Evaluation of Factors Influencing the Groundwater Chemistry in a Small Tropical Island of Malaysia
Abstract
:1. Introduction
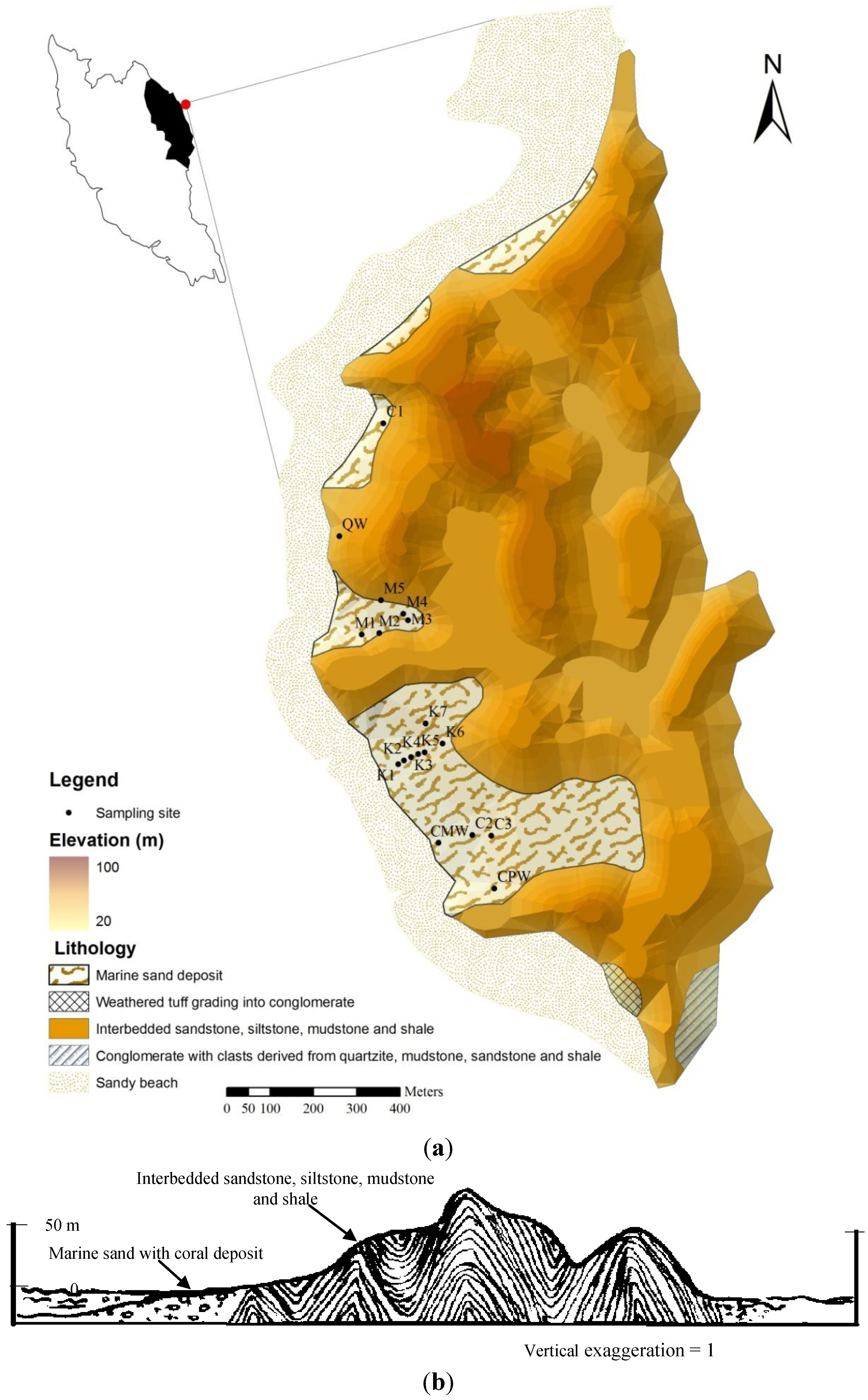
2. Study Area
3. Materials and Methods
3.1. Sampling and Chemical Analysis
3.2. Statistical Analysis
3.3. Principal Component Analysis
| r-value | Interpretation |
|---|---|
| 0.0 to 0.29 | Negligible or little correlation |
| 0.3 to 0.49 | Low correlation |
| 0.5 to 0.69 | Moderate or marked correlation |
| 0.7 to 0.89 | High correlation |
| 0.9 to 1.00 | Very high correlation |
3.4. Cluster Analysis
4. Results and Discussion
4.1. Statistical Summary
| Variable | Units | Minimum | Maximum | Mean | Std. deviation |
|---|---|---|---|---|---|
| Ca | mg·L−1 | 1.1 | 254.4 | 69.9 | 48.2 |
| Mg | mg·L−1 | 1.0 | 496.2 | 47.7 | 117.2 |
| Na | mg·L−1 | 3.0 | 1,920.7 | 241.3 | 563.6 |
| K | mg·L−1 | 0.5 | 83.3 | 14.0 | 24.2 |
| HCO3 | mg·L−1 | 48.8 | 7,320.0 | 613.1 | 1,511.0 |
| Cl | mg·L−1 | 31.0 | 6,198.1 | 517.7 | 1,455.1 |
| SO4 | mg·L−1 | 1.0 | 500.0 | 70.3 | 134.6 |
| NO3 | mg·L−1 | 0.4 | 10.6 | 1.7 | 2.3 |
| EC | µS·cm−1 | 138.6 | 14,085.0 | 1,756.5 | 3,463.4 |
| Salinity | mg·L−1 | 0.1 | 7.9 | 0.9 | 1.9 |
| pH | - | 4.5 | 7.7 | 6.9 | 0.9 |
| DO | mg·L−1 | 2.3 | 9.7 | 6.5 | 1.5 |
| Tur | NTU | 0.9 | 1,297.0 | 235.8 | 407.9 |
| Temp | °C | 27.4 | 30.5 | 28.8 | 1.0 |
| W/L | m | 1.1 | 3.3 | 2.4 | 0.5 |
| Eh | mV | −20.8 | 195.4 | 25.9 | 58.1 |
| TDS | mg·L−1 | 70.3 | 8,213.1 | 988.3 | 2,020.5 |
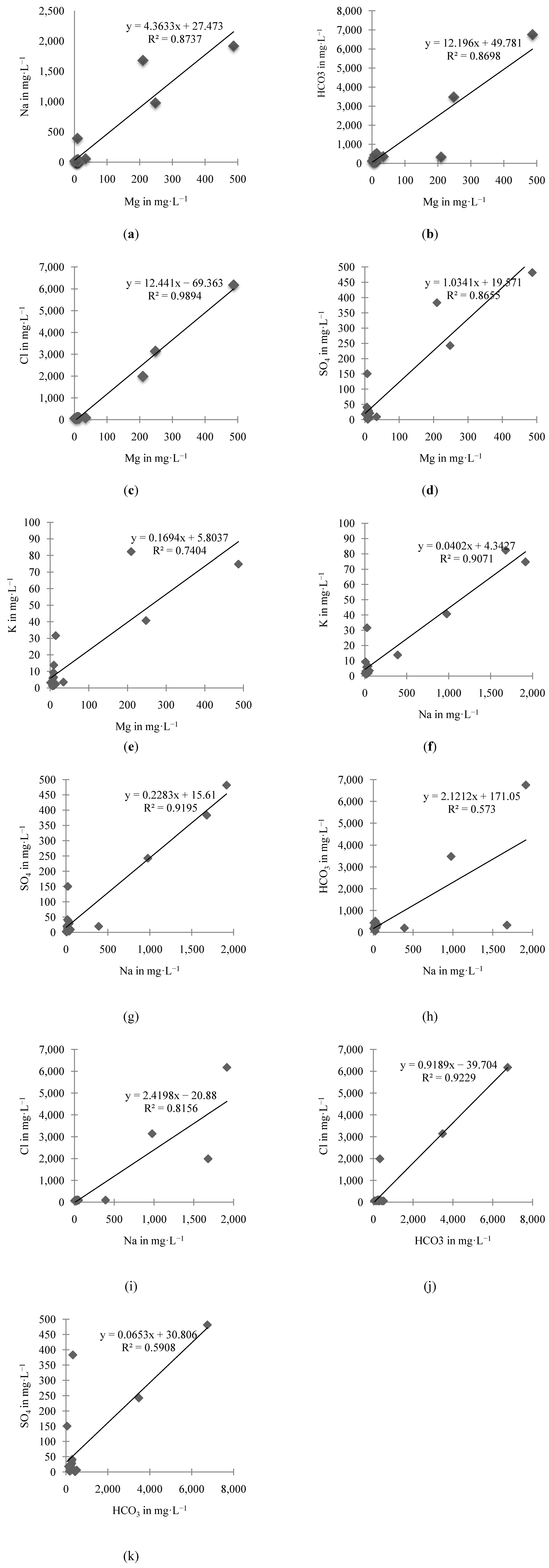
4.2. Correlation
