Fate and Transport of Toxoplasma gondii Oocysts in Seasonally Snow Covered Watersheds: A Conceptual Framework from a Melting Snowpack to the Canadian Arctic Coasts
Abstract
:1. Introduction
2. The Framework
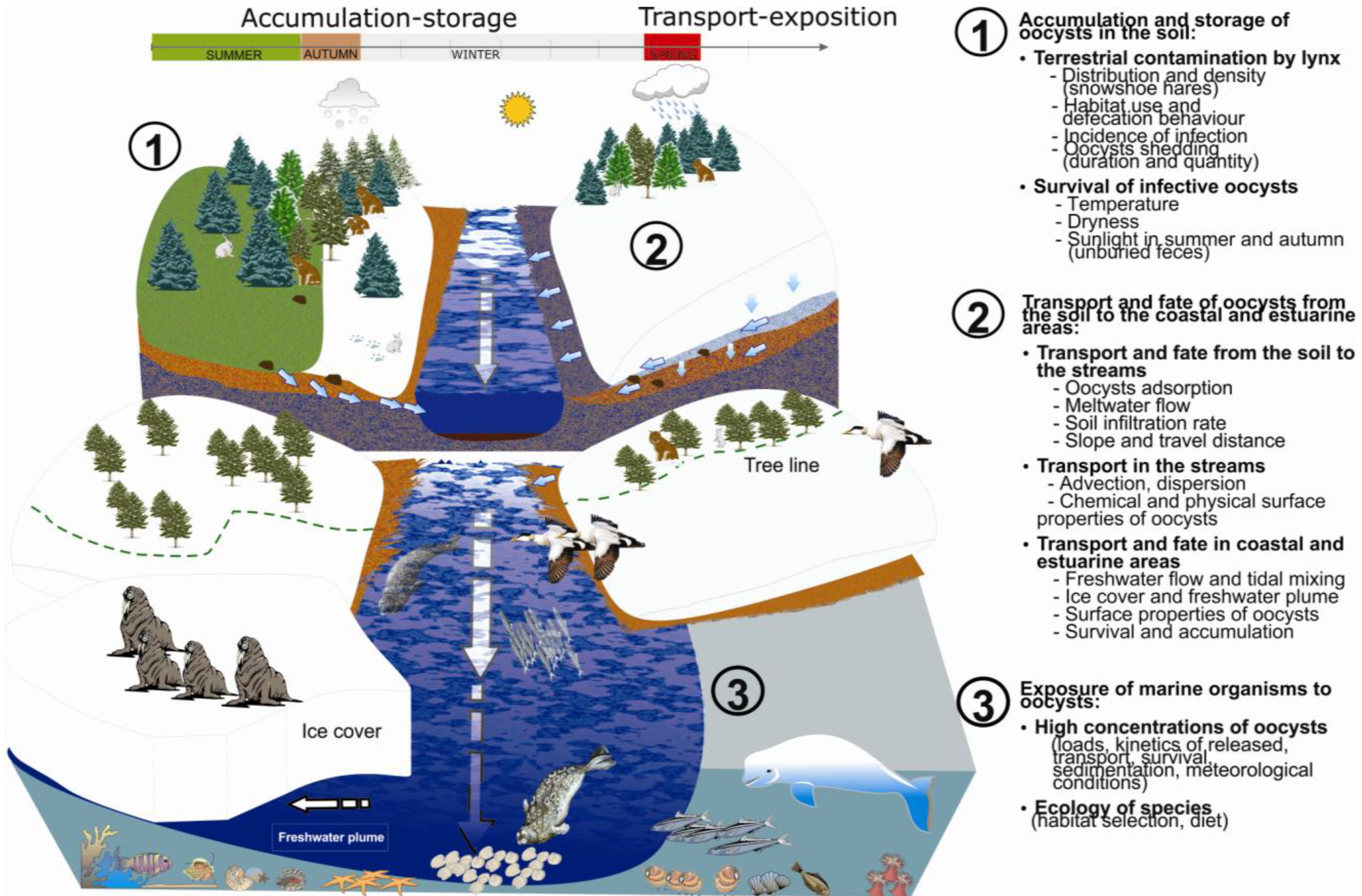
2.1. Accumulation and Storage of T. gondii Oocysts in the Soil and within the Snowpack
2.1.1. Environmental Contamination by T. gondii oocysts
2.1.2. Survival of T. gondii Oocysts
2.2. Transport and Fate of T. gondii Oocysts
2.2.1. Overland Transport from the Soil to the Streams
2.2.2. From the Streams to the Coastal and Estuarine Areas
2.3. Exposure of Marine Organisms to T. gondii Oocysts
2.3.1. Meteorological Conditions and Concentrations of T. gondii Oocysts in Estuaries
2.3.2. Ecology of Marine Species and Dietary Pathway
3. Conclusions
Acknowledgments
Conflict of Interest
References
- Ferguson, C.; Husman, A.M.D.; Altavilla, N.; Deere, D.; Ashbolt, N. Fate and transport of surface water pathogens in watersheds. Crit. Rev. Environ. Sci. Tech. 2003, 33, 299–361. [Google Scholar] [CrossRef]
- Thomas, M.K.; Charron, D.F.; Waltner-Toews, D.; Schuster, C.; Maarouf, A.R.; Holt, J.D. A role of high impact weather events in waterborne disease outbreaks in Canada, 1975–2001. Int. J. Environ. Health Rev. 2006, 16, 167–180. [Google Scholar] [CrossRef]
- Baldursson, S.; Karanis, P. Waterborne transmission of protozoan parasites: Review of worldwide outbreaks—An update 2004–2010. Water Res. 2011, 45, 6603–6614. [Google Scholar] [CrossRef]
- Bowie, W.R.; King, A.S.; Werker, D.H.; Isaac-Renton, J.L.; Bell, A.; Eng, S.B.; Marion, S.A. Outbreak of toxoplasmosis associated with municipal drinking water. The BC Toxoplasma investigation team. Lancet 1997, 350, 173–177. [Google Scholar]
- Whitman, R.L.; Przybyla-Kelly, K.; Shively, D.A.; Nevers, M.B.; Byappanahalli, M.N. Sunlight, season, snowmelt, storm, and source affect E. coli populations in an artificially ponded stream. Sci. Total Environ. 2008, 390, 448–455. [Google Scholar] [CrossRef]
- Zhu, Z.X.; Broersma, K.; Mazumder, A. Model assessment of cattle and climate impacts on stream fecal coliform pollution in the salmon river watershed, British Columbia, Canada. Water Air Soil Pollut. 2011, 215, 155–176. [Google Scholar] [CrossRef]
- Meyer, T.; Wania, F. Organic contaminant amplification during snowmelt. Water Res. 2008, 42, 1847–1865. [Google Scholar] [CrossRef]
- Simon, A.; Chambellant, M.; Ward, B.J.; Simard, M.; Proulx, J.F.; Levesque, B.; Bigras-Poulin, M.; Rousseau, A.N.; Ogden, N.H. Spatio-temporal variations and age effect on Toxoplasma gondii seroprevalence in seals from the Canadian Arctic. Parasitology 2011, 138, 1362–1368. [Google Scholar] [CrossRef]
