Asellus aquaticus as a Potential Carrier of Escherichia coli and Other Coliform Bacteria into Drinking Water Distribution Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Locations
2.2. Sampling
2.3. Analyses
2.4. Increased Concentrations of Coliform Bacteria
2.5. Calculations
3. Results and Discussion
3.1. A. aquaticus and Associated Coliform Bacteria from Surface Waters
| Pond 1 | Pond 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| January 2010 0 °C (ice cover) | October 2010 11 °C | September 2010 5 °C | October 2010 6 °C | |||||
| E | T | E | T | E | T | E | T | |
| Water phase (MPN·mL−1) | <0.01 | 0.03 | 0.01 ± 0.01 | 5 ± 1.9 | 2 ± 1.4 | 5 ± 1.9 | 0.1 ± 0.03 | 1 ± 0.05 |
| Sediment phase (MPN·mL−1) | <1 | <1 | 14 ± 9.7 | >240 | 200 ± 6 | >240 | >86 | >170 |
| Associated (MPN· A. aquaticus−1) | <1 | <1 | <1 | 6 ± 4 | 3 ± 2 | 5 ± 2 | 1 ± 1 | 2 ± 1.5 |
3.2. A. aquaticus Associated Coliform Bacteria after Incubation
| E. coli | Total coliform bacteria | HPC (R2A, 20 °C) | |
|---|---|---|---|
| Total conc. in beaker (MPN·mL−1) | 0.4 | >170 | NA |
| Water phase (MPN·mL−1) | <0.01 | 58 | NA |
| Sediment phase (MPN·mL−1) | 4 | >1,200 | NA |
| Associated (MPN or CFU· A. aquaticus−1) | <1 | 350 | 1.3 ± 0.09 × 105 |
| Start sediment (MPN or CFU·mL−1) | <1 | <1 | 3.3 ± 0.14 × 104 |
3.3. Intrusion Scenarios
3.3.1. Scenario 1: Do A. aquaticus Contribute to the Transport of E. coli and Other Coliforms into Distribution Systems during Contaminations with Incoming Water and A. aquaticus Together?
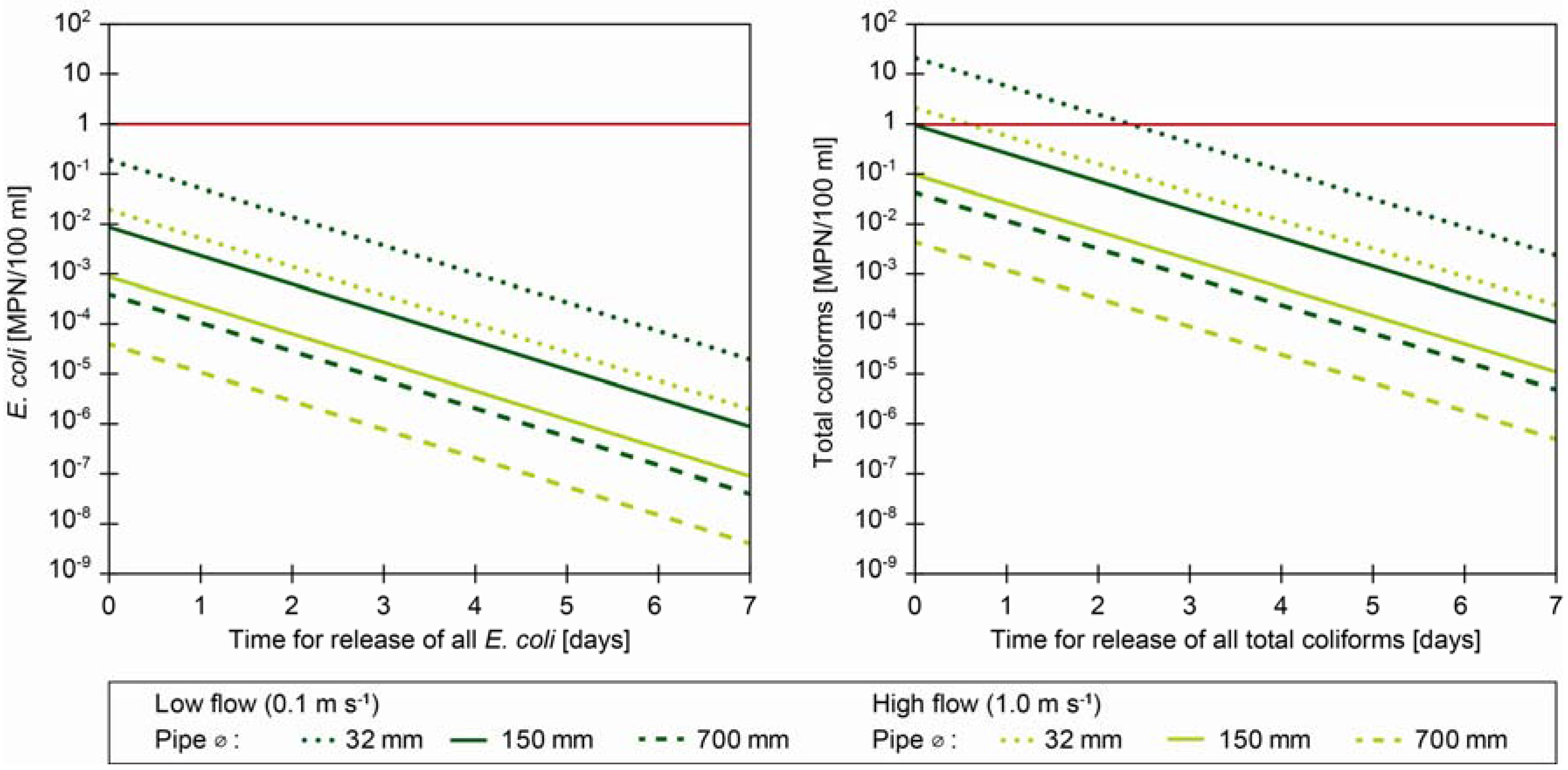
3.3.2. Scenario 2: Does Intrusion of Single A. aquaticus Specimens without Inflow of Water Cause Exceedings of Drinking Water Guideline Values for E. coli and Total Coliforms?
