Hypothyroxinemia Induced by Mild Iodine Deficiency Deregulats Thyroid Proteins during Gestation and Lactation in Dams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Iodine Deficient Diet
2.3. Measurement of Thyroid Iodine Content
2.4. Western Blotting
2.5. Statistics
3. Results
3.1. Animal Model
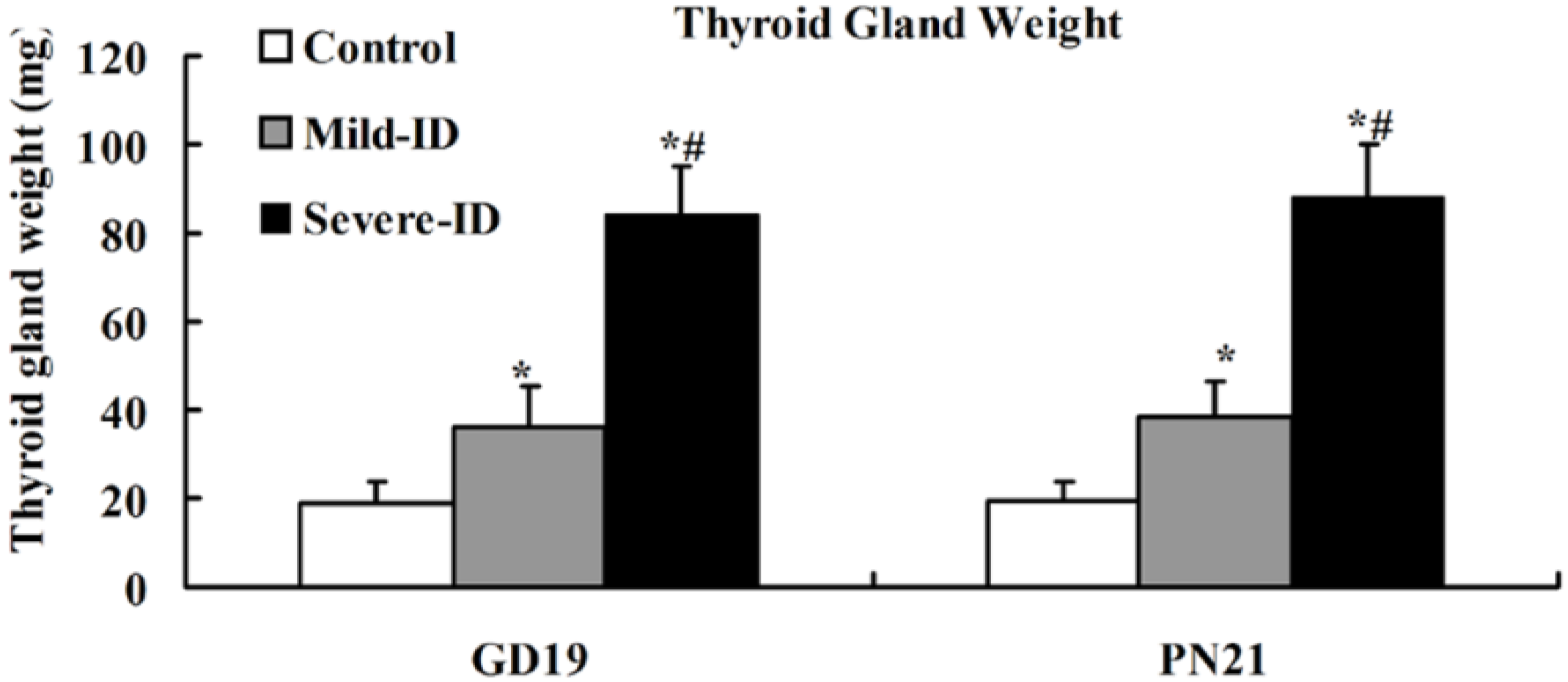
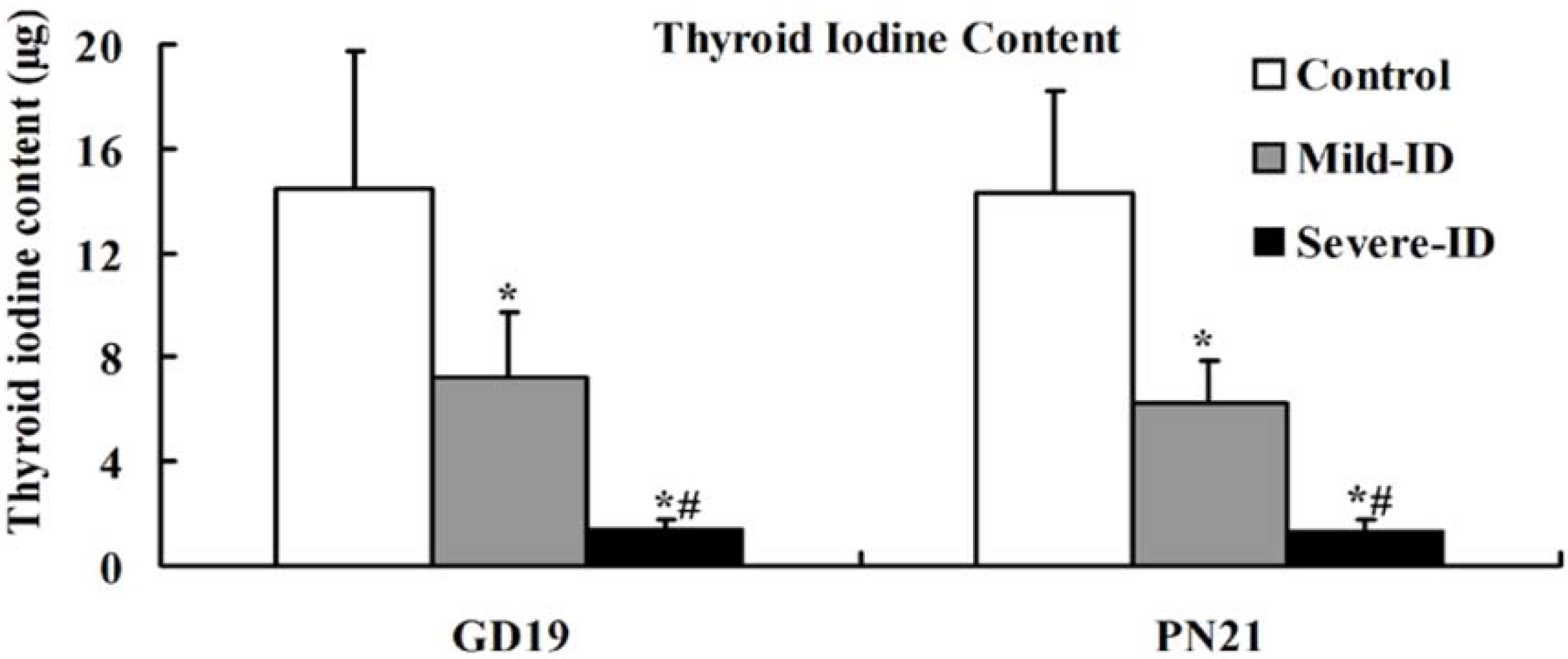
3.2. Increased Thyroid Gland Weight and Decreased Thyroid Iodine Content
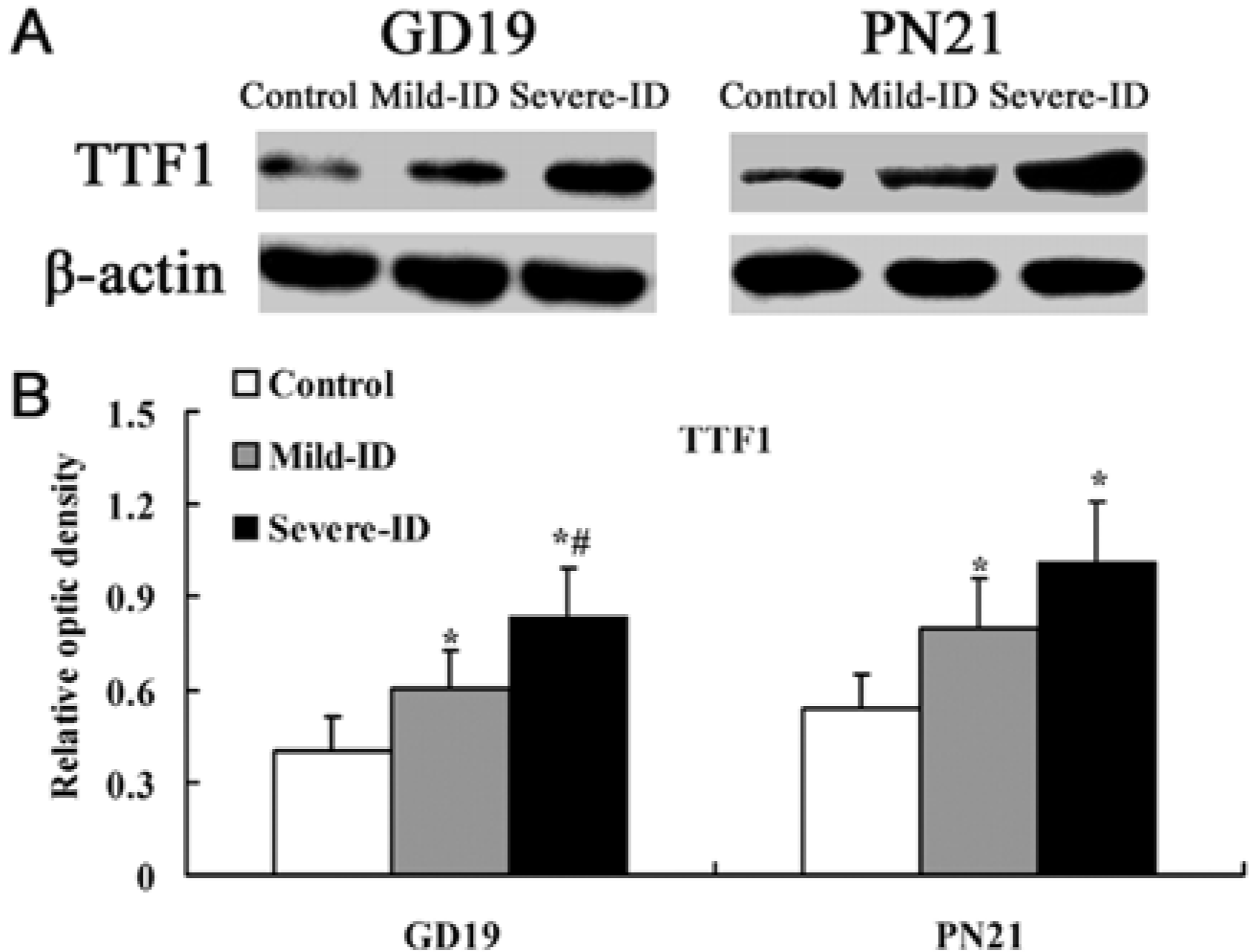
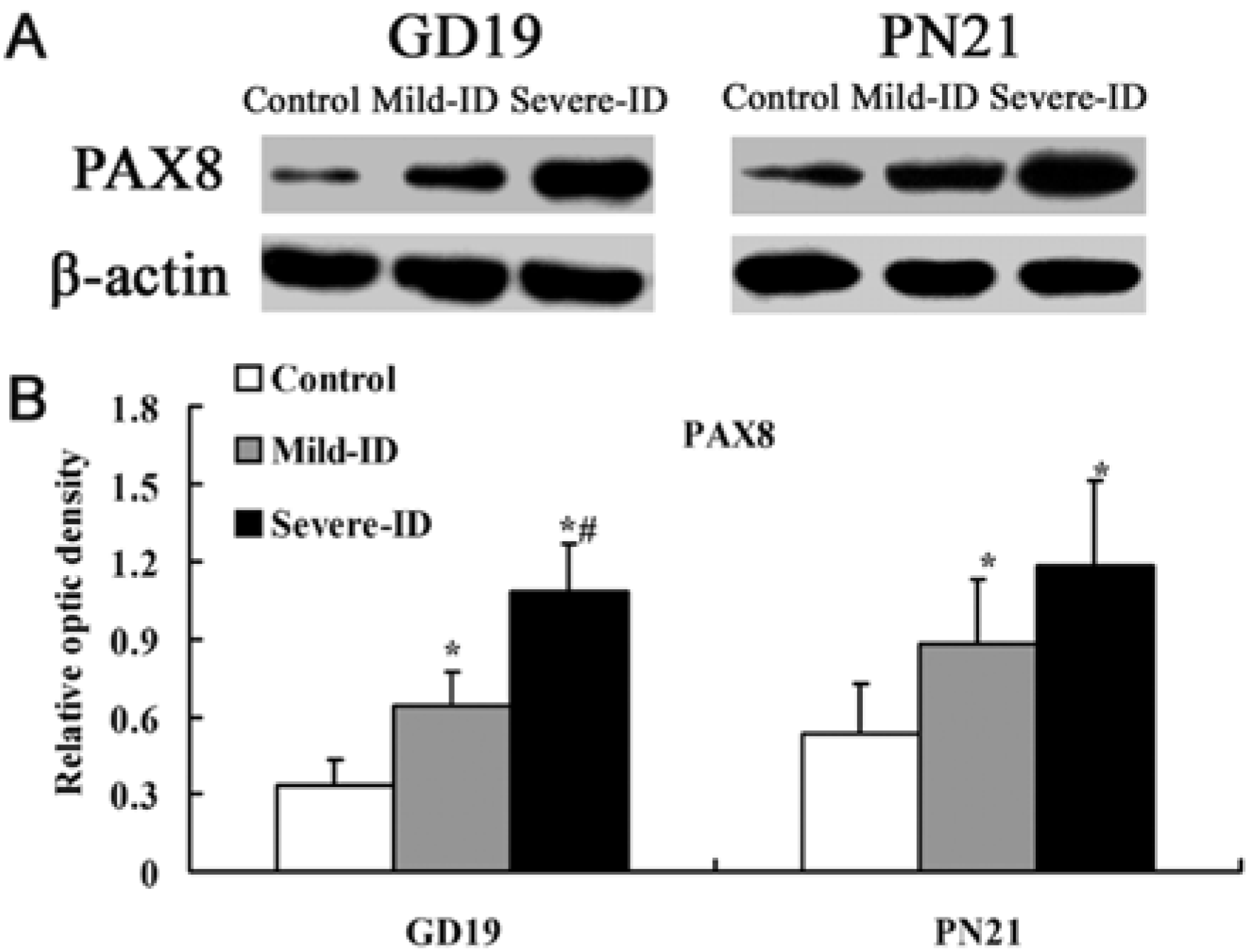
3.3. Up-Regulated Protein Levels of TTF1 and PAX8 in the Thyroid
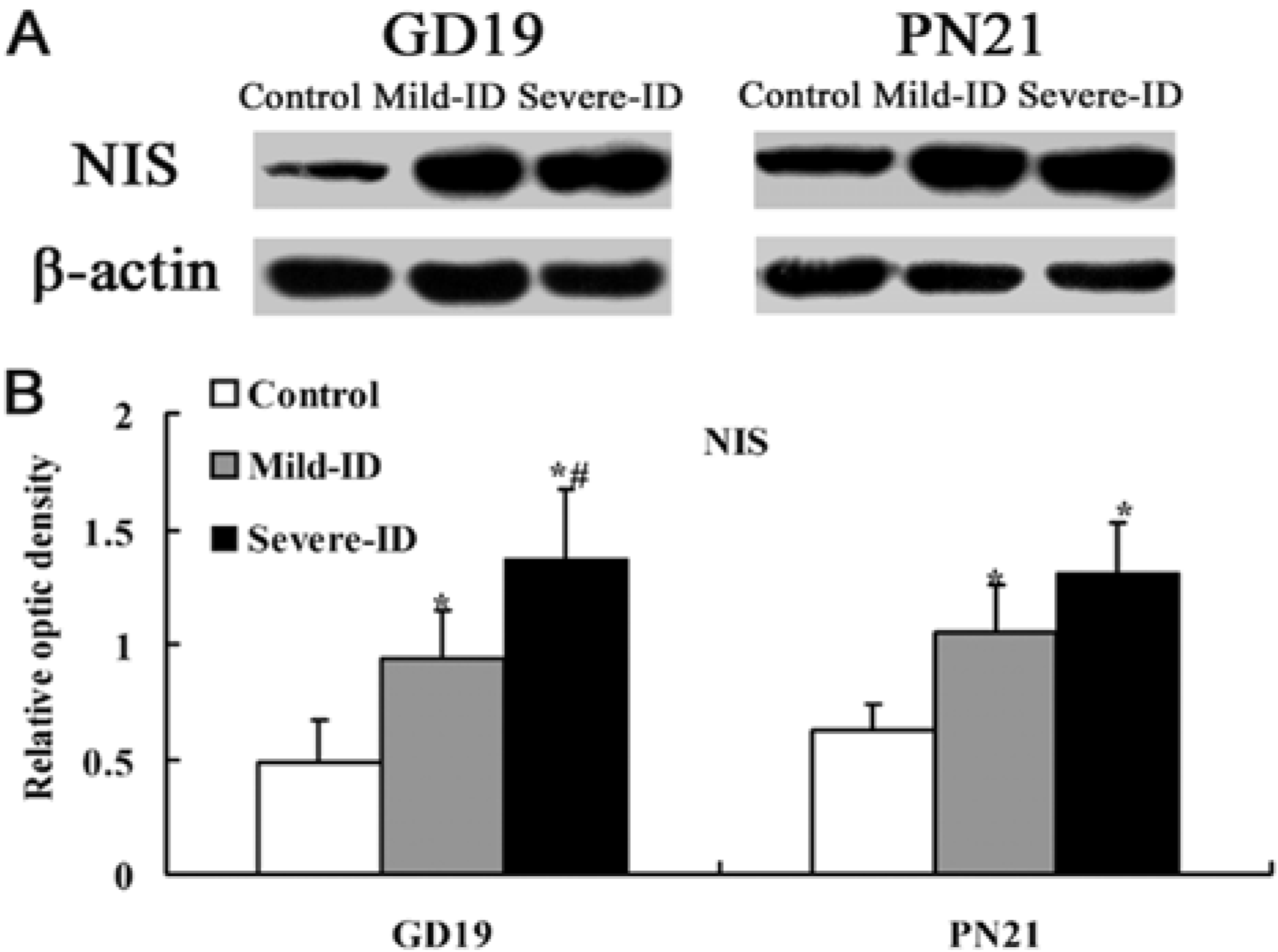
3.4. Up-Regulated Protein Levels of NIS in the Thyroid
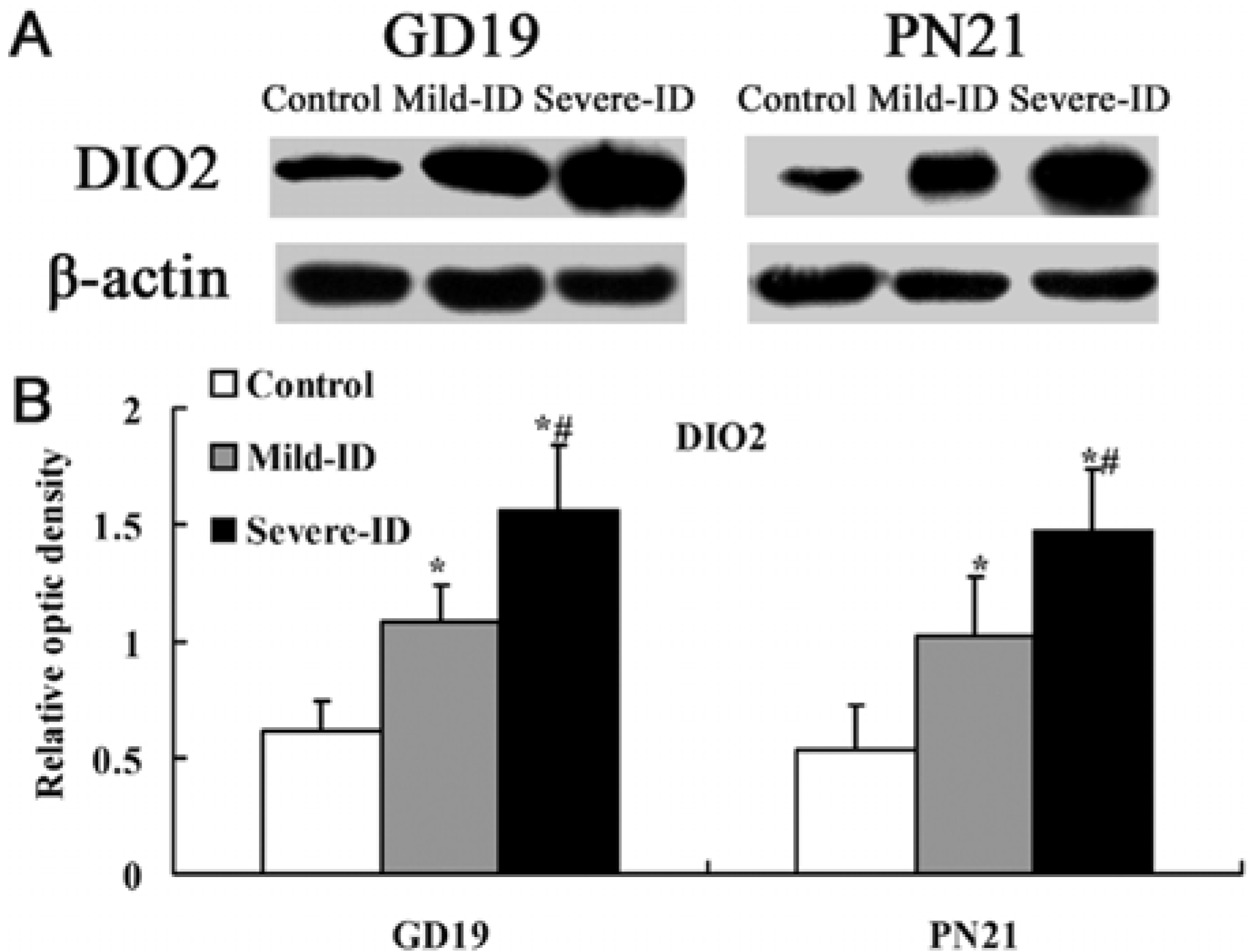
3.5. Up-Regulated Protein Levels of DIO1 and DIO2 in the Thyroid

