Swine Dysentery: Aetiology, Pathogenicity, Determinants of Transmission and the Fight against the Disease
Abstract
:1. Introduction
2. The Etiological Agent: General Considerations and Virulence Traits; Lessons from the Genome
3. Environmental Determinants of SD Transmission
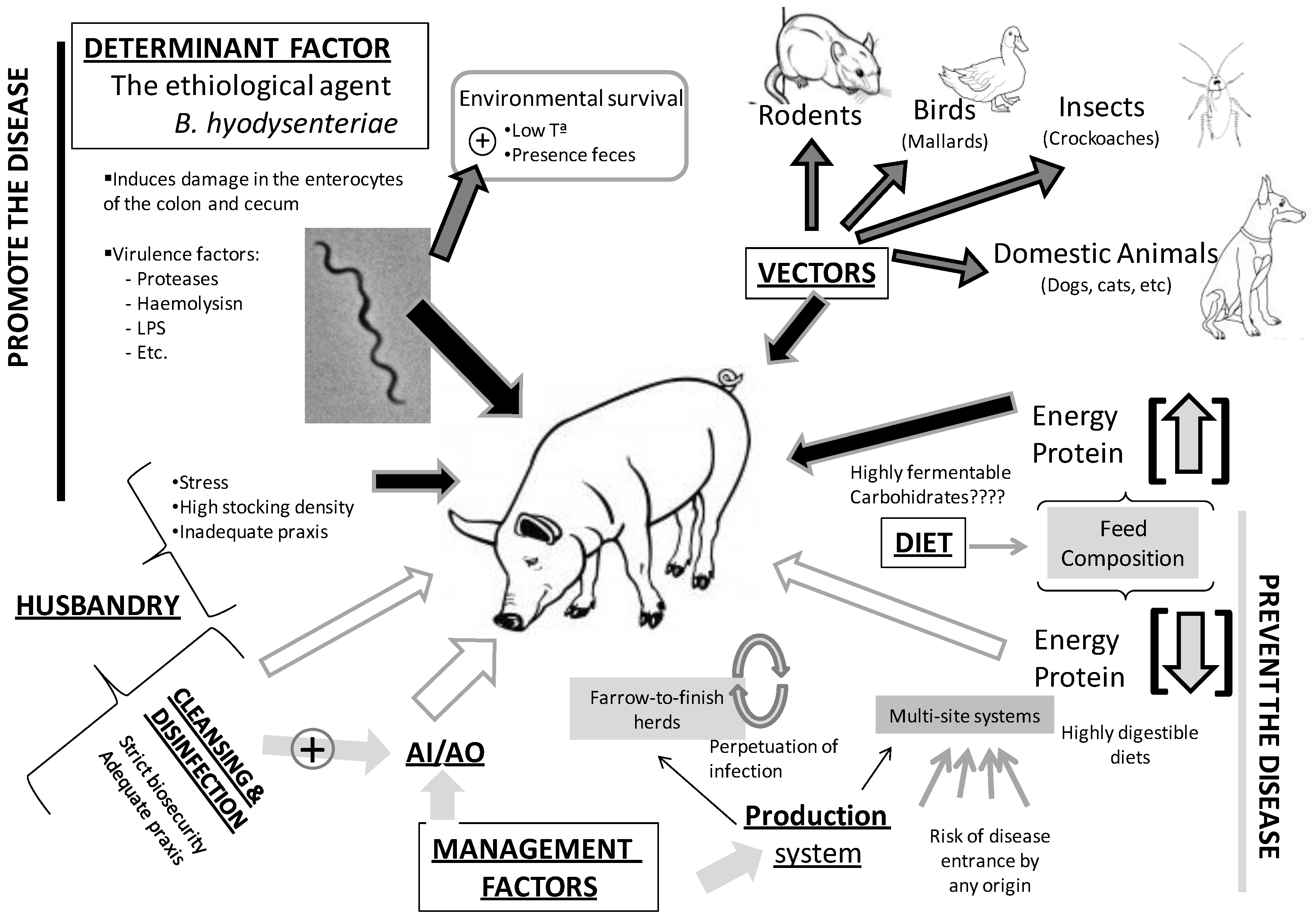
3.1. Environmental Survival
3.2. Biosecurity and Husbandry Factors
3.3. Vectors
3.4. The Role of Diet and Intestinal Microbiota
4. The Fight against SD
4.1. Therapy with Antibiotics
| Drug | Dosage in SD treatment a | Dosage in SD prevention | Point mutations associated to decreased susceptibility b | Wild type MIC cutoff values c | Clinical MIC breakpoint d |
|---|---|---|---|---|---|
| Tiamulin | Im: 10 mg/kg bw for 1–3 days | In feed medication: 30–40 ppm | 23S rRNA gene position 2058 and 2032 | >0.25 µg/mL | >2 µg/mL |
| Po: 8 mg/kg bw for 5–7 days in drinking water | L3 ribosomal protein gene 148 | ||||
| In feed medication: 100 ppm for 7–10 days | |||||
| Valnemulin | In feed medication: 3–4 mg/kg bw for 1–4 weeks | In feed medication: 25 ppm | 23S rRNA gene position 2058 and 2032 L3 ribosomal protein gene position 149 | >0.125 µg/mL | >5 µg/mL |
| Tylosin | Im: 10 mg/kg bw for 3–5 days | - | 23S rRNA gene position 2058 | >16 µg/mL | >32 µg/mL |
| Po: 5–10 mg/kg bw in drinking water for 5–7 days | |||||
| Tylvalosin | In feed medication: 4.25 mg/kg bw for 10–14 days | In feed medication: 2.125 mg/kg·bw | 23S rRNA gene position 2058 and 2059 | >1 µg/mL | >32 µg/mL |
| Lincomycin | Po: 8 mg/kg bw in drinking water for 1 to 10 days | In feed medication: 44 ppm | 23S rRNA gene position 2058, 2059 and 2032 | >1 µg/mL | >36 µg/mL |
| In feed medication: 100 ppm until clinical signs disappear followed by 40 ppm |
4.2. Vaccination
4.3. Dietary Interventions
4.4. Probiotics
4.5. Natural Antimicrobials
5. Conclusions and Future Prospects
Acknowledgments
Conflicts of interest
References
- Wills, R.W. Diarrhea in growing-finishing swine. Vet. Clin. North Am. Food Anim. Pract. 2000, 16, 135–161. [Google Scholar]
- Whiting, R.A.; Doyle, L.P.; Spray, R.S. Swine dysentery. Bulletin 1937, 257, 1–15. [Google Scholar]
- Taylor, D.J.; Alexander, T.J.L. The production of dysentery in swine by feeding cultures containing a spirochaete. Br. Vet. J. 1971, 127, 58–61. [Google Scholar]
- Harris, D.L.; Glock, R.D.; Christensen, C.R.; Kinyon, J.M. Inoculation of pigs with Treponema hyodysenteriae (new species) and reproduction of the disease. Vet. Med. Small Anim. Clin. 1972, 67, 61–64. [Google Scholar]
- Hampson, D.J.; Fellstrom, C.; Thomson, J.R. Swine dysentery. In Diseases of Swine; Straw, B.E., Zimmerman, J.J., D’Allaire, S., Eds.; Blackwell Publishing Professional: Ames, IA, USA, 2006; pp. 785–805. [Google Scholar]
- Suh, D.K.; Song, J.C. Simultaneous detection of Lawsonia intracellularis, Brachyspira hyodysenteriae and Salmonella spp. in swine intestinal specimens by multiplex polymerase chain reaction. J. Vet. Sci. 2005, 6, 231–237. [Google Scholar]
- Fellstrom, C.; Pettersson, B.; Johansson, K.E.; Lundeheim, N.; Gunnarsson, A. Prevalence of Serpulina species in relation to diarrhea and feed medication in pig-rearing herds in Sweden. Am. J. Vet. Res. 1996, 57, 807–811. [Google Scholar]
- Møller, K.; Jensen, T.K.; Jorsal, S.E.; Leser, T.D.; Carstensen, B. Detection of Lawsonia intracellularis, Serpulina hyodysenteriae, weakly beta-hemolytic intestinal spirochaetes, Salmonella enterica, and Escherichia coli from swine herds with and without diarrhoea among growing pigs. Vet. Microbiol. 1998, 62, 59–72. [Google Scholar] [CrossRef]