| Variables | Ca | Mg | Na | K | HCO3 | Cl | SO4 | NO3 | EC | Salinity | pH | DO | Tur | Temp | W/L | Eh | TDS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | 1 | ||||||||||||||||
| Mg | 0.700 ** | 1 | |||||||||||||||
| Na | 0.606 ** | 0.931 ** | 1 | ||||||||||||||
| K | 0.524 * | 0.853 ** | 0.949 ** | 1 | |||||||||||||
| HCO3 | 0.737 ** | 0.920 ** | 0.731 ** | 0.632 * | 1 | ||||||||||||
| Cl | 0.713 ** | 0.994 ** | 0.897 ** | 0.809 ** | 0.950 ** | 1 | |||||||||||
| SO4 | 0.550 * | 0.924 ** | 0.954 ** | 0.904 ** | 0.743 ** | 0.899 ** | 1 | ||||||||||
| NO3 | −0.056 | 0.101 | 0.164 | 0.440 | 0.036 | 0.069 | 0.127 | 1 | |||||||||
| EC | 0.688 ** | 0.990 ** | 0.955 ** | 0.913 ** | 0.878 ** | 0.976 ** | 0.941 ** | 0.206 | 1 | ||||||||
| Salinity | 0.689 ** | 0.992 ** | 0.954 ** | 0.909 ** | 0.881 ** | 0.978 ** | 0.942 ** | 0.195 | 1.000 ** | 1 | |||||||
| pH | 0.246 | −0.109 | −0.058 | −0.037 | −0.134 | −0.142 | −0.228 | 0.029 | −0.083 | −0.091 | 1 | ||||||
| DO | 0.052 | 0.042 | 0.039 | −0.142 | 0.071 | 0.069 | 0.062 | −0.622 ** | −0.019 | −0.007 | −0.391 | 1 | |||||
| Tur | −0.086 | −0.200 | −0.217 | −0.252 | −0.151 | −0.181 | −0.187 | −0.114 | −0.211 | −0.216 | −0.287 | 0.086 | 1 | ||||
| Temp | −0.152 | −0.224 | −0.323 | −0.305 | −0.126 | −0.190 | −0.286 | 0.058 | −0.225 | −0.226 | 0.057 | −0.379 | 0.048 | 1 | |||
| W/L | 0.128 | −0.055 | −0.110 | −0.114 | 0.050 | −0.025 | −0.185 | 0.031 | −0.063 | −0.072 | 0.308 | −0.493 * | 0.249 | 0.351 | 1 | ||
| Eh | −0.328 | 0.039 | −0.024 | −0.047 | 0.078 | 0.071 | 0.184 | −0.075 | 0.005 | 0.012 | −0.964 ** | 0.329 | 0.287 | −0.073 | −0.235 | 1 | |
