011, 85, 178–181. [Google Scholar] [CrossRef]
- Hakim, J.A.; Bitto, A.C. Comprehensive surveillance, prevention, and control measures for West Nile virus in Monroe county, Pennsylvania. Environ. Pract. 2004, 6, 36–49. [Google Scholar]
- Elnaiem, D.E.; Kelley, K.; Wright, S.; Laffey, R.; Yoshimura, G.; Reed, M.; Goodman, G.; Thiemann, T.; Reimer, L.; Reisen, W.K.; et al. Impact of aerial spraying of pyrethrin insecticide on Culex pipiens and Culex tarsalis (diptera: Culicidae) abundance and West Nile virus infection rates in an urban/suburban area of Sacramento county, California. J. Med. Entomol. 2008, 45, 741–747. [Google Scholar] [CrossRef]
- Carney, R.M.; Husted, S.; Jean, C.; Glaser, C.; Kramer, V. Efficacy of aerial spraying of mosquito adulticide in reducing incidence of West Nile virus, California, 2005. Emerg. Infect. Dis. 2008, 14, 747–754. [Google Scholar]
- Barber, L.M.; Schleier, J.J.; Peterson, R.K. Economic cost analysis of West Nile virus outbreak, Sacramento county, California, USA, 2005. Emerg. Infect. Dis. 2010, 16, 480–486. [Google Scholar] [CrossRef]
- Macedo, P.A.; Schleier, J.J.; Reed, M.; Kelley, K.; Goodman, G.W.; Brown, D.A.; Peterson, R.K. Evaluation of efficacy and human health risk of aerial ultra-low volume applications of pyrethrins and piperonyl butoxide for adult mosquito management in response to West Nile virus activity in Sacramento county, California. J. Amer. Mosq. Control. Assoc. 2010, 26, 57–66. [Google Scholar] [CrossRef]
- Lothtrop, H.D.; Lothtrop, B.B.; Gomsi, D.E.; Reisen, W.K. Intensive early season adulticide applications decrease arbovirus transmission throughout the Coachella Valley, Riverside County, California. Vector-Borne Zoonotic Dis. 2008, 8, 475–489. [Google Scholar] [CrossRef]
- Tedesco, C.; Ruiz, M.; McLafferty, S. Mosquito politics: Local vector control policies and the spread of West Nile virus in the Chicago region. Health Place 2010, 16, 1188–1195. [Google Scholar] [CrossRef]
- Piakis, N.; Iatrou, G.; Mourelatos, S.; Gewehr, S. Five years of mosquito control in northern Greece. In Area-wide Control of Insect Pests; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Chaskopoulou, A.; Latham, M.D.; Pereira, R.M.; Connelly, R.; Bonds, J.A.; Koehler, P.G. Efficacy of aerial ultra-low volume applications of two novel water-based formulations of unsynergized pyrethroids against riceland mosquitoes in Greece. J. Amer. Mosq. Control. Assoc. 2011, 27, 414–422. [Google Scholar] [CrossRef]
- West Nile Virus Infection Outbreak in Humans in Central Macedonia, Greece; ECDC: Stockholm, Sweden, 2010.
- Diuk-Wasser, M.A.; Brown, H.E.; Andreadis, T.G.; Fish, D. Modeling the spatial distribution of mosquito vectors for West Nile virus in Connecticut, USA. Vector-Borne Zoonotic Dis. 2006, 6, 283–295. [Google Scholar] [CrossRef]
- Winters, A.M.; Bolling, B.G.; Beaty, B.J.; Blair, C.D.; Eisen, R.J.; Meyer, A.M.; Pape, W.J.; Moore, C.G.; Eisen, L. Combining mosquito vector and human disease data for improved assessment of spatial West Nile virus disease risk. Amer. J. Trop. Med. Hyg. 2008, 78, 654–665. [Google Scholar]
- Angelini, P.; Tamba, M.; Finarelli, A.C.; Bellini, R.; Albieri, A.; Bonilauri, P.; Cavrini, F.; Dottori, M.; Gaibani, P.; Martini, E.; et al. West Nile virus circulation in Emilia-Romagna, Italy: The integrated surveillance system 2009. Eur. Surveill. 2010, 15. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19547 (accessed on 29 July 2013).
- Chaskopoulou, A.; Dovas, C.I.; Chaintoutis, S.C.; Kashefi, J.; Koehler, P.; Papanastassopoulou, M. Detection and early warning of West Nile virus circulation in Central Macedonia, Greece, using sentinel chickens and mosquitoes. Vector Borne Zoonotic Dis. 2013, 13, 723–732. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Marka, A.; Diamantidis, A.; Papa, A.; Valiakos, G.; Chaintoutis, S.C.; Doukas, D.; Tserkezou, P.; Giannakopoulos, A.; Papaspyropoulos, K.; Patsoula, E.; et al. West Nile Virus State of the Art Report of MALWEST Project. Int. J. Environ. Res. Public Health 2013, 10, 6534-6610. https://doi.org/10.3390/ijerph10126534
Marka A, Diamantidis A, Papa A, Valiakos G, Chaintoutis SC, Doukas D, Tserkezou P, Giannakopoulos A, Papaspyropoulos K, Patsoula E, et al. West Nile Virus State of the Art Report of MALWEST Project. International Journal of Environmental Research and Public Health. 2013; 10(12):6534-6610. https://doi.org/10.3390/ijerph10126534
Chicago/Turabian StyleMarka, Andriani, Alexandros Diamantidis, Anna Papa, George Valiakos, Serafeim C. Chaintoutis, Dimitrios Doukas, Persefoni Tserkezou, Alexios Giannakopoulos, Konstantinos Papaspyropoulos, Eleni Patsoula, and et al. 2013. "West Nile Virus State of the Art Report of MALWEST Project" International Journal of Environmental Research and Public Health 10, no. 12: 6534-6610. https://doi.org/10.3390/ijerph10126534








