Protective Effects of Sodium Selenite against Aflatoxin B1-Induced Oxidative Stress and Apoptosis in Broiler Spleen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Lipid Peroxidation and Antioxidant Defense System Assays
2.3. Annexin V Apoptosis Detection by Flow Cytometry
2.4. TUNEL
2.5. Statistical Analysis
3. Results
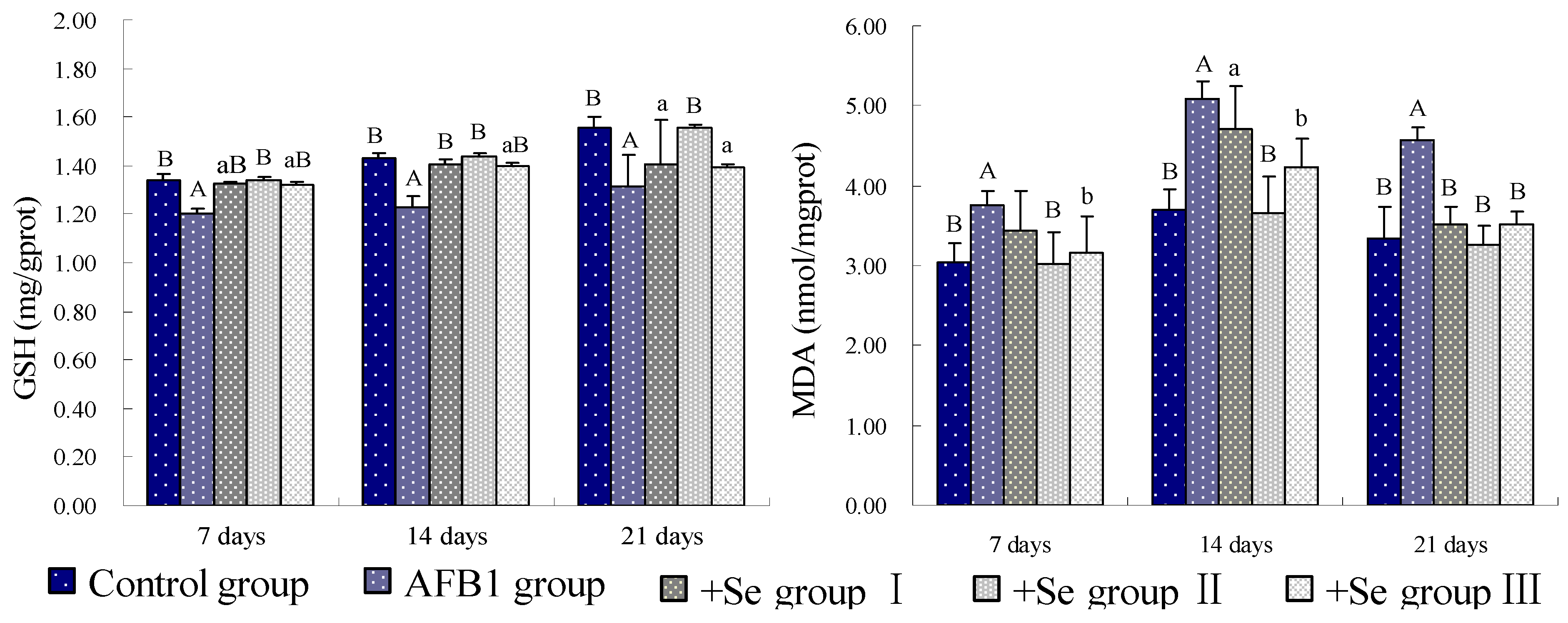
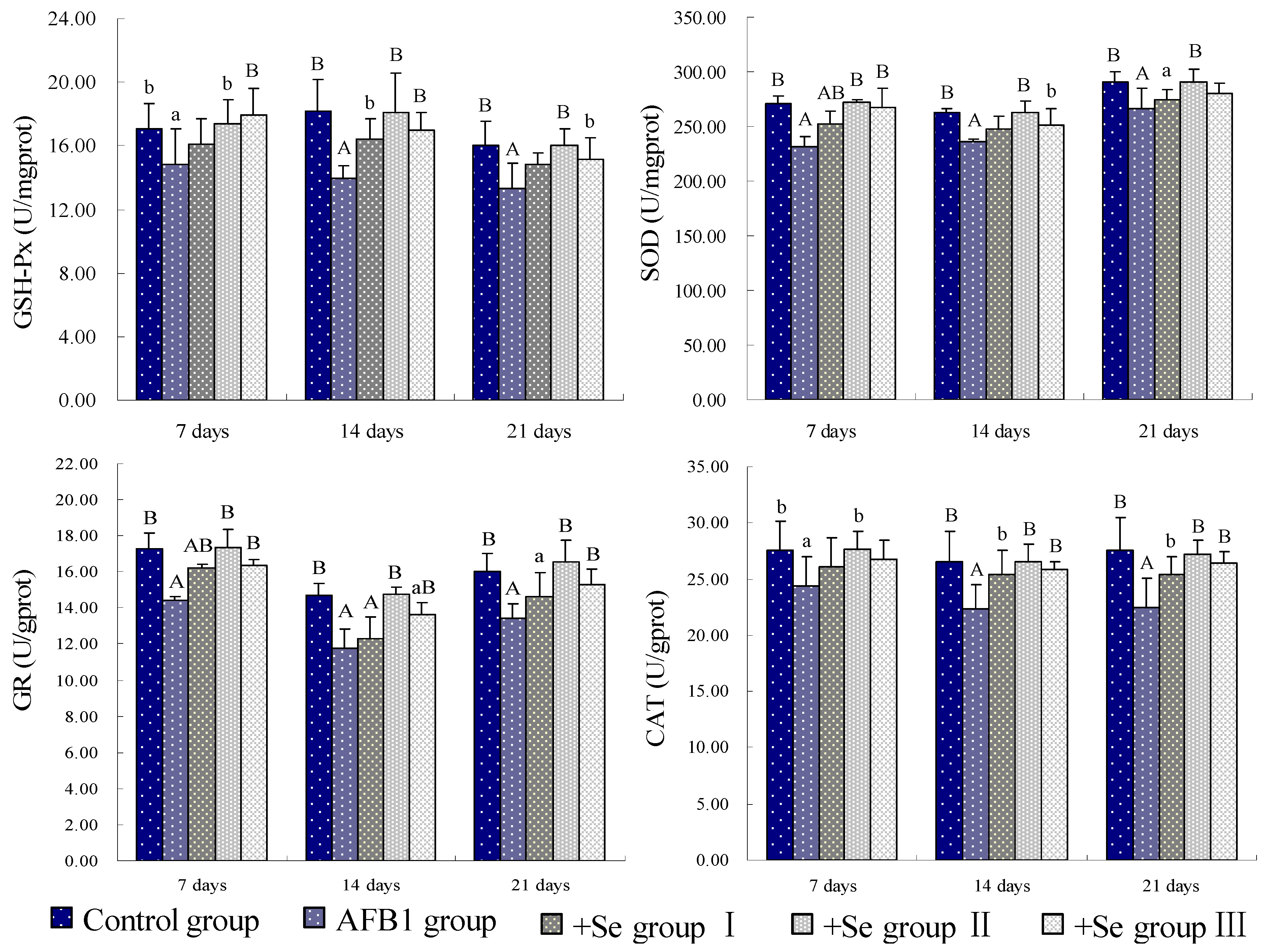
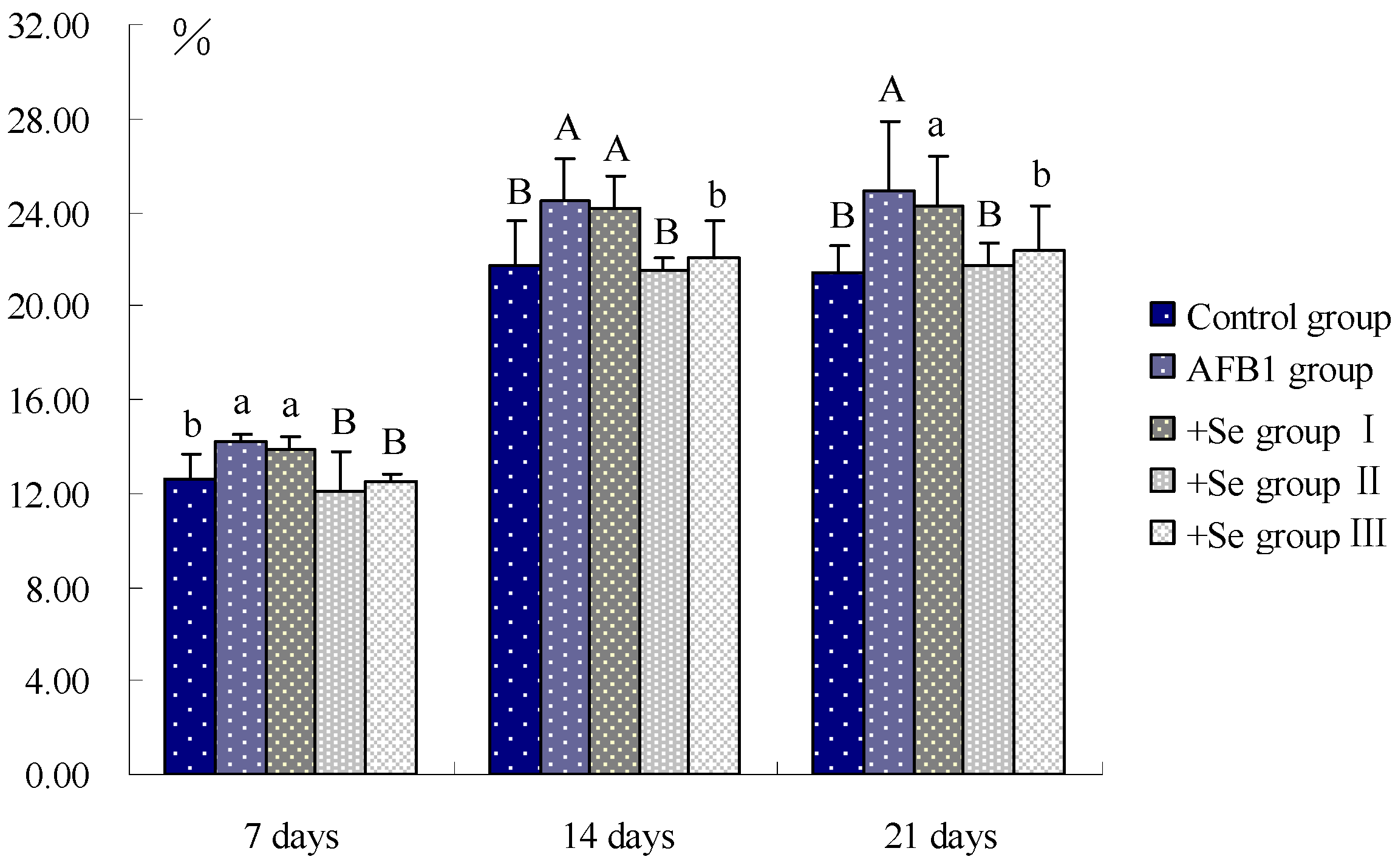
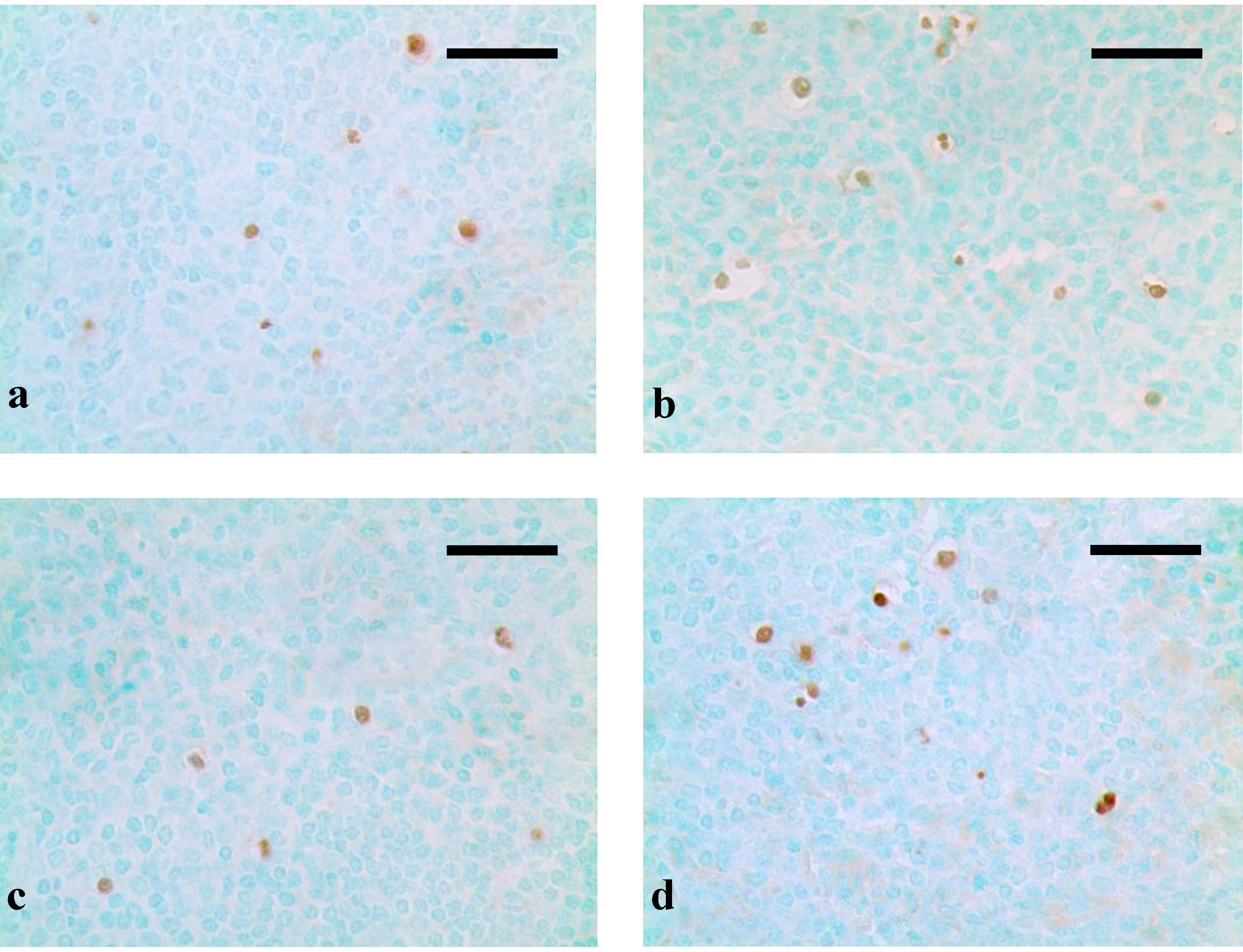
4. Discussion and Conclusions
Acknowledgments
Conflict of Interest
References
- Partanen, H.A.; El-Nezami, H.S.; Leppänen, J.M.; Myllynen, P.K.; Woodhouse, H.J.; Vähäkangas, K.H. Aflatoxin B1 transfer and metabolism in human placenta. Toxicol. Sci. 2010, 113, 216–225. [Google Scholar] [CrossRef]
- Wu, H.C.; Wang, Q.; Yang, H.I.; Ahsan, H.; Tsai, W.Y.; Wang, L.Y.; Chen, S.Y.; Chen, C.J.; Santella, R.M. Aflatoxin B1 exposure, hepatitis B virus infection, and hepatocellular carcinoma in Taiwan. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 846–853. [Google Scholar] [CrossRef]
- Wild, C.P.; Turner, P.C. The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis 2002, 17, 471–481. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, M.Z.; Khan, A.; Javed, I.; Saleemi, M.K.; Mahmood, S.; Asi, M.R. Residues of aflatoxin B1 in broiler meat: Effect of age and dietary aflatoxin B1 levels. Food Chem. Toxicol. 2010, 48, 3304–3307. [Google Scholar] [CrossRef]
- Verma, J.; Johri, T.S.; Swain, B.K.; Ameena, S. Effect of graded levels of aflatoxin, ochratoxin and their combinations on the performance and immune response of broilers. Brit. Poultry Sci. 2004, 45, 512–518. [Google Scholar] [CrossRef]