- Dubey, J.P. Toxoplasmosis of Animals and Humans, 2nd ed; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Cole, R.A.; Lindsay, D.S.; Howe, D.K.; Roderick, C.L.; Dubey, J.P.; Thomas, N.J.; Baeten, L.A. Biological and molecular characterizations of Toxoplasma gondii strains obtained from southern sea otters (Enhydra lutris nereis). J. Parasitol. 2000, 86, 526–530. [Google Scholar]
- Conrad, P.A.; Miller, M.A.; Kreuder, C.; James, E.R.; Mazet, J.; Dabritz, H.; Jessup, D.A.; Gulland, F.; Grigg, M.E. Transmission of Toxoplasma: Clues from the study of sea otters as sentinels of Toxoplasma gondii flow into the marine environment. Int. J. Parasitol. 2005, 35, 1155–1168. [Google Scholar] [CrossRef]
- Miller, M.A.; Gardner, I.A.; Kreuder, C.; Paradies, D.M.; Worcester, K.R.; Jessup, D.A.; Dodd, E.; Harris, M.D.; Ames, J.A.; Packham, A.E.; et al. Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis). Int. J. Parasitol. 2002, 32, 997–1006. [Google Scholar] [CrossRef]
- Miller, M.A.; Miller, W.A.; Conrad, P.A.; James, E.R.; Melli, A.C.; Leutenegger, C.M.; Dabritz, H.A.; Packham, A.E.; Paradies, D.; et al. Type X Toxoplasma gondii in a wild mussel and terrestrial carnivores from coastal California: New linkages between terrestrial mammals, runoff and toxoplasmosis of sea otters. Int. J. Parasitol. 2008, 38, 1319–1328. [Google Scholar] [CrossRef]
- Vanwormer, E.; Fritz, H.; Shapiro, K.; Mazet, J.A.; Conrad, P.A. Molecules to modeling: Toxoplasma gondii oocysts at the human-animal-environment interface. Comp. Immunol Microbiol. Infect. Dis. 2012. [Google Scholar] [CrossRef]
- Simon, A.; Poulin, M.B.; Rousseau, A.N.; Dubey, J.P.; Ogden, N.H. Spatiotemporal dynamics of Toxoplasma gondii infection in Canadian lynx (Lynx canadensis) in Western Quebec, Canada. J. Wildlife Dis. 2013, 49, 39–48. [Google Scholar]
- Jokelainen, P.; Isomursu, M.; Nareaho, A.; Oksanen, A. Natural Toxoplasma gondii infections in european brown hares and mountain hares in Finland: Proportional mortality rate, antibody prevalence, and genetic characterization. J. Wildlife Dis. 2011, 47, 154–163. [Google Scholar]
- Frenkel, J.K.; Dubey, J.P. Effects of freezing on the viability of Toxoplasma oocysts. J. Parasitol. 1973, 59, 587–588. [Google Scholar] [CrossRef]
- Yilmaz, S.M.; Hopkins, S.H. Effects of different conditions on duration of infectivity of Toxoplasma gondii oocysts. J. Parasitol. 1972, 58, 938–939. [Google Scholar] [CrossRef]
