Decreased IgA+ B Cells Population and IgA, IgG, IgM Contents of the Cecal Tonsil Induced by Dietary High Fluorine in Broilers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diets and Broilers
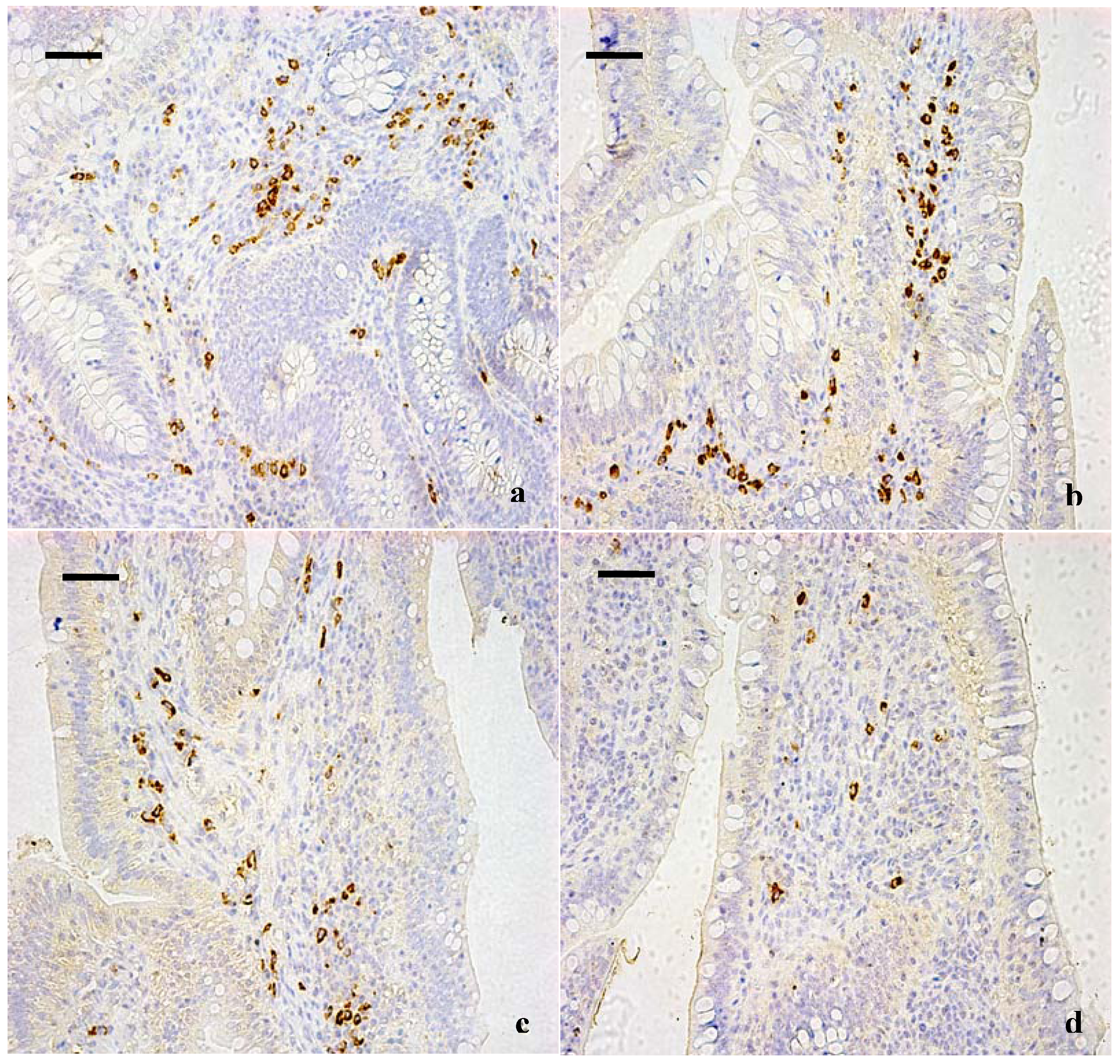
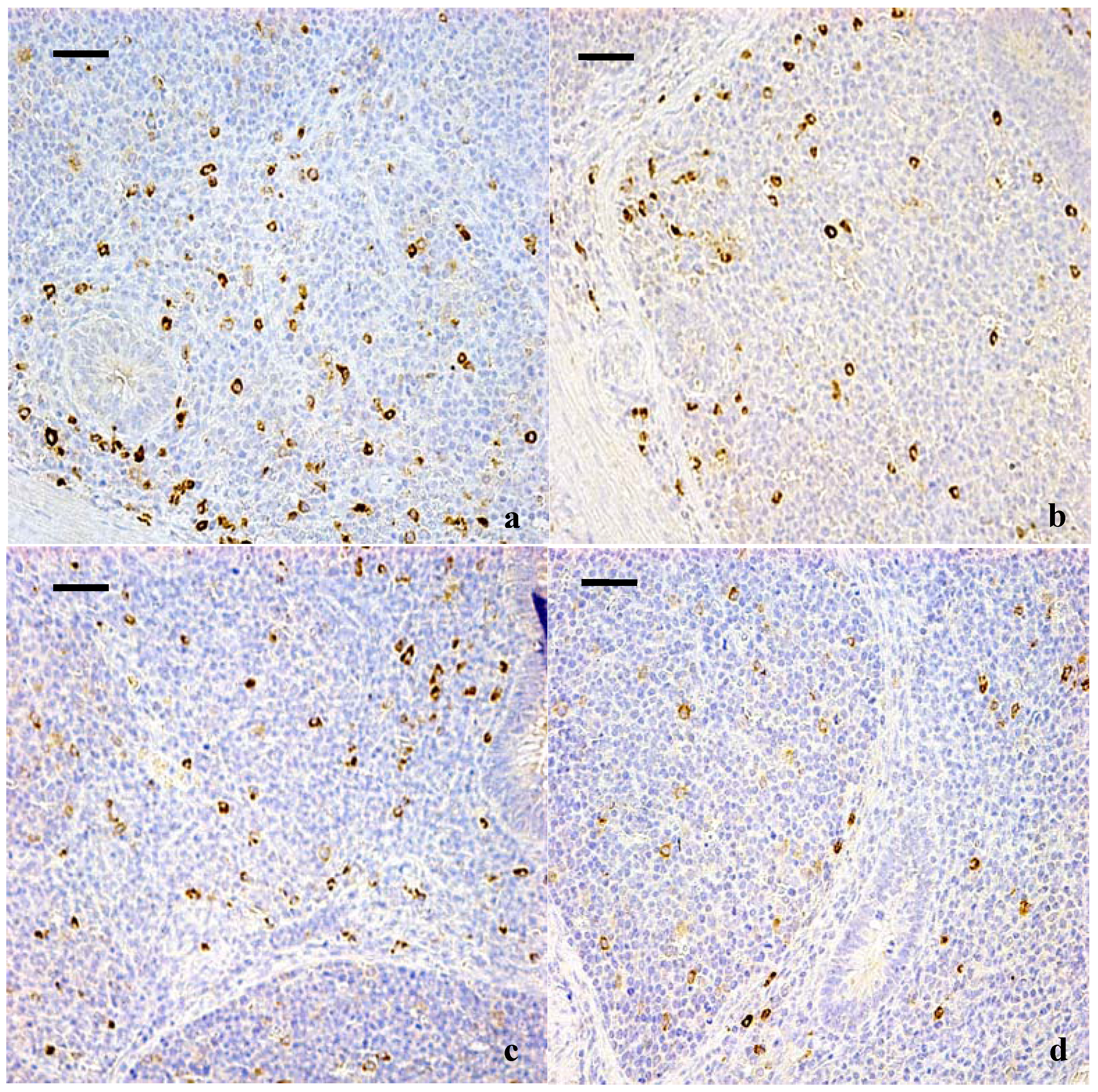
2.2. Immunohistochemical Examination for IgA+ B Cells in Cecal Tonsil
2.3. Determination of the IgA, IgG and IgM Contents in the Cecal Tonsil by ELISA
2.4. Statistical Analysis
3. Results
3.1. Changes of the IgA+ B Cells in the Cecal Tonsil
| Groups | Cecal Tonsil (μg/mL) | ||
|---|---|---|---|
| 14 days | 28 days | 42 days | |
| Control group | 190 ± 15 | 210 ± 18 | 311 ± 12 |
| High fluorine group I | 184 ± 12 | 189 ± 14 | 281 ± 14 ** |
| High fluorine group II | 97 ± 6 ** | 147 ± 13 ** | 134 ± 13 ** |
| High fluorine group III | 85 ± 8 ** | 120 ± 10 ** | 118 ± 11 ** |
3.2. Changes of the IgA Content in the Cecal Tonsil
| Groups | Cecal Tonsil (μg/mL) | ||
|---|---|---|---|
| 14 days | 28 days | 42 days | |
| Control group | 15.25 ± 0.65 | 17.03 ± 1.58 | 19.60 ± 1.00 |
| High fluorine group I | 14.23 ± 1.60 | 17.66 ± 1.41 | 18.91 ± 1.18 |
| High fluorine group II | 13.24 ± 1.25 | 13.00 ± 1.45 * | 12.58 ± 1.38 ** |
| High fluorine group III | 12.71 ± 1.50 * | 12.13 ± 0.72 ** | 11.50 ± 1.22 ** |
| Groups | Cecal Tonsil (μg/mL) | ||
|---|---|---|---|
| 14 days | 28 days | 42 days | |
| Control group | 7.30 ± 0.49 | 9.09 ± 0.33 | 8.70 ± 0.49 |
| High fluorine group I | 6.83 ± 0.31 | 8.83 ± 0.56 | 8.09 ± 0.51 * |
| High fluorine group II | 6.58 ± 0.60 * | 6.18 ± 0.38 ** | 5.44 ± 0.30 ** |
| High fluorine group III | 6.41 ± 0.30 * | 5.56 ± 0.10 ** | 5.22 ± 0.10 ** |
3.3. Changes of the IgG Content in the Cecal Tonsil
3.4. Changes of the IgM Content in the Cecal Tonsil
| Groups | Cecal Tonsil (μg/mL) | ||