4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- De La Vieja, A.; Dohan, O.; Levy, O.; Carrasco, N. Molecular analysis of the sodium iodide symporter impact on thyroid and extrathyroid pathophysiology. Physiol. Rev. 2000, 80, 1083–1105. [Google Scholar]
- Delange, F. The disorders induced by iodine deficiency. Thyroid 1994, 4, 107–128. [Google Scholar] [CrossRef]
- Pearce, E.N.; Andersson, M.; Zimmermann, M.B. Global iodine nutrition: Where do we stand in 2013? Thyroid 2013, 23, 523–528. [Google Scholar] [CrossRef]
- Kibirige, M.S.; Hutchison, S.; Owen, C.J.; Delves, H.T. Prevalence of maternal dietary iodine insufficiency in the north east of England: Implications for the fetus. Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, 436–439. [Google Scholar] [CrossRef]
- Stagnaro-Green, A.; Pearce, E. Thyroid disorders in pregnancy. Nat. Rev.Endocrinol. 2012, 18, 650–658. [Google Scholar] [CrossRef]
- Zimmermann, M.B. Iodine deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef]
- Morreale de Escobar, G.; Obregón, M.J.; Escobar del Rey, F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J. Clin. Endocrinol. Metab. 2000, 85, 3975–3987. [Google Scholar] [CrossRef]
- Vermiglio, F.; Lo Presti, V.P.; Moleti, M.; Sidoti, M.; Tortorella, G.; Scaffidi, G.; Castagna, M.G.; Mattina, F.; Violi, M.A.; Crisà, A.; et al. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild–moderate iodine deficiency: A possible novel iodine deficiency disorder in developed countries. J. Clin. Endocrinol. Metab. 2004, 89, 6054–6060. [Google Scholar] [CrossRef]