- Stege, H.; Jensen, T.K.; Møller, K.; Bakbo, P.; Jorsal, S.E. Prevalence of intestinal pathogens in Danish finishing pig herds. Prev. Vet. Med. 2000, 46, 279–292. [Google Scholar] [CrossRef]
- Carvajal, A.; de Arriba, M.L.; Rodríguez, H.; Vidal, A.B.; Duhamel, G.E.; Rubio, P. Prevalence of Brachyspira species in pigs with diarrhoea in Spain. Vet. Rec. 2006, 158, 700–701. [Google Scholar] [CrossRef]
- Johnston, W.T.; Dewey, C.E.; Friendship, R.M.; Smart, N.; McEwen, B.J.; Stalker, M.; de Lange, C.F.M. An investigation of the aetiology of a mild diarrhea observed in a group of grower/finisher pigs. Can. Vet. J. 2001, 42, 33–37. [Google Scholar]
- Jacobson, M.; Gerth Lofstedt, M.; Holmgren, N.; Lundeheim, N.; Fellstrom, C. The prevalences of Brachyspira spp. and Lawsonia intracellularis in Swedish piglet producing herds and wild board population. J. Vet. Med. 2005, 52, 386–391. [Google Scholar] [CrossRef]
- Hampson, D.J. The Serpulina Story. In Proceedings of the 16th International Pig Veterinary Society Congress, Melbourne, Australia, 17–21 September 2000; pp. 1–5.
- Fries, R. Conclusions and activities of previous expert groups: The scientific steering committee of the EU. J. Vet. Med. 2004, 51, 403–407. [Google Scholar] [CrossRef]
- Råsbäck, T.; Jansson, D.S.; Johansson, K.E.; Fellström, C. A novel enteropathogenic, strongly haemolytic spirochaete isolated from pig and mallard, provisionally designated “Brachyspira suanatina”sp. nov. Environ. Microbiol. 2007, 9, 983–991. [Google Scholar] [CrossRef]
- Burrough, E.R.; Strait, E.L.; Kinyon, J.M.; Bower, L.P.; Madson, D.M.; Wilberts, B.L.; Schwartz, K.J.; Frana, T.S.; Songer, J.G. Comparative virulence of clinical Brachyspira spp. isolates in inoculated pigs. J. Vet. Diagn. Invest. 2012, 24, 1025–1034. [Google Scholar] [CrossRef]
- Chander, Y.; Primus, A.; Oliveira, S.; Gebhart, C.J. Phenotypic and molecular characterization of a novel strongly hemolytic Brachyspira species, provisionally designated “Brachyspira hampsonii”. J. Vet. Diagn. Invest. 2012, 24, 903–910. [Google Scholar]
- Rubin, J.E.; Costa, M.O.; Hill, J.E.; Kittrell, H.E.; Fernando, C.; Huang, Y.; O’Connor, B.; Harding, J.C.S. Reproduction of mucohaemorrhagic diarrhea and colitis indistinguishable from swine dysentery following experimental inoculation with “Brachyspira hampsonii” strain 30446. PloS One 2013, 8, e57146. [Google Scholar] [CrossRef]
- Desrosiers, R. Transmission of swine pathogens: Different means, different needs. Anim. Health Res. Rev. 2011, 12, 1–13. [Google Scholar] [CrossRef]
- Songer, J.G.; Glock, R.D.; Schwartz, K.J.; Harris, D.L. Isolation of Treponema hyodysenteriae from sources other than swine. J. Am. Vet. Med. Assoc. 1978, 172, 464–466. [Google Scholar]
- Jensen, N.S.; Stanton, T.B.; Swayne, D.E. Identification of the swine pathogen Serpulina hyodysenteriae in rheas (Rhea Americana). Vet. Microbiol. 1996, 52, 259–269. [Google Scholar] [CrossRef]
- Boye, M.; Baloda, S.B.; Leser, T.D.; Møller, K. Survival of Brachyspira hyodysenteriae and B. pilosicoli in terrestrial microcosms. Vet. Microbiol. 2001, 81, 33–40. [Google Scholar] [CrossRef]
- Jansson, D.S.; Johansson, K.E.; Olofson, T.; Rasback, T.; Vagsholm, I.; Petterson, B.; Gunnarson, A.; Fellstrom, C. Brachyspira hyodysenteriae and other strongly betahemolytic and indole-positive spirochaetes isolated from mallards (Anas platyrhynchos). J. Vet. Microbiol. 2004, 53, 293–300. [Google Scholar]
- Blunt, R.; McOrist, S. On-Farm Insect Vector Carriage of Swine Pathogens—Brachyspira and Lawsonia. In Proceedings of the 20th International Pig Veterinary Society Congress, Durban, South Africa, 22–25 June 2008; p. 291.