|---|---|---|---|
| 14 days | 28 days | 42 days | |
| Control group | 0.56 ± 0.04 | 0.52 ± 0.01 | 0.51 ± 0.01 |
| High fluorine group I | 0.54 ± 0.03 | 0.49 ± 0.01 * | 0.50 ± 0.01 |
| High fluorine group II | 0.54 ± 0.02 | 0.49 ± 0.01 * | 0.49 ± 0.01 * |
| High fluorine group III | 0.49 ± 0.01 * | 0.48 ± 0.02 * | 0.48 ± 0.01 * |
4. Discussion
5. Conclusions
Acknowledgment
Conflict of Interest
References
- Kanbur, M.; Eraslan, G.; Silici, S.; Karabacak, M. Effects of sodium fluoride exposure on some biochemical parameters in mice: Evaluation of the ameliorative effect of royal jelly applications on these parameters. Food Chem. Toxicol. 2009, 47, 1184–1189. [Google Scholar] [CrossRef]
- Mameri, N.; Yeddou, A.R.; Lounici, H.; Belhocine, D.; Grib, H.; Bariou, B. Defluoridation of septentrional Sahara water of North Africa by electrocoagulation process using bipolar aluminium electrodes. Water Res. 1998, 32, 1604–1610. [Google Scholar] [CrossRef]
- Ba, Y.; Huang, H.; Yang, Y.J.; Cui, L.X.; Zhu, J.Y.; Zhu, C.R.; Liu, J.; Zhang, Y.W. The association between osteocalcin gene polymorphism and dental fluorosis among children exposed to fluoride in People’s Republic of China. Ecotoxicol. Environ. Saf. 2009, 72, 2158–2161. [Google Scholar] [CrossRef]
- Garrott, R.A.; Eberhardt, L.L.; Otton, J.K.; White, P.J.; Chaffee, M.A. A geochemical trophic cascade in Yellow stone’s geothermal environments. Ecosystems 2002, 5, 659–666. [Google Scholar] [CrossRef]
- Sosroseno, W. Effect of sodium fluoride on the murine splenic immune response to porphyromonas gingivalis in vitro. Immunopharmacol. Immunotoxicol. 2003, 25, 123–127. [Google Scholar] [CrossRef]