- Henrichs, J.; Bongers-Schokking, J.J.; Schenk, J.J.; Ghassabian, A.; Schmidt, H.G.; Visser, T.J.; Hooijkaas, H.; de Muinck Keizer-Schrama, S.M.; Hofman, A.; Jaddoe, V.V.; et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: The generation R study. J. Clin. Endocrinol. Metab. 2010, 95, 4227–4234. [Google Scholar] [CrossRef]
- Pop, V.J.; Brouwers, E.P.; Vader, H.L.; Vulsma, T.; van Baar, A.L.; de Vijlder, J.J. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: A 3-year follow-up study. Clin. Endocrinol. 2003, 59, 282–288. [Google Scholar] [CrossRef]
- Berbel, P.; Obregón, M.J.; Bernal, J.; Escobar del Rey, F.; Morreale de Escobar, G. Iodine supplementation during pregnancy: A public health challenge. Trends Endocrinol. Metab. 2007, 18, 338–343. [Google Scholar] [CrossRef]
- Di Palma, T.; Nitsch, R.; Mascia, A.; Nitsch, L.; di Lauro, R.; Zannini, M. The paired domain-containing factor Pax8 and the homeodomain-containing factor TTF-1 directly interact and synergistically activate transcription. J. Biol. Chem. 2003, 278, 3395–3402. [Google Scholar]
- Di Palma, T.; Zampella, E.; Filippone, M.G.; Macchia, P.E.; Ris-Stalpers, C.; de Vroede, M.; Zannini, M. Characterization of a novel loss-of-function mutation of PAX8 associated with congenital hypothyroidism. Clin. Endocrinol. 2010, 73, 808–814. [Google Scholar] [CrossRef]
- Plachov, D.; Chowdhury, K.; Walther, C.; Simon, D.; Guenet, J.L.; Gruss, P. Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development 1990, 110, 643–651. [Google Scholar]
- Lazzaro, D.; Price, M.; de Felice, M.; di Lauro, R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the fetal brain. Development 1991, 113, 1093–1104. [Google Scholar]