- Feberwee, A.; Hampson, D.J.; Philips, N.D.; La, T.; van der Heijden, H.M.; Wellenberg, G.J.; Dwars, R.M.; Landman, W.J. Identification of Brachyspira hyodysenteriae and other Brachyspira species in chickens from laying flocks with diarrhea or reduced production or both. J. Clin. Microbiol. 2008, 46, 593–600. [Google Scholar] [CrossRef]
- Gallie, A.; Chesworth, M.; Blunt, R.; McOrist, S. Identification of Harmful Dipteroid Communities on Pig Farms. In Proceedings of the American Association of Swine Veterinarians, Dallas, TX, USA, 7–10 March 2009; p. 323.
- Phillips, N.D.; La, T.; Adams, P.J.; Harland, B.L.; Fenwick, S.G.; Hampson, D.J. Detection of Brachyspira hyodysenteriae, Lawsonia intracellularis and Brachyspira pilosicoli in feral pigs. Vet. Microbiol. 2009, 134, 294–299. [Google Scholar] [CrossRef]
- Paster, B.J.; Dewhirst, F.E. Phylogenetic foundation of spirochaetes. J. Mol. Microb. Biotech. 2000, 2, 341–344. [Google Scholar]
- Ter Huurne, A.A.; Gaastra, W. Swine dysentery: More unknown than known. Vet. Microbiol. 1995, 46, 347–360. [Google Scholar] [CrossRef]
- Bellgard, M.I.; Wanchanthuek, P.; La, T.; Ryan, K.; Moolhuijzen, P.; Albertyn, Z.; Shaban, B.; Motro, Y.; Dunn, D.S.; Schibeci, D.; et al. Genome sequence of the pathogenic intestinal spirochaete Brachyspira hyodysenteriae reveals adaptations to its lifestyle in the porcine large intestine. PLoS One 2009, 4, e4641. [Google Scholar] [CrossRef]
- Halter, M.R.; Joens, L.A. Lipooligosaccharides from Treponema hyodysenteriae and Treponema innocens. Infect. Immun. 1988, 56, 3152–3156. [Google Scholar]
- Kennedy, M.J.; Rosnick, D.K.; Ulrich, R.G.; Yancey, R.J. Association of Treponema hyodysenteriae with porcine intestinal mucosa. J. Gen. Microbiol. 1988, 134, 1565–1576. [Google Scholar]
- Ter Huurne, A.A.; Muir, S.; van Houten, M.; van der Zeijst, B.A.; Gaastra, W.; Kusters, J.G. Characterization of three putative Serpulina hyodysenteriae hemolysins. Microb. Pathog. 1994, 16, 269–282. [Google Scholar] [CrossRef]
- Rosey, E.L.; Kennedy, M.J.; Yancey, R.J. Dual flaA1 flaB1 mutant of Serpulina hyodysenteriae expressing periplasmic flagella is severely attenuated in a murine model of swine dysentery. Infect. Immun. 1996, 64, 4154–4162. [Google Scholar]
- Wassenaar, T.M.; Gaastra, W. Bacterial virulence: Can we draw the line? FEMS Microbiol. Lett. 2001, 201, 1–7. [Google Scholar] [CrossRef]
- Barth, S.; Gommel, M.; Baljer, G.; Herbst, W. Demonstration of genes encoding virulence and virulence life-style factors in Brachyspira spp. isolates from pigs. Vet. Microbiol. 2012, 155, 438–443. [Google Scholar] [CrossRef]
- La, T.; Phillips, N.D.; Wanchanthuek, P.; Bellgard, M.I.; O’Hara, A.J.; Hampson, D.J. Evidence that the 36 kb plasmid of Brachyspira hyodysenteriae contributes to virulence. Vet. Microbiol. 2011, 153, 150–155. [Google Scholar]
- Olson, L.D. Clinical and pathological observations on the experimental passage of swine dysentery. Can. J. Comp. Med. 1974, 38, 7–13. [Google Scholar]
- Moreng, N.T.; Quarles, C.L.; Fagerberg, D.J.; Moeller, D.J. Pathogenesis and lesions of swine dysentery induced by artificial methods in early weaned pigs. Vet. Med. Small Anim. Clin. 1980, 75, 1841–1844. [Google Scholar]
- Savage, D.C. Colonization by and survival of pathogenic bacteria on intestinal mucosal surfaces. In Adsorption of Microorganisms to Surfaces; Bitton, B., Marshall, K.C., Eds.; Wiley: New York, NY, USA, 1980; pp. 175–206. [Google Scholar]
- Kinyon, J.M.; Harris, D.L.; Glock, R.D. Isolation of Treponema hyodysenteriae from Experimentally Infected Pigs at Various Intervals Post-Inoculation. In Proceedings of the 6th International Pig Veterinary Society Congress, Copenhagen, Denmark, 30 June–3 July 1980; p. 232.