| TDS | 0.689 ** | 0.991 ** | 0.953 ** | 0.910 ** | 0.882 ** | 0.977 ** | 0.940 ** | 0.202 | 1.000 ** | 1.000 ** | −0.088 | −0.015 | −0.210 | −0.223 | −0.063 | 0.010 | 1 |

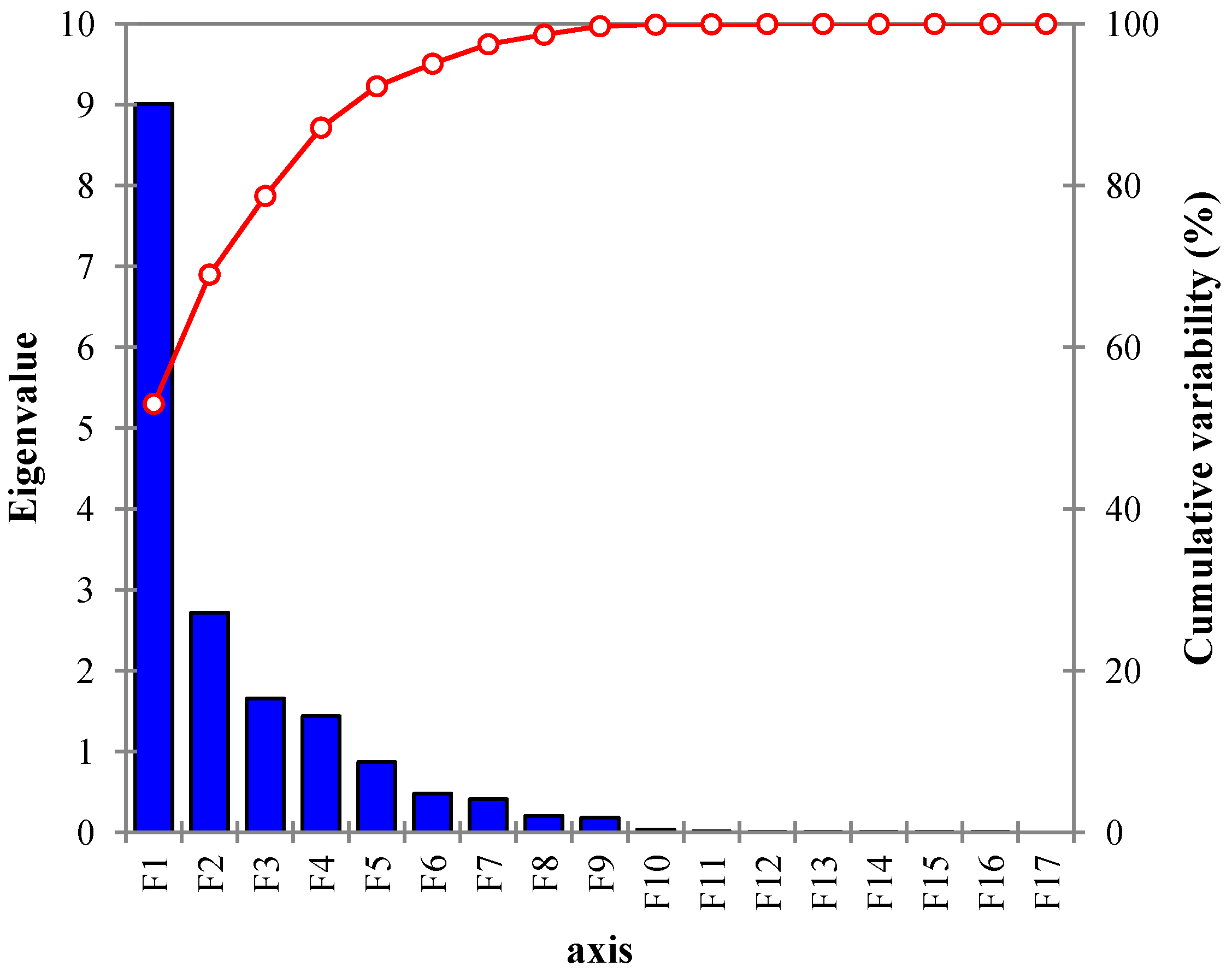
4.3. Extraction of Components
| Components | Eigenvalue | Variability (%) | Cumulative % |
|---|---|---|---|
| PC1 | 9.008 | 52.986 | 52.986 |
| PC2 | 2.725 | 16.028 | 69.015 |
| PC3 | 1.654 | 9.732 | 78.747 |
| PC4 | 1.418 | 8.340 | 87.087 |
| PC5 | 0.872 | 5.129 | 92.216 |
| PC6 | 0.474 | 2.786 | 95.002 |
| PC7 | 0.407 | 2.394 | 97.396 |
| PC8 | 0.215 | 1.266 | 98.662 |
| PC9 | 0.175 | 1.027 | 99.690 |
| PC10 | 0.035 | 0.206 | 99.896 |
| PC11 | 0.009 | 0.054 | 99.950 |
| PC12 | 0.005 | 0.027 | 99.977 |
| PC13 | 0.002 | 0.014 | 99.990 |
| PC14 | 0.001 | 0.007 | 99.997 |
| PC15 | 0.000 | 0.003 | 100.000 |
| PC16 | 0.000 | 0.000 | 100.000 |
| PC17 | 0.000 | 0.000 | 100.000 |
| Variables | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Ca | 0.752 | −0.362 | −0.280 | 0.135 |
| Mg | 0.991 | 0.017 | −0.004 | −0.070 |
| Na | 0.934 | −0.027 | 0.097 | −0.211 |
| K | 0.865 | −0.033 | 0.364 | −0.232 |
| HCO3 | 0.912 | 0.036 | −0.130 | 0.093 |
| Cl | 0.987 | 0.045 | −0.049 | −0.023 |
| SO4 | 0.922 | 0.154 | 0.091 | −0.215 |
| NO3 | 0.123 | 0.007 | 0.908 | 0.001 |
| EC | 0.989 | −0.004 | 0.100 | −0.091 |
| Sal | 0.989 | 0.001 | 0.089 | −0.098 |
| pH | −0.088 | −0.968 | 0.054 | 0.112 |
| DO | 0.012 | 0.299 | −0.762 | −0.467 |
| Tur | −0.147 | 0.444 | −0.246 | 0.503 |
| Temp | −0.205 | 0.000 | 0.197 | 0.635 |
| W/L | 0.019 | −0.226 | 0.053 | 0.848 |
| Eh | 0.016 | 0.966 | −0.053 | −0.074 |
| TDS | 0.990 | 0.000 | 0.094 | −0.089 |