- Pak, C.V.C.; Sakhre, K.; Zorwekh, J.E.; Parcel, C.; Peterson, R.; Johnson, K. Safe and effective treatment of osteoporosis with intermittent slow release of sodium fluoride: Augmentation of vertebral bone mass and inhibition of fractures. J. Clin. Endocrinol. Metab. 1989, 68, 150–159. [Google Scholar] [CrossRef]
- Whitford, G.M. Acute and chronic fluoride toxicity. J. Dent. Res. 1992, 71, 1249–1254. [Google Scholar] [CrossRef]
- Gibson, S. Effect of fluoride on immune system function. Complement. Med. Res. 1992, 6, 111–113. [Google Scholar]
- Liu, X.L.; Li, C.C.; Liu, K.J.; Cui, C.Y.; Zhang, Y.Z.; Liu, Y. The influence of fluoride on the expression of inhibitors of Wnt/β-Catenin signaling pathway in rat skin fibroblast cells. Biol. Trace Elem. Res. 2012, 148, 117–121. [Google Scholar] [CrossRef]
- Bai, C.M.; Chen, T.; Cui, Y.; Gong, T.; Peng, X.; Cui, H.M. Effect of high fluoride on the cell cycle and apoptosis of renal cells in chickens. Biol. Trace Elem. Res. 2010, 138, 173–180. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Moghaddam, A.H.; Eslamim, S.; Nabavi, S.M. Protective effects of curcumin against sodium fluoride-induced toxicity in rat kidneys. Biol. Trace Elem. Res. 2012, 145, 369–374. [Google Scholar] [CrossRef]
- Chen, T.; Cui, H.M.; Cui, Y.; Bai, C.M.; Gong, T. Decreased antioxidase activities and oxidative stress in the spleen of chickens fed on high-fluorine diets. Hum. Exp. Toxicol. 2010, 30, 1282–1286. [Google Scholar]
- Chen, T.; Cui, H.M.; Cui, Y.; Bai, C.M.; Gong, T. Cell-cycle blockage associated with increased apoptotic cells in the thymus of chickens fed on diets high in fluorine. Hum. Exp. Toxicol. 2010, 30, 685–692. [Google Scholar]
- Chen, T.; Cui, Y.; Bai, C.M.; Gong, T.; Peng, X.; Cui, H.M. Decreased percentages of the peripheral blood T-cell subsets and the serum IL-2 contents in chickens fed on diets excess in fluorine. Biol. Trace Elem. Res. 2009, 132, 122–128. [Google Scholar] [CrossRef]