- Tedesco, D.; Steidler, S.; Galletti, S.; Tameni, M.; Sonzogni, O.; Ravarotto, L. Efficacy of silymarin-phospholipid complex in reducing the toxicity of aflatoxin B1 in broiler chicks. Poultry Sci. 2004, 83, 1839–1843. [Google Scholar]
- Mary, V.S.; Theumer, M.G.; Arias, S.L.; Rubinstein, H.R. Reactive oxygen species sources and biomolecular oxidative damage induced by aflatoxin B1 and fumonisin B1 in rat spleen mononuclear cells. Toxicology 2012, 302, 299–307. [Google Scholar]
- Dalel, B.; Chayma, B.; Yousra, A.; Hédi, B.M.; Lazhar, Z.; Hassen, B. Chemopreventive effect of cactus Opuntia ficus indica on oxidative stress and genotoxicity of aflatoxin B1. Nutr. Metab. 2011, 73. [Google Scholar] [CrossRef]
- Combs, G.F.; Gray, W.P. Chemopreventive agents: Selenium. Pharmacol. Therapeut. 1998, 79, 179–192. [Google Scholar] [CrossRef]
- Farzin, L.; Moassesi, M.E.; Sajadi, F.; Amiri, M.; Shams, H. Serum levels of antioxidants (Zn, Cu, Se) in healthy volunteers living in Tehran. Biol. Trace Elem. Res. 2009, 129, 36–45. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.; Walker, S.W.; Arthur, J.R.; Nicol, F.; Pickard, K.; Lewin, M.H.; Howie, A.F.; Beckett, G.J. Selenite protects human endothelial cells from oxidative damage and induces thioredoxin reductase. Clin. Sci. 2001, 100, 543–550. [Google Scholar] [CrossRef]
- Cui, W.; Cui, H.M.; Peng, X.; Fang, J.; Liu, X.D.; Wu, B.Y. Effect of vanadium on splenocyte apoptosis in broilers. Med. Chem. 2012, 2, 57–60. [Google Scholar]