| Eigenvalue | 9.009 | 2.719 | 1.653 | 1.436 |
| Variability (%) | 52.993 | 15.995 | 9.726 | 8.450 |
| Cumulative % | 52.993 | 68.988 | 78.714 | 87.164 |
4.4. Cluster Analysis
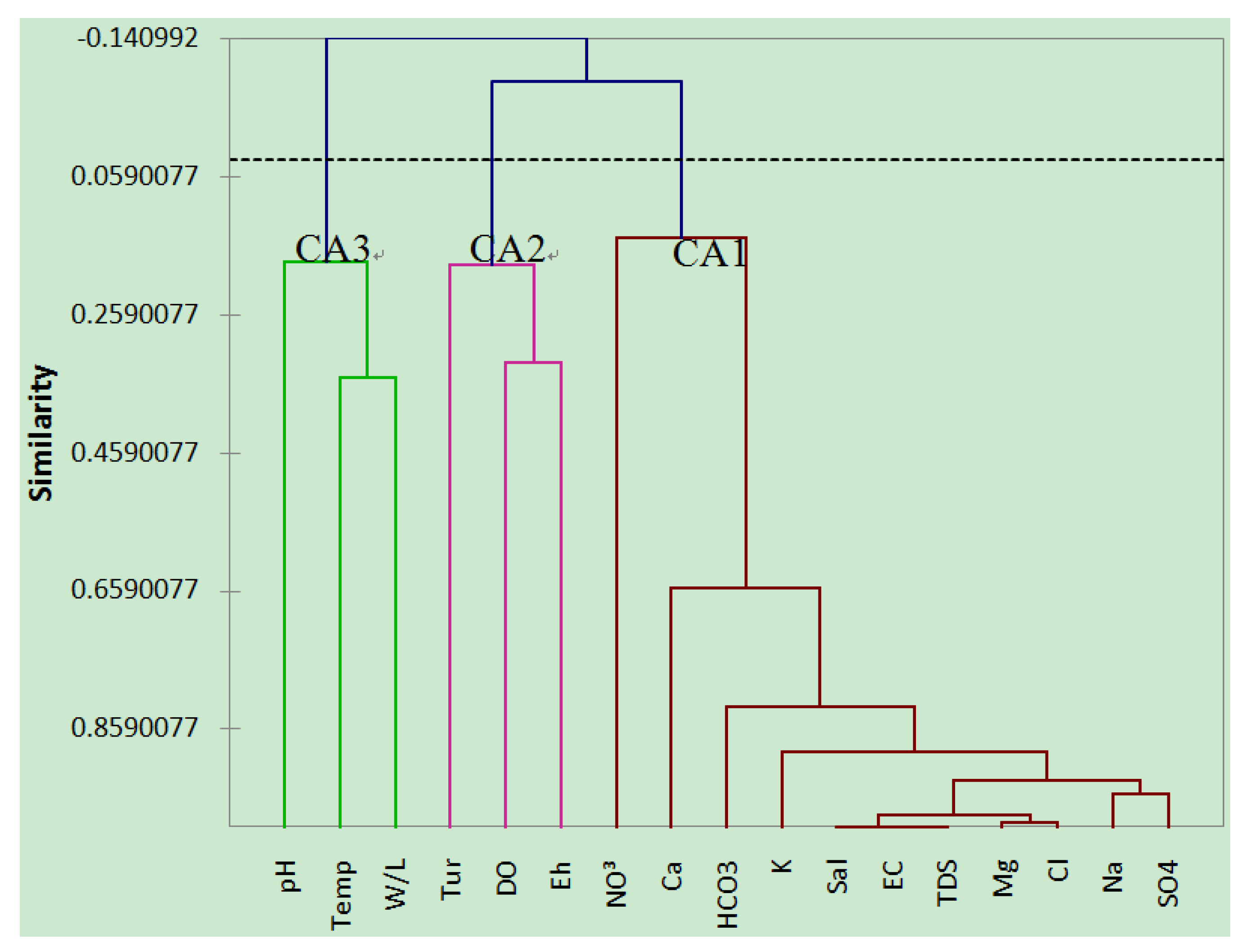
4.5. Groundwater Classification

5. Conclusions
Acknowledgments
Conflict of Interest
References
- Arslan, H. Application of multivariate statistical techniques in the assessment of groundwater quality in seawater intrusion area in Bafra Plain, Turkey. Environ. Monit. Assess. 2013, 185, 2439–2452. [Google Scholar]
- Trabelsi, R.; Zairi, M.; Dhia, H.B. Groundwater salinization of the Sfax superficial aquifer, Tunisia. Hydrogeol. J. 2007, 15, 1341–1355. [Google Scholar]
- Aris, A.Z.; Abdullah, M.H.; Ahmed, A.; Woong, K.K. Controlling factors of groundwater hydrochemistry in a small Island’s aquifer. Int. J. Environ. Sci. Tech. 2007, 4, 441–450. [Google Scholar]
- Praveena, S.M.; Abdullah, M.H.; Bidin, K.; Aris, A.Z. Understanding of groundwater salinity using statistical modeling in a small tropical island, East Malaysia. Environmentalist 2011, 31, 279–287. [Google Scholar]
- Aris, A.Z.; Abdullah, M.H.; Woong, K.K.; Praveena, S.M. Hydrochemical changes in small tropical island’s aquifer: Manukan Island, Sabah, Malaysia. Environ. Geol. 2009, 56, 1721–1732. [Google Scholar]
- Mohapatra, P.; Vijay, R.; Pujari, P.; Sundaray, S.; Mohanty, B. Determination of processes affecting groundwater quality in the coastal aquifer beneath Puri city, India: A multivariate statistical approach. Water Sci. Tech. 2011, 64, 809–817. [Google Scholar] [CrossRef]