- Hampson, D.J.; Cutler, R.; Lee, B.J. Virulent Serpulina hyodysenteriae from a pig in a herd free of clinical swine dysentery. Vet. Rec. 1992, 131, 318–319. [Google Scholar]
- Chia, S.P.; Taylor, D.J. Factors affecting the survvial of Treponema hyodysenteriae in dysenteric pig faeces. Vet. Rec. 1978, 103, 68–70. [Google Scholar]
- Harris, D.L.; Hampson, D.J.; Glock, R.D. Diseases of Swine; Straw, B.E., D’Allaire, S.D., Mengelling, W.D., Taylor, D.J., Eds.; Iowa State University Press: Ames, IA, USA, 1999; pp. 579–600. [Google Scholar]
- Songer, J.G.; Harris, D.L. Transmission of swine dysentery by carrier pigs. Am. J. Vet. Res. 1978, 39, 913–916. [Google Scholar]
- Hidalgo, A.; Osorio, J.; Papaterra, G.J.; Llanos, A.; Marca, J.; Ferro, A.; Hernandez-Caravaca, I.; Carvajal, A.; Rubio, P. Control of Swine Dysentery with an Innactivated Autovaccine against Brachyspira hyodysenteriae in a Multiplier Herd. In Proceedings of the 20th International Pig Veterinary Society Congress, Durban, South Africa, 22–25 June 2008; p. 242.
- Osorio, J.; Hidalgo, A.; Papaterra, G.J.; Llanos, A.; Marca, J.; Ferro, A.; Hernandez-Dávila, C.; Carvajal, A.; Rubio, P. Control of Swine Dysentery with An Autogenous Bacterin of Brachyspira hyodysenteriae in Iberian Pigs. In Proceedings of the 20th International Pig Veterinary Society Congress, Durban, South Africa, 22–25 June 2008; p. 249.
- Kanora, A.; de Groote, S.; Fockedey, M.; Velesova, S.; Karanikolova, M. Brachyspira Causing Enteric Disorder in Young Fattening Pigs Coming from Same Reproduction Pyramid. In Proceedings of the 20th International Pig Veterinary Society Congress, Durban, South Africa, 22–25 June 2008; p. 249.
- Robertson, I.D.; Mhoma, J.R.; Hampson, D.J. Risk factors associated with the occurrence of swine dysentery in Western Australia: Results of a postal survey. Aust. Vet. J. 1992, 69, 92–101. [Google Scholar] [CrossRef]
- Fellström, C; Rasbäck, T. How to Handle Swine Dysentery-the Swedish Approach. In Proceedings of the 5th Spiroconference, León, Spain, 8–10 June 2009; pp. 41–43.
- Bano, L.; Merialdi, G.; Bonilauri, P.; Dall’Anese, G.; Capello, K.; Comin, D.; Cattoli, G.; Sanguinetti, V.; Hampson, D.J.; Agnoletti, F. Prevalence, disease associations and risk factors for colonization with intestinal spirochaetes (Brachyspira spp.) in flocks of laying hens in north-eastern Italy. Avian Pathol. 2008, 37, 281–286. [Google Scholar]
- McOrist, S.; Bennett, C. Eradication of Swine Dysentery on Large-Scale Breeder Farms by Partial Depopulation/Medication. In Proceedings of the 20th International Pig Veterinary Society Congress, Durban, South Africa, 22–25 June 2008; p. 319.
- Phillips, N.D.; La, T.; Hampson, D.J. Survival of intestinal spirochaete strains from chickens in the presence of disinfectants and in faeces held at different temperatures. Avian Pathol. 2003, 32, 639–643. [Google Scholar] [CrossRef]
- Pico, L.; Szancer, J.; Pique, J.; Domeneque, A.; Rodriguez-Sierra, E.; Vidal, A. Swine Dysentery Eradication Programme in a Large Farm with Three Site Production by Strategic Management and Medication. In Proceedings of the 20th International Pig Veterinary Society Congress, Durban, South Africa, 22–25 June 2008; p. 131.
- Rajska, M.; Kempa, W.; Wilczynski, K. Experiences with Control Programme of Swine Dysentery in A Typical POLISH Pig Unit. In Experiences with Control Programme of Swine Dysentery in A Typical POLISH Pig Unit., Durban, South Africa, 22–25 June 2008; p. 250.
- Hampson, D.J.; Combs, B.G.; Harders, S.J.; Connaughton, I.D.; Fahy, V.A. Isolation of Treponema hyodysenteriae from a wild rat living on a piggery. Aust. Vet. J. 1991, 68, 308. [Google Scholar] [CrossRef]
- Backhans, A.; Johansson, K.E.; Fellström, C. Spirochaetes Isolated from Wild Rodents. In Proceedings of the 5th Spiroconference, León, Spain, 8–10 June 2009; pp. 58–59.