- Sandford, E.E.; Orr, M.; Balfanz, E.; Bowerman, N.; Li, X.Y.; Zhou, H.J.; Johnson, T.J.; Kariyawasam, S.; Liu, P.; Nolan, L.K.; Lamont, S.J. Spleen transcriptome response to infection with avian pathogenic Escherichia coli in broiler chickens. BMC Genomics 2011, 12, 469. [Google Scholar]
- Guo, S.N.; Liao, S.Q.; Su, R.S.; Lin, R.Q.; Chen, Y.Z.; Tang, Z.X.; Wu, H.; Shi, D.Y. Influence of Longdan Xiegan decoction on body weights and immune organ indexes in ducklings intoxicated with aflatoxin B1. J. Anim. Vet. Adv. 2012, 11, 1162–1165. [Google Scholar] [CrossRef]
- Guo, S.N.; Shi, D.Y.; Liao, S.Q.; Su, R.S.; Lin, Y.C.; Pan, J.Q.; Tang, Z.X. Influence of selenium on body weights and immune organ indexes in ducklings intoxicated with aflatoxin B1. Biol. Trace Elem. Res. 2012, 146, 167–170. [Google Scholar] [CrossRef]
- Ghosh, R.C.; Chauhan, H.V.S.; Roy, S. Immunosuppression in broilers under experimental aflatoxicosis. Br. Vet. J. 1990, 146, 457–462. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.X.; Shi, D.Z.; Chen, Y.X. Adult exposure to sasanguasaponin induces spermatogenic cell apoptosis in vivo through increased oxidative stress in male mice. Toxicol. Ind. Health 2010, 26, 691–700. [Google Scholar] [CrossRef]
- Chen, T.; Cui, H.M.; Cui, Y.; Bai, C.M.; Gong, T.; Peng, X. Cell-cycle blockage associated with increased apoptotic cells in the thymus of chickens fed on diets high in fluorine. Hum. Exp. Toxicol. 2011, 30, 685–692. [Google Scholar] [CrossRef]