- Ohno, M.; Zannini, M.; Levy, O.; Carrasco, N.; di Lauro, R. The paired-domain transcription factor Pax8 binds to the upstream enhancer of the rat sodium/iodide symporter gene and participates in both thyroid-specific and cyclic-AMP dependent transcription. Mol. Cell. Biol. 1990, 19, 2051–2060. [Google Scholar]
- Endo, T.; Kaneshige, M.; Nakazato, M.; Ohmori, M.; Harii, N.; Onaya, T. Thyroid transcription factor-1 activates the promoter activity of rat thyroid Na+/I− symporter gene. Mol. Endocrinol. 1997, 11, 1747–1755. [Google Scholar] [CrossRef]
- Harun-Or-Rashid, M.; Asai, M.; Sun, X.Y.; Hayashi, Y.; Sakamoto, J.; Murata, Y. Effect of thyroid statuses on sodium iodide symporter (NIS) gene expression in the extrathyroidal tissues in mice. Thyroid Res. 2010, 3, 3–8. [Google Scholar] [CrossRef]
- Polenz, J.; Medeiros-Neto, G.; Gross, J.L.; Silveiro, S.P.; Knobel, M.; Refetoff, S. Hypothyroidism in a Brazilian kindred due to iodine trapping defect caused by a homozygous mutation in the sodium/iodide symporter gene. Biochem. Biophys. Res. Commun. 1997, 240, 488–491. [Google Scholar] [CrossRef]
- Uyttersprot, N.; Pelgrims, N.; Carrasco, N.; Gervy, C.; Maenhaut, C.; Dumont, J.E.; Miot, F. Moderate doses of iodide in vivo inhibit cell proliferation and the expression of thyroperoxidase and Na+/I− symporter mRNAs in dog thyroid. Mol. Cell. Endocrinol. 1997, 131, 195–203. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar]
- Assessment of Iodine Deficiency Disorders and Menitoring Their Limenation, 3rd ed.; Geneva, WHO/UNICEF/ICCIDD: Geneva, Switzerland, 2007.