- Trott, D.J.; Atyeo, R.F.; Lee, J.I.; Swayne, D.A.; Stoutenburg, J.W.; Hampson, D.J. Genetic relatedness amongst intestinal spirochaetes isolated from rats and birds. Lett. Appl. Microbiol. 1996, 23, 431–436. [Google Scholar] [CrossRef]
- Fellstrom, C.; Holmgren, N. Mice as reservoirs for swine dysentery in a fattening herd. Svensk Veterinartid. 2005, 57, 19–21. [Google Scholar]
- Backhans, A.; Jansson, D.S.; Aspán, A.; Fellström, C. Typing of Brachyspira spp. from rodents, pigs and chickens on Swedish farms., A.; Fellström, C. Vet. Microbiol. 2011, 153, 156–162. [Google Scholar]
- Jensen, N.S.; Stanton, T.B.; Swayne, D.E. Identification of the swine pathogen Serpulina hyodysenteriae in rheas (Rhea americana). Vet. Microbiol. 1996, 52, 259–269. [Google Scholar] [CrossRef]
- Swayne, D.E.; McLaren, A.J. Avian intestinal spirochaetes and avian intestinal spirochaetosis. In Intestinal Spirochaetes in Domestic Animals and Humans; Hampson, D.J., Stanton, T.B., Eds.; CAB International: New York, NY, USA, 1997; pp. 267–300. [Google Scholar]
- Oxberry, S.L.; Trott, D.J.; Hampson, D.J. Serpulina pilosicoli, waterbirds and water: Potential sources of infection for humans and other animals. Epidemiol. Infect. 1998, 121, 219–225. [Google Scholar] [CrossRef]
- Duhamel, G.E. Comparative pathology and pathogenesis of naturally acquired and experimentally induced colonic spirochetosis. Anim. Health Res. Rev. 2001, 2, 3–17. [Google Scholar]
- Jansson, D.S.; Rasback, T.; Fellstrom, C.; Feinstein, R. Experimental challenge of mallards (Anas platyrhynchos) with Brachyspira hyodysenteriae and “Brachyspira suanatina” isolated from pigs and mallards. J. Comp. Pathol. 2009, 141, 211–222. [Google Scholar] [CrossRef]
- Jansson, D.S.; Persson, M.; Zimmerman, U.; Johansson, K.E. Phenotypic and genetic diversity among intestinal spirochaetes (genus Brachyspira) in free-living wild mallards (Anas platyrhynchos) sampled in southern Sweden. Syst. Appl. Microbiol. 2011, 34, 566–575. [Google Scholar] [CrossRef]
- Sagartz, J.E.; Swayne, D.E.; Eaton, K.A.; Hayes, J.R.; Amass, K.D.; Wack, R.; Kramer, L. Necrotizing typhlocolitis associated with a spirochaete in rheas (Rhea americana). Avian Dis. 1992, 36, 282–289. [Google Scholar]
- Buckles, E.L. Inoculation of Neonatal Common Rheas (Rhea americana) and Mallard Ducklings (Anas platyrhynchos) with Three Bacterial Species Associated with Necrotizing Typhlitis. M.Sc. Thesis, Ohio State University, Columbus, OH, USA, 1996; pp. 43–66. [Google Scholar]
- Buckles, E.L.; Eaton, K.A.; Swayne, D.E. Cases of spirochaete associated necrotizing typhlitis in captive common rheas (Rhea americana). Avian Dis. 1997, 41, 144–148. [Google Scholar] [CrossRef]
- Stanton, T.B.; Jensen, N.S.; Bosworth, B.T.; Kunkle, R.A. Evaluation of the Virulence of Rhea S. hyodysenteriae Strains for Swine. Brachyspira hyodysenteriae in Mallards. In Proceedings of the 1st International Virtual Conference on Infectious Diseases of Animals, National Animal Disease Center, Ames, IA, USA, 20 April–2 May 1997.
- Blunt, R.; Hancox, L.; Mellits, K.; McOrist, S. Likely Carriage of Brachyspira hyodysenteriae in Cockroaches and Flies on Pig Farms. In Proceedings of the 21st International Pig Veterinary Society Congress, Vancouver, BC, Canada, 18–21 July 2010; p. 93.
- Blunt, R.; McOrist, S. The Potential Transmission of Swine Dysentery by Cockroach Vectors. In Proceedings of the 5th Spiroconference, León, Spain, 8–10 June 2009; p. 14.
- Fellström, C.; Jacobsson, M. Screening for Brachyspira spp. and Lawsonia intracellularis in European Wild Pigs. In Proceedings of the 17th International Pig Veterinary Society Congress, Ames, IA, USA, 2–5 June 2002; p. 367.