- Sun, L.; Lam, W.P.; Wong, Y.W.; Lam, L.H.; Tang, H.C.; Wai, M.S.; Mak, Y.T.; Pan, F.; Yew, D.T. Permanent deficits in brain functions caused by long-term ketamine treatment in mice. Hum. Exp. Toxicol. 2011, 30, 1287–1296. [Google Scholar] [CrossRef]
- Peng, X.; Cui, Y.; Cui, W.; Deng, J.L.; Cui, H.M. The decrease of relative weight, lesions, and apoptosis of bursa of fabricius induced by excess dietary selenium in chickens. Biol. Trace Elem. Res. 2009, 131, 33–42. [Google Scholar] [CrossRef]
- Shen, H.M.; Shi, C.Y.; Lee, H.P.; Ong, C.N. Aflatoxin B1-induced lipid peroxidation in rat liver. Toxicol. Appl. Pharm. 1994, 127, 145–150. [Google Scholar] [CrossRef]
- Alpsoy, L.; Yildirim, A.; Agar, G. The antioxidant effects of vitamin A, C, and E on aflatoxin B1-induced oxidative stress in human lymphocytes. Toxicol. Ind. Health 2009, 25, 121–127. [Google Scholar] [CrossRef]
- Kotan, E.; Alpsoy, L.; Anar, M.; Aslan, A.; Agar, G. Protective role of methanol extract of Cetraria islandica (L.) against oxidative stress and genotoxic effects of AFB1 in human lymphocytes in vitro. Toxicol. Ind. Health 2011, 27, 599–605. [Google Scholar] [CrossRef]
- Farombi, E.O.; Adepoju, B.F.; Ola-Davies, O.E.; Emerole, G.O. Chemoprevention of aflatoxin B1-induced genotoxicity and hepatic oxidative damage in rats by kolaviron, a natural biflavonoid of Garcinia kola seeds. Eur. J. Cancer Prev. 2005, 14, 207–214. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Introduction. In Oxidative Stress: Oxidants and Antioxidants; Academic Press: San Diego, CA, USA, 1991; pp. 15–22. [Google Scholar]
- Verma, R.J.; Nair, A. Ameliorative effect of vitamin E on aflatoxin-induced lipid peroxidation in the testis of mice. Asian J. Androl. 2001, 3, 217–221. [Google Scholar]