- (1)
- All associated bacteria are not likely to be released from A. aquaticus instantly. Based on previous experiments we assume that they will be released over time, most likely days [12,21]. We have applied a variable, describing a uniform release over a time frame of one minute to seven days for total release of all coliform bacteria (Figure 1). However, due to dispersion, sedimentation and inactivation, not accounted for in this scenario, the approach is conservative and coliform concentrations are expected to be reduced further.
- (2)
- Pipe diameters of transmission and distribution pipes in most distribution systems vary by an order of magnitude (40–700 mm with an average of 150 mm in a large Danish distribution system). Pipes of 32–40 mm are typically connection pipes to private premises.
- (3)
- The flow rates in distribution systems vary according to the water demands and may even be stagnant e.g., during night. We have calculated the release of bacteria at two flow velocities (0.1 and 1 m·s−1).
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Christensen, S.C.B.; Nissen, E.; Arvin, E.; Albrechtsen, H.-J. Distribution of Asellus aquaticus and microinvertebrates in a non-chlorinated drinking water supply system—Effects of pipe material and sedimentation. Water Res. 2011, 45, 3215–3224. [Google Scholar]
- Van Lieverloo, J.H.M.; van der Kooij, D.; Hoogenboezem, W. Invertebrates and protozoans (free-living) in drinking water distribution systems. In Encyclopedia of Environmental Microbiology; Bitton, G., Ed.; Wiley: New York, NY, USA, 2002; pp. 1718–1733. [Google Scholar]
- Gauthier, V.; Gérard, B.; Portal, J.-M.; Block, J.-C.; Gatel, D. Organic matter as loose deposits in a drinking water distribution system. Water Res. 1999, 33, 1014–1026. [Google Scholar] [CrossRef]
- Walker, A.P. The microscopy of consumer complaints. J. Inst. Water Eng. Sci. 1983, 37, 200–214. [Google Scholar]
- Levy, R.V.; Hart, F.L.; Cheethan, R.D. Occurrence and public health significance of invertebrates in drinking water systems. Am. Water Works Assoc. J. 1986, 78, 105–110. [Google Scholar]
- Locas, A.; Barbeau, B.; Gauthier, V. 2007 Nematodes as a source of total coliforms in a distribution system. Can. J. Microbiol. 2007, 53, 580–585. [Google Scholar] [CrossRef]
- Lupi, E.; Ricci, V.; Burrini, D. Recovery of bacteria in nematodes isolated from a drinking water supply. J. Water SRT Aqua. 1995, 44, 212–218. [Google Scholar]
- Evins, C. Small animals in drinking water distribution systems. In Safe. Piped Water: Managing Microbial Water Quality in Piped Distribution Systems; Ainsworth, R., Ed.; World Health Organization, IWA Publishing: London, UK, 2004; pp. 101–120. [Google Scholar]
- Van Lieverloo, J.H.M.; van Buuren, R.; Veenendaal, G.; van der Kooij, D. How to Control Invertebrates in Distribution Systems: By Starvation or by Flushing? In Proceedings of the AWWA Water Quality Technology Conference, Denver, CO, USA, 9–12 November 1997.
- Holland, G.J. The eradication of Asellus aquaticus from water supply mains. J. Inst. Water Eng. 1956, 10, 220–241. [Google Scholar]
- Økland, K.A. Life history and growth of Asellus aquaticus (L.) in relation to environment in a eutrophic lake in Norway. Hydrobiologia 1978, 59, 243–259. [Google Scholar] [CrossRef]
- Levy, R.V.; Cheetham, R.D.; Davis, J.; Winer, G.; Hart, F.L. Novel method for studying the public health significance of macroinvertebrates occurring in potable water. Appl. Environ. Microbiol. 1984, 47, 889–894. [Google Scholar]
- Wang, Y.; Brune, A.; Zimmer, M. Bacterial symbionts in the hepatopancreas of isopods: Diversity and environmental transmission. FEMS Microbiol. Ecol. 2007, 61, 141–152. [Google Scholar] [CrossRef]
- Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2008.