- Woo, M.-K. Northern hydrology. In Canada’s Cold Environments; French, H.M., Slaymaker, O., Eds.; McGill-Queen’s University Press: Montréal, Canada, 1993; pp. 117–142. [Google Scholar]
- Mann, M.E.; Schmidt, G.A. Ground vs. surface air temperature trends: Implications for borehole surface temperature reconstructions. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Blagburn, B.L.; Dubey, J.P. Survival of nonsporulated Toxoplasma gondii oocysts under refrigerator conditions. Vet. Parasitol. 2002, 103, 309–313. [Google Scholar] [CrossRef]
- Mowat, G.; Poole, K.G.; O’Donoghue, M. Ecology of lynx in northern Canada and Alaska. In Ecology and Conservation of Lynx in the United States; Ruggerio, L.F., Aubry, K.B., Buskirk, S.W., Koehler, G.M., Krebs, C.J., McKelvey, K.S., Squires, J.R., Eds.; University of Colorado Press: Boulder, Co, USA, 2000; pp. 265–306. [Google Scholar]
- Dubey, J.P. Toxoplasma gondii oocyst survival under defined temperatures. J. Parasitol 1998, 84, 862–865. [Google Scholar] [CrossRef]
- Frenkel, J.K.; Ruiz, A.; Chinchilla, M. Soil survival of Toxoplasma oocysts in Kansas and Costa Rica. Am. J. Trop Med. Hyg. 1975, 24, 439–443. [Google Scholar]
- Lelu, M.; Villena, I.; Darde, M.L.; Aubert, D.; Geers, R.; Dupuis, E.; Marnef, F.; Poulle, M.L.; Gotteland, C.; Dumetre, A.; et al. Quantitative estimation of the viability of Toxoplasma gondii oocysts in soil. Appl. Environ. Microbol. 2012, 78, 5127–5132. [Google Scholar] [CrossRef]
- Shapiro, K.; Largier, J.; Mazet, J.A.; Bernt, W.; Ell, J.R.; Melli, A.C.; Conrad, P.A. Surface properties of Toxoplasma gondii oocysts and surrogate microspheres. Appl. Environ. Microbiol. 2009, 75, 1185–1191. [Google Scholar]
- Jamieson, R.; Gordon, R.; Joy, D.; Lee, H. Assessing microbial pollution of rural surface waters—A review of current watershed scale modeling approaches. Agr. Water Manag. 2004, 70, 1–17. [Google Scholar] [CrossRef]
- Davies, C.M.; Ferguson, C.M.; Kaucner, C.; Krogh, M.; Altavilla, N.; Deere, D.A.; Ashbolt, N.J. Dispersion and transport of Cryptosporidium oocysts from fecal pats under simulated rainfall events. Appl. Environ. Microbol. 2004, 70, 1151–1159. [Google Scholar] [CrossRef]
- McGechan, M.B.; Lewis, D.R. Transport of particulate and colloid-sorbed contaminants through soil, part 1: General principles. Biosyst. Eng. 2002, 83, 255–273. [Google Scholar] [CrossRef]
- Fraser, R.H.; Barten, P.K.; Pinney, D.A.K. Predicting stream pathogen loading from livestock using a geographical information system-based delivery model. J. Environ. Qual. 1998, 27, 935–945. [Google Scholar] [CrossRef]