- Liu, J.; Li, N.; Ma, L.; Duan, Y.M.; Wang, J.; Zhao, X.Y.; Wang, S.S.; Wang, H.; Hong, F.S. Oxidative injury in the mouse spleen caused by lanthanides. J. Alloy. Compd. 2010, 489, 708–713. [Google Scholar] [CrossRef]
- Gagliano, N.; Donne, I.D.; Torri, C.; Migliori, M.; Grizzi, F.; Milzani, A.; Filippi, C.; Annoni, G.; Colombo, P.; Costa, F.; et al. Early cytotoxic effects of ochratoxin A in rat liver: A morphological, biochemical and molecular study. Toxicology 2006, 225, 214–224. [Google Scholar] [CrossRef]
- Abdel-Aziema, S.H.; Hassana, A.M.; Abdel-Wahhab, M.A. Dietary supplementation with whey protein and ginseng extract counteracts oxidative stress and DNA damage in rats fed an aflatoxin-contaminated diet. Mutat. Res. 2011, 723, 65–71. [Google Scholar] [CrossRef]
- Bernabucci, U.; Colavecchia, L.; Danieli, P.P.; Basiricò, L.; Lacetera, N.; Nardone, A.; Ronchi, B. Aflatoxin B1 and fumonisin B1 affect the oxidative status of bovine peripheral blood mononuclear cells. Toxicol. In Vitro 2011, 25, 684–691. [Google Scholar] [CrossRef]
- El-Nekeety, A.A.; Mohamed, S.R.; Hathout, A.S.; Hassan, N.S.; Aly, S.E.; Abdel-Wahhab, M.A. Antioxidant properties of thymus vulgaris oil against aflatoxin-induce oxidative stress in male rats. Toxicon 2011, 57, 984–991. [Google Scholar] [CrossRef]
- Meki, A.R.; Esmail, E.E.D.F.; Hussein, A.A.; Hassanein, H.M. Caspase-3 and heat shock protein-70 in rat liver treated with aflatoxin B1: Effect of melatonin. Toxicon 2004, 43, 93–100. [Google Scholar] [CrossRef]