- Bharti, V.K.; Srivastava, R.S. Effect of pineal proteins at different dose level on fluoride-induced changes in plasma biochemical and blood antioxidants enzymes in rats. Biol. Trace Elem. Res. 2012, 141, 275–282. [Google Scholar] [CrossRef]
- Blaszczyk, L.; Birkner, E.; Gutowska, I.; Romuk, E.; Chlubek, D. Influence of methionine and vitamin E on fluoride concentration in bones and teeth of rats exposed to sodium fluoride in drinking water. Biol. Trace Elem. Res. 2012, 146, 335–339. [Google Scholar] [CrossRef]
- Susheela, A.K.; Kharp, P. Aortic calcification in chronic fluoride poisoning: Biochemical electron microscopic evidence. Exp. Mol. Pathol. 1990, 53, 72–80. [Google Scholar] [CrossRef]
- Gharzouli, K.; Amira, S.; Khennouf, S.; Gharzouli, A. Effects of sodium fluoride on water and acid secretion, soluble mucus and adherent mucus of the rat stomach. Can. J. Gastroenterol. 2010, 14, 493–498. [Google Scholar]
- Lillehoj, H.S.; Trout, J.M. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin. Microbiol. Rev. 1996, 9, 349–360. [Google Scholar]
- Liu, J.; Cui, H.M.; Peng, X.; Fang, J.; Zuo, Z.C.; Wang, H.S.; Wu, B.Y.; Deng, Y.X.; Wang, K.P. Dietary high fluorine induces oxidative damage in the cecal tonsil of broilers. Fluoride 2012, 45, 47–52. [Google Scholar]
- Liu, J.; Cui, H.M.; Peng, X.; Fang, J.; Zuo, Z.C.; Wang, H.S.; Wu, B.Y.; Deng, Y.X.; Wang, K.P. Decreased percentages of T-cell subsets and IL-2 contents in the cecal tonsil of broilers fed diets high in fluorine. Fluoride 2012, 45, 53–57. [Google Scholar]
- Subcommittee on Poultry Nutrition, Committee on Animal Nutrition, Board on Agriculture, National Research Council—Ninth Revised Edition; National Academy Press: Washington, DC, USA, 1994.