- Singh, C.K.; Shashtri, S.; Mukherjee, S. Integrating multivariate statistical analysis with GIS for geochemical assessment of groundwater quality in Shiwaliks of Punjab, India. Environ. Earth Sci. 2011, 62, 1387–1405. [Google Scholar]
- Belkhiria, L.; Mounib, L.; Boudoukha, A. Geochemical evolution of groundwater in an alluvial aquifer: Case of El Eulma aquifer, East Algeria. J. Afr. Earth Sci. 2012, 66, 46–55. [Google Scholar] [CrossRef]
- Akbal, F.; Gürel, L.; Bahadır, T.; Güler, İ.; Bakan, G.; Büyükgüngör, H. Water and sediment quality assessment in the mid-Black Sea coast of Turkey using multivariate statistical techniques. Environ. Earth Sci. 2011, 64, 1387–1395. [Google Scholar]
- Glynn, P.D.; Plummer, L.N. Geochemistry and the understanding of ground-water systems. Hydrogeol. J. 2005, 13, 263–287. [Google Scholar] [CrossRef]
- Aris, A.Z.; Abdullah, M.H.; Woong, K.K. Hydrogeochemistry of groundwater in Manukan Island, Sabah. Malays. J. Anal. Sci. 2007, 11, 407–413. [Google Scholar]
- Jalali, M. Application of Multivariate analysis to study water chemistry of groundwater in a semi-arid aquifer, Malayer, Western Iran. Desalination Water Treat. 2010, 19, 307–317. [Google Scholar]
- Singh, E.J.K.; Gupta, A.; Singh, N.R. Groundwater quality in Imphal West district, Manipur, India, with multivariate statistical analysis of data. Environ. Sci. Pollut. Res. 2012. [Google Scholar] [CrossRef]
- Tlili-Zrelli, B.; Hamzaoui-Azaza, F.; Gueddari, M.; Bouhlila, R. Geochemistry and quality assessment of groundwater using graphical and multivariate statistical methods. A case study: Grombalia phreatic aquifer (Northeastern Tunisia). Arab. J. Geosci. 2012. [Google Scholar] [CrossRef]