- European Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. Available online: http://www.iss.it/binary/publ/publi/0016.1109850012.pdf (accessed on 25 February 2013).
- Paton, J.C.; Paton, A.W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 1998, 11, 450–79. [Google Scholar]
- LeChevallier, M.W. Coliform regrowth in drinking water—A review. Am. Water Works Assoc. J. 1990, 82, 74–86. [Google Scholar]
- Van der Kooij, D. Potential for biofilm development in drinking water distribution systems. J. Appl. Microbiol. 1998, 85, 39–44. [Google Scholar] [CrossRef]
- Hammes, F.; Berger, C.; Koster, O.; Egli, T. Assessing biological stability of drinking water without disinfectant residuals in a full-scale water supply system. J. Water SRT Aqua. 2010, 59, 31–40. [Google Scholar] [CrossRef]
- Bichai, F.; Barbeau, B.; Payment, P. Protection against UV disinfection of E. coli bacteria and B. subtilis spores ingested by C. elegans nematodes. Water Res. 2009, 43, 3397–3406. [Google Scholar] [CrossRef]
- Christensen, S.C.B.; Nissen, E.; Arvin, E.; Albrechtsen, H.-J. Influence of Asellus. aquaticus on the indicator organisms Escherichia coli and Klebsiella. pneumoniae and the pathogen Campylobacter jejuni in drinking water. Water Res. 2012, 46, 5279–5286. [Google Scholar]
- Reasoner, D.J.; Geldreich, E.E. A new medium for the enumeration and subculture of bacteria from potable water. Appl. Env. Microbiol. 1985, 49, 1–7. [Google Scholar]
- Raghubeer, E.V.; Matches, J.R. Temperature range for growth of Escherichia coli stereotype O157, H7 and selected coliforms in E. coli medium. J. Clin. Microbiol. 1990, 28, 803–805. [Google Scholar]
- Flint, K.P. The long term survival of Escherichia coli in river water. J. Appl. Bacteriol. 1987, 63, 261–270. [Google Scholar] [CrossRef]
- Bichai, F.; Hijnen, W.; Baars, E.; Rosielle, M.; Dullemont, Y.; Barbeau, B. Preliminary study on the occurrence and risk arising from bacteria internalized in zooplankton in drinking water. Water Sci. Technol. 2011, 63, 108–14. [Google Scholar] [CrossRef]
- Heidelberg, J.F.; Heidelberg, K.B.; Colwell, R.R. Bacteria of the gamma-subclass Proteobacteria associated with zooplankton in Chesapeake Bay. Society 2002, 68, 5498–5507. [Google Scholar]
- Tang, K.W.; Turk, V.; Grossart, H.-P. Linkage between crustacean zooplankton and aquatic bacteria. Aqua. Microb. Ecol. 2010, 61, 261–277. [Google Scholar] [CrossRef]
- WHO and UNICEF. Global Water Supply and Sanitation Assessment 2000 Report. Available online: http://www.who.int/water_sanitation_health/monitoring/jmp2000.pdf (accessed on 25 February 2013).
- Vewin 2012. The Association of Dutch Water Companies. Available online: http://www.vewin.nl/english/Dutch%20water%20sector/Pages/default.aspx (accessed on 23 November 2012).
- DANVA’s Benchmarking and Water Statistics 2010. Available online: http://aoa.ew.eea.europa.eu/virtual-library-viewer/answer_8384288221 (accessed on 25 February 2013).
- Karim, M.R.; Abbaszadegan, M.; Le Chevallier, M. Potential for pathogen intrusion during pressure transients. Am. Water Works Assoc. J. 2003, 95, 134–146. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Christensen, S.C.B.; Arvin, E.; Nissen, E.; Albrechtsen, H.-J. Asellus aquaticus as a Potential Carrier of Escherichia coli and Other Coliform Bacteria into Drinking Water Distribution Systems. Int. J. Environ. Res. Public Health 2013, 10, 845-855. https://doi.org/10.3390/ijerph10030845
Christensen SCB, Arvin E, Nissen E, Albrechtsen H-J. Asellus aquaticus as a Potential Carrier of Escherichia coli and Other Coliform Bacteria into Drinking Water Distribution Systems. International Journal of Environmental Research and Public Health. 2013; 10(3):845-855. https://doi.org/10.3390/ijerph10030845
Chicago/Turabian StyleChristensen, Sarah C. B., Erik Arvin, Erling Nissen, and Hans-Jørgen Albrechtsen. 2013. "Asellus aquaticus as a Potential Carrier of Escherichia coli and Other Coliform Bacteria into Drinking Water Distribution Systems" International Journal of Environmental Research and Public Health 10, no. 3: 845-855. https://doi.org/10.3390/ijerph10030845