- Sun, E.W.; Shi, Y.F. Apoptosis: The quiet death silences the immune system. Pharmacol. Therapeut. 2001, 92, 135–145. [Google Scholar] [CrossRef]
- Deng, S.X.; Tian, L.X.; Liu, F.J.; Jin, S.J.; Liang, G.Y.; Yang, H.J.; Du, Z.Y.; Liu, Y.J. Toxic effects and residue of aflatoxin B1 in tilapia (Oreochromis niloticus × O. aureus) during long-term dietary exposure. Aquaculture 2010, 307, 233–240. [Google Scholar] [CrossRef]
- Raja, H.G.; Kohlia, E.; Rohila, V.; Dwarakanathb, B.S.; Parmarc, V.S.; Malika, S.; Adhikarib, J.S.; Tyagia, Y.K.; Goela, S.; Guptaa, K.; Bosea, M.; Olsend, C.E. Acetoxy-4-methylcoumarins confer differential protection from aflatoxin B1-induced micronuclei and apoptosis in lung and bone marrow cells. Mutat. Res. 2001, 494, 31–40. [Google Scholar] [CrossRef]
- Yang, X.J.; Lu, H.Y.; Li, Z.Y.; Bian, Q.; Qiu, L.L.; Li, Z.; Liu, Q.Z.; Li, J.M.; Wang, X.R.; Wang, S.L. Cytochrome P450 2A13 mediates aflatoxin B1-induced cytotoxicity and apoptosis in human bronchial epithelial cells. Toxicology 2012, 300, 138–148. [Google Scholar] [CrossRef]
- Raza, H.; John, A.; Benedict, S. Acetylsalicylic acid-induced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells. Eur. J. Pharmacol. 2011, 668, 15–24. [Google Scholar] [CrossRef]
- Kume, S.; Haneda, M.; Kanasaki, K.; Sugimoto, T.; Araki, S.; Isono, M.; Isshiki, K.; Uzu, T.; Kashiwagi, A.; Koya, D. Silent information regulator 2 (SIRT1) attenuates oxidative stress-induced mesangial cell apoptosis via p53 deacetylation. Free Radic. Bio. Med. 2006, 40, 2175–2182. [Google Scholar] [CrossRef]