- Shyu, G.-S.; Cheng, B.-Y.; Chiang, C.-T.; Yao, P.-H.; Chang, T.-K. Applying factor analysis combined with kriging and information entropy theory for mapping and evaluating the stability of groundwater quality variation in Taiwan. Int. J. Environ. Res. Public Health 2011, 8, 1084–1109. [Google Scholar]
- White, I.; Falkland, T.; Perez, P.; Dray, A.; Metutera, T.; Metai, E.; Overmars, M. Challenges in freshwater management in low coral atolls. J. Cleaner Prod. 2007, 15, 1522–1528. [Google Scholar]
- Isa, N.M.; Aris, A.Z.; Sulaiman, W.N.A. Extent and severity of groundwater contamination based on hydrochemistry mechanism of sandy tropical coastal aquifer. Sci. Total Environ. 2012, 438, 414–425. [Google Scholar]
- Abdullah, M. Geological Survey Report of Kapas Island; Geological Survey Headquaters: Terengganu, Malaysia, 1981. [Google Scholar]
- Association, A.W.W.; Federation, W.E. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Parizi, H.S.; Samani, N. Geochemical evolution and quality assessment of water resources in the Sarcheshmeh copper mine area (Iran) using multivariate statistical techniques. Environ. Earth Sci. 2012. [Google Scholar] [CrossRef]
- Ranjan, R.K.; Ramanathan, A.; Parthasarathy, P.; Kumar, A. Hydrochemical charasteristics of groundwater in the plains of Phalgu River in Gaya, Bihar, India. Arab. J. Geosci. 2012. [Google Scholar] [CrossRef]
- Smith, L.I. A Tutorial on Principal Component Analysis. Available online: http://csnet.otago.ac.nz/cosc453/student_tutorials/principal_components.pdf (accessed on 17 November 2012).