- Tate, K.W.; Pereira, M.D.; Atwill, E.R. Efficacy of vegetated buffer strips for retaining Cryptosporidium parvum. J. Environ. Qual. 2004, 33, 2243–2251. [Google Scholar] [CrossRef]
- Dumetre, A.; Aubert, D.; Puech, P.H.; Hohweyer, J.; Azas, N.; Villena, I. Interaction forces drive the environmental transmission of pathogenic protozoa. Appl. Environ. Microbol. 2012, 78, 905–912. [Google Scholar] [CrossRef]
- Shapiro, K.; Conrad, P.A.; Mazet, J.A.; Wallender, W.W.; Miller, W.A.; Largier, J.L. Effect of estuarine wetland degradation on the transport of Toxoplasma gondii surrogates from land to sea. Appl. Environ. Microbiol. 2010, 76, 6821–6828. [Google Scholar] [CrossRef]
- Mann, K.H.; Lazier, J.R.N. Dynamics of Marine Ecosystems Biological-Physical Interactions in the Oceans, 3rd ed; Blackwell Pub.: Malden, MA, USA, 2006. [Google Scholar]
- Shapiro, K.; Silver, M.W.; Largier, J.L.; Conrad, P.A.; Mazet, J.A.K. Association of Toxoplasma gondii oocysts with fresh, estuarine, and marine macroaggregates. Limnol. Oceanogr. 2012, 57, 449–456. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Collins, M.V.; Mitchell, S.M.; Cole, R.A.; Flick, G.J.; Wetch, C.N.; Lindquist, A.; Dubey, J.P. Sporulation and survival of Toxoplasma gondii oocysts in seawater. J. Eukaryot. Microbiol. 2003, 50, 687–688. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Dubey, J.P. Long-term survival of Toxoplasma gondii sporulated oocysts in seawater. J. Parasitol. 2009, 95, 1019–1020. [Google Scholar] [CrossRef]
- Johnson, C.K.; Tinker, M.T.; Estes, J.A.; Conrad, P.A.; Staedler, M.; Miller, M.A.; Jessup, D.A.; Mazet, J.A. Prey choice and habitat use drive sea otter pathogen exposure in a resource-limited coastal system. Proc. Natl. Acad. Sci. USA 2009, 106, 2242–2247. [Google Scholar] [CrossRef]
- Jones, J.L.; Dargelas, V.; Roberts, J.; Press, C.; Remington, J.S.; Montoya, J.G. Risk factors for Toxoplasma gondii infection in the United States. Clin. Infect. Dis. 2009, 49, 878–884. [Google Scholar] [CrossRef]
- Arkush, K.D.; Miller, M.A.; Leutenegger, C.M.; Gardner, I.A.; Packham, A.E.; Heckeroth, A.R.; Tenter, A.M.; Barr, B.C.; Conrad, P.A. Molecular and bioassay-based detection of Toxoplasma gondii oocyst uptake by mussels (Mytilus galloprovincialis). Int. J. Parasitol. 2003, 33, 1087–1097. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Phelps, K.K.; Smith, S.A.; Flick, G.; Sumner, S.S.; Dubey, J.P. Removal of Toxoplasma gondii oocysts from sea water by eastern oysters (Crassostrea virginica). J. Eukaryot. Microbiol. 2001, 48, 197S–198S. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Collins, M.V.; Mitchell, S.M.; Wetch, C.N.; Rosypal, A.C.; Flick, G.J.; Zajac, A.M.; Lindquist, A.; Dubey, J.P. Survival of Toxoplasma gondii oocysts in Eastern oysters (Crassostrea virginica). J. Parasitol. 2004, 90, 1054–1057. [Google Scholar] [CrossRef]