- Forceville, X. Effects of high doses of selenium, as sodium selenite, in septic shock patients a placebo-controlled, randomized, double-blind, multi-center phase II study—Selenium and sepsis. J. Trace Elem. Med. Bio 2007, 21, 62–65. [Google Scholar] [CrossRef]
- Peng, X.; Cui, H.M.; Deng, J.L.; Zuo, Z.C.; Lai, W.M. Histological lesion of spleen and inhibition of splenocyte proliferation in broilers fed on diets excess in selenium. Biol. Trace Elem. Res. 2011, 140, 66–72. [Google Scholar] [CrossRef]
- Cemek, M.; Büyükokuroðlu, M.E.; Hazman, Ö.; Bulut, S.; Konuk, M.; Birdane, Y. Antioxidant enzyme and element status in heroin addiction or heroin withdrawal in rats: Effect of melatonin and vitamin E plus Se. Biol. Trace Elem. Res. 2011, 139, 41–54. [Google Scholar] [CrossRef]
- Taskin, E.; Dursun, N. The protection of selenium on adriamycin-induced mitochondrial damage in rat. Biol. Trace Elem. Res. 2012, 147, 165–171. [Google Scholar] [CrossRef]
- Markoviæ, S.D.; Djaèiæ, D.S.; Cvetkoviæ, D.M.; Obradoviæ, A.D.; Žižiæ, J.B.; Ognjanoviæ, B.I.; Štajn, A.Š. Effects of acute in vivo cisplatin and selenium treatment on hematological and oxidative stress parameters in red blood cells of rats. Biol. Trace Elem. Res. 2011, 142, 660–670. [Google Scholar] [CrossRef]
- Ding, L.; Li, X.; Liu, P.; Li, S.Q.; Lv, J.L. Study of the action of Se and Cu on the growth metabolism of Escherichia coli by microcalorimetry. Biol. Trace Elem. Res. 2010, 137, 364–372. [Google Scholar] [CrossRef]
- Battin, E.E.; Perron, N.R.; Brumaghim, J.L. The central role of metal coordination in selenium antioxidant activity. Inorg. Chem. 2006, 45, 499–501. [Google Scholar]