- Pitkänen, P.; Partamies, S.; Luukkonen, A. Hydrogeochemical Interpretation of Baseline Groundwater Conditions at the Olkiluoto Site; Posiva: Olkiluoto, Finland, 2004. [Google Scholar]
- Farnhama, I.; Johannessonb, V.; Singhc, A.; Hodged, V.; Stetzenbach, K. Factor analytical approaches for evaluating groundwater trace element chemistry data. Anal. Chim. Acta 2003, 490, 123–138. [Google Scholar] [CrossRef]
- Guildford, J.P. Fundamental Statistics in Psychology and Education; McGraw-Hill: New York, NY, USA, 1973. [Google Scholar]
- Mustapha, A.; Aris, A.Z. Multivariate statistical analysis and environmental modeling of heavy metals pollution by industies. Pol. J. Environ. Stud. 2012, 21, 1359–1367. [Google Scholar]
- Mohapatra, P.; Vijay, R.; Pujari, P.; Sundaray, S.; Mohanty, B. Determination of processes affecting groundwater quality in the coastal aquifer beneath Puri city, India: A multivariate statistical approach. Water Sci. Tech. 2011, 64, 809–817. [Google Scholar] [CrossRef]
- Nelson, D. Natural Variations in the Composition of Groundwater; Drinking Water Program Oregon Department of Human Services: Springfield, OR, USA, 2002. [Google Scholar]
- Abrahams, R.H.; Loague, K. A compartmentalized solute transport model for redox zones in contaminated aquifers: 2. Field-scale simulations. Water Resour. Res. 2008, 36, 2015–2029. [Google Scholar] [CrossRef]
- Mor, S.; Singh, S.; Yadav, P.; Rani, V.; Rani, P.; Sheoran, M.; Singh, G.; Ravindra, K. Appraisal of salinity and fluride in a semi-arid region of India using statistical and multivariate techniques. Environ. Geochem. Health 2009, 31, 643–655. [Google Scholar] [CrossRef] [Green Version]
- Sundaray, S.K. Application of multivariate statistical techniques in hydrogeological studies—A case study of Brahmani-Koel River (India). Environ. Monit. Assess. 2010, 164, 297–310. [Google Scholar] [CrossRef]
- Wollast, R.; Mackenzie, F. Global Cycle of Silica. In Silicon Geochemistry and Biogeochemistry; Academic: New York, NY, USA, 1983; pp. 39–76. [Google Scholar]
- Panteleit, B.; Kessels, W.; Kantor, W.; Schulz, H. Geochemical Characteristics of Salinization-Zones in the Coastal Aquifer Test Field (CAT-Field) in North-Germany. In Proceeding of 5th International Conference on Saltwater Intrusion and Coastal Aquifers-Monitoring, Modeling, and Management, Essaouira, Morocco, 23–25 April 2001.