- Songer, J.G.; Glock, R.D.; Schwartz, K.J.; Harris, D.L. Isolation of Treponema hyodysenteriae from sources other than swine. J. Am. Vet. Med. Assoc. 1978, 172, 464–466. [Google Scholar]
- Huisman, J.; Jansman, A.J.M. Dietary effects and some analytical aspects of antinutritional factors in pea (Pisum sativum), common beans (Phaseolus vulgaris) and soyabeans (Glycine max L.) in monogastric farm animals. A literature review. Nutr. Abstr. Rev. 1991, 61, 901–921. [Google Scholar]
- Dréau, D.; Lallés, J.P.; Philouze-Romé, V.; Toullec, R.; Salmon, H. Local and systemic immune responses to soybean protein ingestion in early-weaned pigs. J. Anim. Sci. 1994, 72, 2090–2098. [Google Scholar]
- Whipp, S.C.; Robinson, I.M.; Harris, D.L.; Glock, R.D.; Matthews, P.J.; Alexander, T.J. Pathogenic synergism between Treponema hyodysenteriae and other selected anaerobes in gnotobiotic pigs. Infect. Immun. 1979, 26, 1042–1047. [Google Scholar]
- Dréau, D.; Lallés, J.P.; Toullec, R.; Salmon, H. B and T lymphocytes are enhanced in the gut of piglets fed heat-treated soyabean proteins. Vet. Immunol. Immunopathol. 1995, 47, 69–79. [Google Scholar] [CrossRef]
- Neef, N.A.; McOrist, S.; Lysons, R.J.; Bland, A.P.; Miller, B.G. Development of large intestinal attaching and effacing lesions in pigs in association with the feeding of a particular diet. Infect. Immun. 1994, 62, 4325–4332. [Google Scholar]
- Siba, P.M.; Pethick, D.W.; Hampson, D.J. Pigs experimentally infected with Serpulina hyodysenteriae can be protected from developing swine dysentery by feeding them a highly digestible diet. Epidemiol. Infect. 1996, 116, 207–216. [Google Scholar] [CrossRef]
- Durmic, Z.; Pethick, D.; Mullan, B.; Schulze, H.; Accioly, J.; Hampson, D. Extrusion of wheat or sorghum and/or addition of exogenous enzymes to pig diets influences the large intestinal microbiota but does not prevent development of swine dysentery following experimental challenge. J. Appl. Microbiol. 2000, 89, 678–686. [Google Scholar] [CrossRef]
- Lindecrona, R.H.; Jensen, T.K.; Jensen, B.B.; Leser, T.D.; Jiufeng, W.; Møller, K. The influence of diet on the development of swine dysentery upon experimental infection. Anim. Sci. 2003, 76, 81–87. [Google Scholar]
- Thomsen, L.E.; Bach Knudsen, K.E.; Jensen, T.K.; Christensen, A.S.; Møller, K.; Roepstorff, A. The effect of fermentable carbohydrates on experimental swine dysentery and whip worm infections in pigs. Vet. Microbiol. 2007, 119, 152–163. [Google Scholar] [CrossRef]
- Hansen, C.F.; Phillips, N.D.; La, T.; Hernández, A.; Mansfield, J.; Kim, J.C.; Mullan, B.P.; Hampson, D.J.; Pluske, J.R. Diets containing inulin but not lupins help to prevent swine dysentery in experimentally challenged pigs. J. Anim. Sci. 2010, 88, 3327–3336. [Google Scholar] [CrossRef]
- Roberfroid, M.B.; van Loo, J.A.E.; Gibson, G.R. The bifidogenic nature of chicory inulin and its hydrolysis products. J. Nutr. 1998, 128, 11–19. [Google Scholar]
- Hansen, C.; Hernández, A.; Mansfield, J.; Hidalgo, A.; La, T.; Phillips, N.; Hampson, D.; Pluske, J. A high dietary concentration of inulin is necessary to reduce the incidence of swine dysentery in pigs experimentally challenged with Brachyspira hyodysenteriae. Br. J. Nutr. 2011, 106, 1506–1513. [Google Scholar] [CrossRef]
- Cummings, J.H.; Macfarlane, G.T. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 1991, 70, 443–459. [Google Scholar] [CrossRef]
- Mølbak, L.; Thomsen, L.E.; Jensen, T.K.; Bach Knudsen, K.E.; Boye, M. Increased amount of Bifidobacterium thermacidophilum and Megasphaera elsdenii in the colonic microbiota of pigs fed a swine dysentery preventive diet containing chicory roots and sweet lupine. J. Appl. Microbiol. 2007, 103, 1853–1867. [Google Scholar] [CrossRef]
- Kowalski, C.; Zarí, R.; Rolinski, Z. Pleuromutilin derivatives and their usage in veterinary treatment. Med. Weter. 2004, 60, 22–26. [Google Scholar]
- Molnar, L. Sensitivity of strains of Serpulina hyodysenteriae isolated in Hungary to chemotherapeutic drugs. Vet. Rec. 1996, 138, 158–160. [Google Scholar] [CrossRef]
- Gresham, A.C.; Hunt, B.W.; Dalziel, R.W. Treatment of swine dysentery—Problems of antibiotic resistance and concurrent salmonellosis. Vet. Rec. 1998, 143, 619. [Google Scholar]
- Karlsson, M.; Gunnarsson, A.; Franklin, A. Susceptibility to pleuromutilins in Brachyspira (Serpulina) hyodysenteriae. Anim. Health Res. Rev. 2001, 2, 59–65. [Google Scholar]
- Karlsson, M.; Fellstrom, C.; Gunnarsson, A.; Landen, A.; Franklin, A. Antimicrobial susceptibility testing of porcine Brachyspira (Serpulina) species isolates. J. Clin. Microbiol. 2003, 41, 2596–2604. [Google Scholar] [CrossRef]
- Lobova, D.; Smola, J.; Cizek, A. Decreased susceptibility to tiamulin and valnemulin among Czech isolates of Brachyspira hyodysenteriae. J. Med. Microbiol. 2004, 53, 287–291. [Google Scholar] [CrossRef]