- Fayer, R.; Dubey, J.P.; Lindsay, D.S. Zoonotic protozoa: From land to sea. Trends Parasitol. 2004, 20, 531–536. [Google Scholar] [CrossRef]
- Massie, G.N.; Ware, M.W.; Villegas, E.N.; Black, M.W. Uptake and transmission of Toxoplasma gondii oocysts by migratory, filter-feeding fish. Vet. Parasitol. 2010, 169, 296–303. [Google Scholar] [CrossRef]
- Dubey, J.P.; Lunney, J.K.; Shen, S.K.; Kwok, O.C.H.; Ashford, D.A.; Thulliez, P. Infectivity of low numbers of Toxoplasma gondii oocysts to pigs. J. Parasitol. 1996, 82, 438–443. [Google Scholar] [CrossRef]
- Dubey, J.P.; Speer, C.A.; Shen, S.K.; Kwok, O.C.H.; Blixt, J.A. Oocyst-induced murine toxoplasmosis: Life cycle, pathogenicity, and stage conversion in mice fed Toxoplasma gondii oocysts. J. Parasitol. 1997, 83, 870–882. [Google Scholar] [CrossRef]
- Karanis, P.; Aldeyarbi, H.M.; Mirhashemi, M.E.; Khalil, K.M. The impact of the waterborne transmission of Toxoplasma gondii and analysis efforts for water detection: An overview and update. Environ. Sci. Pollut. Res. Int. 2013, 20, 86–99. [Google Scholar] [CrossRef]
- Ferguson, C.M.; Charles, K.; Deere, D.A. Quantification of microbial sources in drinking-water catchments. Crit. Rev. Envron. Sci. Tech. 2009, 39, 1–40. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Simon, A.; Poulin, M.B.; Rousseau, A.N.; Ogden, N.H. Fate and Transport of Toxoplasma gondii Oocysts in Seasonally Snow Covered Watersheds: A Conceptual Framework from a Melting Snowpack to the Canadian Arctic Coasts. Int. J. Environ. Res. Public Health 2013, 10, 994-1005. https://doi.org/10.3390/ijerph10030994
Simon A, Poulin MB, Rousseau AN, Ogden NH. Fate and Transport of Toxoplasma gondii Oocysts in Seasonally Snow Covered Watersheds: A Conceptual Framework from a Melting Snowpack to the Canadian Arctic Coasts. International Journal of Environmental Research and Public Health. 2013; 10(3):994-1005. https://doi.org/10.3390/ijerph10030994
Chicago/Turabian StyleSimon, Audrey, Michel Bigras Poulin, Alain N. Rousseau, and Nicholas H. Ogden. 2013. "Fate and Transport of Toxoplasma gondii Oocysts in Seasonally Snow Covered Watersheds: A Conceptual Framework from a Melting Snowpack to the Canadian Arctic Coasts" International Journal of Environmental Research and Public Health 10, no. 3: 994-1005. https://doi.org/10.3390/ijerph10030994