- Thilagavathi, R.; Chidambaram, S.; Prasanna, M.V.; Thivya, C.; Singaraja, C. A study of groundwater geochemistry in layered aquifers system of Pondicherry region, southeast India. Appl. Water Sci. 2012, 2, 253–269. [Google Scholar] [CrossRef]
- Chidambaram, S.; Anandhan, P.; Prasanna, M.V.; Srinivasamoorthy, K.; Vasanthavigar, M. Major ion chemistry and identification of hydrogeochemical processes controlling groundwater in and around Neyveli Lignite Mines, Tamil Nadu, South India. Arab. J. Geosci. 2012. [Google Scholar] [CrossRef]
- Srinivasamoorthy, K.; Chidambaram, S.; Prasanna, M.V.; Vasanthavihar, M.; Peter, J.; Anandhan, P. Identification of major sources controlling groundwater chemistry from a hard rock terrain—A case study from Mettur taluk, Salem district, Tamil Nadu, India. J. Earth Syst. Sci. 2008, 117, 49–58. [Google Scholar]
- Deutsch, W.J. Groundwater Geochemistry: Fundamentals and Applications to Contamination; Lewis: New York, NY, USA, 1997. [Google Scholar]
- Pathak, H.; Limaye, S.N. Study of seasonal variation in groundwater quality of sagar city (India) by principal component analysis. J. Chem. 2011, 8, 2000–2009. [Google Scholar]
- Abdi, H. Factor Rotations in Factor Analyses. In Encyclopedia of Social Science Research Methods; SAGE: Thousand Oaks, CA, USA, 2003; pp. 1–4. [Google Scholar]
- Kaiser, H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- O’Rourke, N.; Hatcher, L.; Stepanski, E.J. A Step-by-Step Approach to Using SAS for Univariate & Multivariate Statistics, 2nd ed; SAS Publishing: Cary, NC, USA, 2005. [Google Scholar]
- Yidana, S.M.; Yakubo, B.B.; Akabzaa, T.M. Analysis of groundwater quality using multivariate and spatial analyses in the Keta basin, Ghana. J. Afr. Earth Sci. 2010, 58, 220–234. [Google Scholar] [CrossRef]
- Suk, H.; Lee, K.-K. Characterization of a water hydrochemical system through multivariate analysis: Clustering into groundwater zones. Groudwater 1999, 37, 358–366. [Google Scholar] [CrossRef]
- Kumaresan, P.R.M. Factor analysis and linear regression model (LRM) of metal speciation and physico-chemical characters of groundwater samples. Environ. Monit. Assess. 2008, 138, 65–79. [Google Scholar] [CrossRef]
- Kehew, E.A. Applied Chemical Hydrogeology; Prentice Hall: Upper Saddle River, NJ, USA, 2000. [Google Scholar]
- Dia, A.; Gruau, G.; Olivié-Lauquet, G.; Riou, C.; Molénat, J.; Curmi, P. The distribution of rare earth elements in groundwaters: Assessing the role of source-rock composition, redox changes and colloidal particles. Geochim. Cosmochim. Ac. 2000, 64, 4131–4151. [Google Scholar] [CrossRef]
- Morales, L.A.; Paz-Ferreiro, J.; Vieira, S. R.; Vázquez, E.V. Spatial and temporal variability of Eh and pH over a rice field as related to lime addition. Bragantia 2010, 69, 67–76. [Google Scholar] [CrossRef]
- Mann, A.G.; Tam, C.C.; Craig, H.D.; Rodrigues, L.C. The association between drinking water turbidity and gastrointestinal illness: A systematic review. BMC Public Health 2007, 256, 1–7. [Google Scholar]
- Delpla, I.; Jung, A.-V.; Baures, E.; Clement, M.; Thomas, O. Impacts of climate change on surface water quality in relation to drinking water production. Environ. Int. 2009, 35, 1225–1233. [Google Scholar]
- Piper, A.M. A Graphic Procedure in the Geochemical Interpretation of Water Analysis; U.S. Department of the Interior, Geological Survey, Water Resources Division, Ground Water Branch: Washington, DC, USA, 1953.
- McArthur, J.; Sikdar, P.; Hoque, M.; Ghosal, U. Waste-water impacts on groundwater: Cl/Br ratios and implications for arsenic pollution of groundwater in the Bengal Basin and Red River Basin, Vietnam. Sci. Total Environ. 2012, 437, 390–402. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kura, N.U.; Ramli, M.F.; Sulaiman, W.N.A.; Ibrahim, S.; Aris, A.Z.; Mustapha, A. Evaluation of Factors Influencing the Groundwater Chemistry in a Small Tropical Island of Malaysia. Int. J. Environ. Res. Public Health 2013, 10, 1861-1881. https://doi.org/10.3390/ijerph10051861
Kura NU, Ramli MF, Sulaiman WNA, Ibrahim S, Aris AZ, Mustapha A. Evaluation of Factors Influencing the Groundwater Chemistry in a Small Tropical Island of Malaysia. International Journal of Environmental Research and Public Health. 2013; 10(5):1861-1881. https://doi.org/10.3390/ijerph10051861
Chicago/Turabian StyleKura, Nura Umar, Mohammad Firuz Ramli, Wan Nur Azmin Sulaiman, Shaharin Ibrahim, Ahmad Zaharin Aris, and Adamu Mustapha. 2013. "Evaluation of Factors Influencing the Groundwater Chemistry in a Small Tropical Island of Malaysia" International Journal of Environmental Research and Public Health 10, no. 5: 1861-1881. https://doi.org/10.3390/ijerph10051861