- Rohde, J.; Kessler, M.; Baums, C.G.; Amtsberg, G. Comparison of methods for antimicrobial susceptibility testing and MIC values for pleuromutilin drugs for Brachyspira hyodysenteriae isolated in Germany. Vet. Microbiol. 2004, 102, 25–32. [Google Scholar] [CrossRef]
- Hidalgo, A.; Carvajal, A.; García-Feliz, C.; Osorio, J.; Rubio, P. Antimicrobial susceptibility testing of Spanish field isolates of Brachyspira hyodysenteriae. Res. Vet. Sci. 2009, 87, 7–12. [Google Scholar] [CrossRef]
- Hidalgo, A.; Carvajal, A.; Vester, B.; Pringle, M.; Naharro, G.; Rubio, P. Trends towards lower antimicrobial susceptibility and characterization of acquired resistance among clinical isolates of Brachyspira hyodysenteriae in Spain. Antimicrob. Agents Ch. 2011, 55, 3330–3337. [Google Scholar] [CrossRef]
- Ohya, T.; Sueyoshi, M. In vitro antimicrobial susceptibility of Brachyspira hyodysenteriae strains isolated in Japan from 1985 to 2009. J. Vet. Med. Sci. 2010, 72, 1651–1653. [Google Scholar] [CrossRef]
- Karlsson, M.; Fellstrom, C.; Heldtander, M.U.; Johansson, K.E.; Franklin, A. Genetic basis of macrolide and lincosamide resistance in Brachyspira (Serpulina) hyodysenteriae. FEMS Microbiol. Lett. 1999, 172, 255–260. [Google Scholar] [CrossRef]
- Karlsson, M.; Fellstrom, C.; Johansson, K.E.; Franklin, A. Antimicrobial resistance in Brachyspira pilosicoli with special reference to point mutations in the 23S rRNA gene associated with macrolide and lincosamide resistance. Microb. Drug Resist. 2004, 10, 204–208. [Google Scholar]
- Pringle, M.; Poehlsgaard, J.; Vester, B.; Long, K.S. Mutations in ribosomal protein L3 and 23S ribosomal RNA at the peptidyl transferase centre are associated with reduced susceptibility to tiamulin in Brachyspira spp. isolates. Mol. Microbiol. 2004, 54, 1295–1306. [Google Scholar] [CrossRef]
- Pringle, M.; Landén, A.; Unnerstad, H.E.; Molander, B.; Bengtsson, B. Antimicrobial susceptibility of porcine Brachyspira hyodysenteriae and Brachyspira pilosicoli isolated in Sweden between 1990 and 2010. Acta Vet. Scand. 2012, 54, 54. [Google Scholar] [CrossRef]
- Rønne, H.; Szancer, J. In Vitro Susceptibility of Danish Field Isolates of Treponema hyodysenteriae to Chemotherapeutics in Swine Dysentery (SD) Therapy. Interpretation of MICs Results Based on Pharmacokinetic Properties of the Antibacterial Agents. In Proceedings of the 11th International Pig veterinary Society Congress, Lausanne, Switerzland, 1–5 July 1990; p. 126.
- Burch, D.G.S. Pharmacokinetic, pharmacodynamic and clinical correlations relating to the therapy of colonic infections in the pig and breakpoints determinations. Pig J. 2005, 56, 8–24. [Google Scholar]
- Joens, L.A.; Harris, D.L.; Baum, D.H. Immunity to swine dysentery in recovered pigs. Am. J. Vet. Res. 1979, 40, 1352–1354. [Google Scholar]
- Fernie, D.S.; Ripley, P.H.; Walker, P.D. Swine dysentery: Protection against experimental challenge following single dose parenteral immunisation with inactivated Treponema hyodysenteriae. Res. Vet. Sci. 1983, 35, 217–221. [Google Scholar]
- Parizek, R.; Stewart, R.; Brown, K.; Blevins, D. Protection against swine dysentery with an inactivated Treponema hyodysentariae bacterin. Vet. Med. 1985, 80, 80–86. [Google Scholar]
- Diego, R.; Lanza, I.; Carvajal, A.; Rubio, P.; Carmenes, P. Serpulina hyodysenteriae challenge of fattening pigs vaccinated with an adjuvanted bivalent bacterin against swine dysentery. Vaccine 1995, 13, 663–667. [Google Scholar] [CrossRef]
- Waters, W.R.; Sacco, R.E.; Dorn, A.D.; Hontecillas, R.; Zuckermann, F.A.; Wannemuehler, M.J. Systemic and mucosal immune responses of pigs to parenteral immunization with a pepsin-digested Serpulina hyodysenteriae bacterin. Vet. Immunol. Immunopathol. 1999, 69, 75–87. [Google Scholar] [CrossRef]
- Hudson, M.J.; Alexander, T.L.J.; Lysons, R.J.; Wellstead, P.D. Swine dysentery: Failure of an attenuated strain of spirochaete, given orally, to protect pigs against subsequent challenge. Br. Vet. J. 1974, 130, 37–40. [Google Scholar]
- Lysons, R.; Burrows, M.R.; Jones, P.W.; Collins, P. Swine dysentry, a new and effective vaccine. Proc. Int. Pig Vet. Soc. 1987, 18, 87–91. [Google Scholar]
- Hyatt, D.R.; Ter Huurne, A.A.; van der Zeijst, B.A.; Joens, L.A. Reduced virulence of Serpulina hyodysenteriae hemolysin-negative mutants in pigs and their potential to protect pigs against challenge with a virulent strain. Infect. Immun. 1994, 62, 2244–2248. [Google Scholar]
- Song, Y.; La, T.; Phillips, N.D.; Bellgard, M.I.; Hampson, D.J. A reverse vaccinology approach to swine dysentery vaccine development. Vet. Microbiol. 2009, 137, 111–119. [Google Scholar] [CrossRef]
- Joens, L.A.; Marquez, M.R.; Halter, M. Comparison of outer-membrane fractions of Serpulina (Treponema) hyodysenteriae. Vet. Microbiol. 1993, 35, 119–132. [Google Scholar] [CrossRef]
- La, T.; Phillips, N.D.; Reichel, M.P.; Hampson, D.J. Protection of pigs from swine dysentery by vaccination with recombinant BmpB, a 29.7 kDa outer-membrane lipoprotein of Brachyspira hyodysenteriae. Vet. Microbiol. 2004, 102, 97–109. [Google Scholar]