- Gaca, M.D.A.; Pickering, J.A.; Arthur, M.J.P.; Benyon, R.C. Human and rat hepatic stellate cells produce stem cell factor: A possible mechanism for mast cell recruitment in liver fibrosis. J. Hepatol. 1999, 30, 850–858. [Google Scholar] [CrossRef]
- Kaul, D.; Ogra, P.L. Mucosal responses to parenteral and mucosal vaccines. Dev. Biol. Stand. 1998, 95, 141–146. [Google Scholar]
- Cerutti, A.; Rescigno, M. The biology of intestinal immunoglobulin A responses. Immunity 2008, 28, 740–750. [Google Scholar] [CrossRef]
- Favre, L.; Spertini, F.; Corthesy, B. Secretory IgA possesses intrinsic modulatory properties stimulating mucosal and systemic immune responses. J. Immunol. 2005, 175, 2793–2800. [Google Scholar]
- Mazanec, M.B.; Nedrud, J.G.; Kaetzel, C.S.; Lamm, M.E. A three-tiered view of the role of IgA in mucosal defense. Immunol. Today 1993, 14, 430–435. [Google Scholar] [CrossRef]
- Mazanec, M.B.; Kaetzel, C.S.; Lamm, M.E.; Fletcher, D.; Nedrud, J.G. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc. Natl. Acad. Sci. USA 1992, 89, 6901–6905. [Google Scholar] [CrossRef]
- Janoff, E.N.; Fasching, C.; Orenstein, J.M.; Rubins, J.B.; Opstad, N.L.; Dalmasso, A.P. Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes. J. Clin. Invest. 1999, 104, 1139–1147. [Google Scholar] [CrossRef]
- Wieland, W.H.; Orzaez, D.; Lammers, A.; Parmentier, H.K.; Verstegen, M.W.A.; Schots, A. A functional polymeric immunoglobulin receptor in chicken (Gallus gallus) indicates ancient role of secretory IgA in mucosal immunity. Biochem. J. 2004, 380, 669–676. [Google Scholar] [CrossRef]
- Mowat, A.M. Anatomical basis of tolerance and immunity to intestinal antigens. Nature Rev. Immunol. 2003, 3, 331–341. [Google Scholar] [CrossRef]
- Kudsk, K.A. Importance of enteral feeding in maintaining gut integrity. Tech. Gastrointest. Endosc. 2001, 3, 2–8. [Google Scholar] [CrossRef]
- Liu, J.; Cui, H.M.; Peng, X.; Fang, J.; Zuo, Z.C.; Wang, H.S.; Wu, B.Y.; Deng, Y.X.; Wang, K.P. Changes induced by high dietary fluorine in the cecal tonsil cytokine content of broilers. Fluoride 2012, 45, 102–106. [Google Scholar]
- Noble, A. Do we have memory of danger as well as antigen? Trends Immunol. 2009, 30, 150–156. [Google Scholar] [CrossRef]
- Leslie, G.A.; Martin, L.N. Studies on the secretory immunologic system of fowl. III. Serum and secretory IgA of the chicken. J. Immunol. 1973, 110, 1–9. [Google Scholar]
- Mansikka, A. Chicken IgA H chains. Implications concerning the evolution of H chain genes. J. Immunol. 1992, 149, 855–861. [Google Scholar]
- Zhang, A.J.; Yang, D.M.; Bai, S.Q. The immune toxicity of fluoride. Chin. J. Ctrl. End. 2002, 17, 30–32. [Google Scholar]
- Shen, L.X.; Liu, J.D.; Chen, R.A. The changes of serum IgA, IgG, and IgM content in fluoride exposed worker. Chin. J. Ind. Med. 1996, 9, 325–327. [Google Scholar]
- Jain, S.K.; Susheela, A.K. Effect of sodium fluoride on antibody formation in rabbits. Environ. Res. 1987, 44, 117–125. [Google Scholar] [CrossRef]
- Liu, J.; Cui, H.M.; Peng, X.; Fang, J.; Zuo, Z.C.; Wang, H.S.; Wu, B.Y.; Deng, Y.X.; Wang, K.P. Dietary high fluorine induces apoptosis and alters bcl-2,bax, and caspase-3 protein expression in the cecal tonsil lymphocytes of broilers. Biol. Trace Elem. Res. 2013, 152, 25–30. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, J.; Cui, H.; Peng, X.; Fang, J.; Zuo, Z.; Deng, J.; Wang, H.; Wu, B.; Deng, Y.; Wang, K. Decreased IgA+ B Cells Population and IgA, IgG, IgM Contents of the Cecal Tonsil Induced by Dietary High Fluorine in Broilers. Int. J. Environ. Res. Public Health 2013, 10, 1775-1785. https://doi.org/10.3390/ijerph10051775
Liu J, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang H, Wu B, Deng Y, Wang K. Decreased IgA+ B Cells Population and IgA, IgG, IgM Contents of the Cecal Tonsil Induced by Dietary High Fluorine in Broilers. International Journal of Environmental Research and Public Health. 2013; 10(5):1775-1785. https://doi.org/10.3390/ijerph10051775
Chicago/Turabian StyleLiu, Juan, Hengmin Cui, Xi Peng, Jing Fang, Zhicai Zuo, Junliang Deng, Hesong Wang, Bangyuan Wu, Yuanxin Deng, and Kangping Wang. 2013. "Decreased IgA+ B Cells Population and IgA, IgG, IgM Contents of the Cecal Tonsil Induced by Dietary High Fluorine in Broilers" International Journal of Environmental Research and Public Health 10, no. 5: 1775-1785. https://doi.org/10.3390/ijerph10051775