- Zhou, Y.J.; Zhang, S.P.; Liu, C.W.; Cai, Y.Q. The protection of selenium on ROS mediated-apoptosis by mitochondria dysfunction in cadmium-induced LLC-PK1 cells. Toxicol. In Vitro 2009, 23, 288–294. [Google Scholar] [CrossRef]
- Guérin, P.J.; Furtak, T.; Eng, K.; Gauthiera, E.R. Oxidative stress is not required for the induction of apoptosis upon glutamine starvation of Sp2/0-Ag14 hybridoma cells. Eur. J. Cell. Biol. 2006, 85, 355–365. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, F.; Shu, G.; Peng, X.; Fang, J.; Chen, K.; Cui, H.; Chen, Z.; Zuo, Z.; Deng, J.; Geng, Y.; et al. Protective Effects of Sodium Selenite against Aflatoxin B1-Induced Oxidative Stress and Apoptosis in Broiler Spleen. Int. J. Environ. Res. Public Health 2013, 10, 2834-2844. https://doi.org/10.3390/ijerph10072834
Wang F, Shu G, Peng X, Fang J, Chen K, Cui H, Chen Z, Zuo Z, Deng J, Geng Y, et al. Protective Effects of Sodium Selenite against Aflatoxin B1-Induced Oxidative Stress and Apoptosis in Broiler Spleen. International Journal of Environmental Research and Public Health. 2013; 10(7):2834-2844. https://doi.org/10.3390/ijerph10072834
Chicago/Turabian StyleWang, Fengyuan, Gang Shu, Xi Peng, Jing Fang, Kejie Chen, Hengmin Cui, Zhengli Chen, Zhicai Zuo, Junliang Deng, Yi Geng, and et al. 2013. "Protective Effects of Sodium Selenite against Aflatoxin B1-Induced Oxidative Stress and Apoptosis in Broiler Spleen" International Journal of Environmental Research and Public Health 10, no. 7: 2834-2844. https://doi.org/10.3390/ijerph10072834