- Witchell, T.D.; Coutts, S.A.J.; Bulach, D.M.; Adler, B. Differential expression of the Bhmp39 major outer membrane proteins of Brachyspira hyodysenteriae. Infect. Immun. 2006, 74, 3271–3276. [Google Scholar] [CrossRef]
- Holden, J.; Coloe, P.J.; Smooker, P.M. An evaluation of the immunogenicity and protective responses to Brachyspira hyodysenteriae recombinant SmpB vaccination. Vet. Microbiol. 2008, 128, 354–363. [Google Scholar] [CrossRef]
- Boyden, D.A.; Albert, F.G.; Robinson, C.S. Cloning and characterization of Treponema hyodysenteriae antigens and protection in a CF-1 mouse model by immunization with a cloned endoflagellar antigen. Infect. Immun. 1989, 57, 3808–3815. [Google Scholar]
- Gabe, J.D.; Chang, R.J.; Slomiany, R.; Andrews, W.H.; McCaman, M.T. Isolation of extracytoplasmic proteins from Serpulina hyodysenteriae B204 and molecular cloning of the flaB1 gene encoding a 38-kilodalton flagellar protein. Infect. Immun. 1995, 63, 142–148. [Google Scholar]
- Davis, A.J.; Smith, S.C.; Moore, R.J. The Brachyspira hyodysenteriae ftnA gene: DNA vaccination and real-time PCR quantification of bacteria in a mouse model of disease. Curr. Microbiol. 2005, 50, 285–291. [Google Scholar]
- Pluske, J.; Durmic, Z.; Pethick, D.; Mullan, B.; Hampson, D. Confirmation of the role of rapidly fermentable carbohydrates in the expression of swine dysentery in pigs after experimental infection. J. Nutr. 1998, 128, 1737–1744. [Google Scholar]
- Durmic, Z.; Pethick, D.W.; Mullan, B.P.; Accioly, J.M.; Schulze, H.; Hampson, D.J. Evaluation of large intestinal parameters associated with dietary treatments designed to reduce the occurrence of swine dysentery. Br. J. Nutr. 2002, 88, 159–169. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar]
- Hontecillas, R.; Wannemeulher, M.J.; Zimmerman, D.R.; Hutto, D.L.; Wilson, J.H.; Ahn, D.U.; Bassaganya-Riera, J. Nutritional regulation of porcine bacterial-induced colitis by conjugated linoleic acid. J. Nutr. 2002, 132, 2019–2027. [Google Scholar]
- Bernardeau, M.; Gueguen, M.; Smith, D.G.E.; Corona-Barrera, E.; Vernoux, J.P. In vitro antagonistic activities of Lactobacillus spp. against Brachyspira hyodysenteriae and Brachyspira pilosicoli. Vet. Microbiol. 2009, 138, 184–190. [Google Scholar] [CrossRef]
- Klose, V.; Bayer, K.; Bruckbeck, R.; Schatzmayr, G.; Loibner, A.P. In vitro antagonistic activities of animal intestinal strains against swine-associated pathogens. Vet. Microbiol. 2010, 144, 515–521. [Google Scholar] [CrossRef]
- Klose, V.; Bruckbeck, R.; Henikl, S.; Schatzmayr, G.; Loibner, A.P. Identification and antimicrobial susceptibility of porcine bacteria that inhibit the growth of Brachyspira hyodysenteriae in vitro. J. Appl. Microbiol. 2010, 108, 1271–1280. [Google Scholar] [CrossRef]
- Lyoo, Y.; Park, D.; Lee, S.; Choi, Y.; Jung, J.; Jun, T.; Ahm, H.; Lee, Ch.; Lym, Y. Antibacterial compound against Pasteurella multocida and Actinobacillus pleuropneumoniae causing porcine pneumonia. J. Microbiol. Biotech. 2001, 11, 350–353. [Google Scholar]
- Becker, P.M.; van Wikselaar, P.G.; Mul, M.F.; Pol, A.; Engel, B.; Wijdenes, J.W.; van der Peet-Schwering, C.M.; Wisselink, H.J.; Stockhofe-Zurwieden, N. Actinobacilluspleuropneumoniae is impaired by the garlic volatile allyl methyl sulphide (AMS) in vitro and in-feed garlic alleviates pleuropneumonia in a pig model. Vet. Microbiol. 2012, 154, 316–324. [Google Scholar] [CrossRef]
- Lobova, D.; Cizek, A. Bactericidal efficacy of two disinfectants against Brachyspira hyodysenteriae and one feed supplement against B. hyodysenteriae and B. pilosicoli. Vet. Med. Czech 2004, 49, 156–160. [Google Scholar]
- Alvarez-Ordóñez, A.; Carvajal, A.; Arguello, H.; Martínez-Lobo, F.J.; Naharro, G.; Rubio, P. Antibacterial activity and mode of action of a commercial citrus fruit extract. J. Appl. Microbiol. 2013. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alvarez-Ordóez, A.; Martínez-Lobo, F.J.; Arguello, H.; Carvajal, A.; Rubio, P. Swine Dysentery: Aetiology, Pathogenicity, Determinants of Transmission and the Fight against the Disease. Int. J. Environ. Res. Public Health 2013, 10, 1927-1947. https://doi.org/10.3390/ijerph10051927
Alvarez-Ordóez A, Martínez-Lobo FJ, Arguello H, Carvajal A, Rubio P. Swine Dysentery: Aetiology, Pathogenicity, Determinants of Transmission and the Fight against the Disease. International Journal of Environmental Research and Public Health. 2013; 10(5):1927-1947. https://doi.org/10.3390/ijerph10051927
Chicago/Turabian StyleAlvarez-Ordóez, Avelino, Francisco Javier Martínez-Lobo, Héctor Arguello, Ana Carvajal, and Pedro Rubio. 2013. "Swine Dysentery: Aetiology, Pathogenicity, Determinants of Transmission and the Fight against the Disease" International Journal of Environmental Research and Public Health 10, no. 5: 1927-1947. https://doi.org/10.3390/ijerph10051927