- Wei, W.; Wang, Y.; Wang, Y.; Dong, J.; Min, H.; Song, B.; Teng, W.; Xi, Q.; Chen, J. Developmental hypothyroxinemia induced by maternal mild iodine deficiency delays hippocampal axonal growth in the rat offspring. J. Neuroendocrinol. 2013. [Google Scholar] [CrossRef]
- Ambroziak, M.; Pachucki, J.; Chojnowski, K.; Wiechno, W.; Nauman, J.; Nauman, A. Pax-8 expression correlates with type ii 5′-deiodinase expression in thyroids from patients with Graves’ Disease. Thyroid 2003, 13, 141–148. [Google Scholar] [CrossRef]
- Mansouri, A.; Chowdhury, K.; Gruss, P. Follicular cells of the thyroid gland require Pax8 gene function. Nat. Genet. 1998, 19, 87–90. [Google Scholar] [CrossRef]
- Chen, Z.P. New cretins discovered in southern Xinjiang, China. ICCIDD Newslett. 2007, 23, 18. [Google Scholar]
- Tang, Z.; Liu, W.; Yin, H.; Wang, P.; Dong, J.; Wang, Y.; Chen, J. Investigation of intelligence quotient and psychomotor development in schoolchildren in areas with different degrees of iodine deficiency. Asia Pac. J. Clin. Nutr. 2007, 16, 731–737. [Google Scholar]
- Pedraza, P.E.; Obregon, M.J.; Escobar-Morreale, H.F.; del Rey, F.E.; de Escobar, G.M. Mechanisms of adaptation to iodine deficiency in rats: Thyroid status is tissue specific. Its relevance for man. Endocrinology 2006, 147, 2098–2108. [Google Scholar] [CrossRef]
- Abrams, G.M.; Larsen, P.R. Triiodothyronine and thyroxine in the serum and thyroid glands of iodine deficient rats. J. Clin. Invest. 1973, 52, 2522–2531. [Google Scholar] [CrossRef]
- Riesco, G.; Taurog, A.; Larsen, P.R. Variations in the response of the thyroid gland of the rat to different low-iodine diets: Correlations with iodine content of the diet. Endocrinology 1977, 99, 270–280. [Google Scholar] [CrossRef]
- Damante, G.; Tell, G.; di Lauro, R. A unique combination of transcription factors controls differentiation of thyroid cells. Prog. Nucleic Acid Res. Mol. Biol. 2001, 66, 307–356. [Google Scholar] [CrossRef]
- Rossi, D.L.; Acebrón, A.; Santisteban, P. Function of the homeo and paired domain proteins TTF-1 and Pax-8 in thyroid cell proliferation. J. Biol. Chem. 1995, 270, 23139–23142. [Google Scholar] [CrossRef]
- Fabbro, D.; di Loreto, C.; Beltrami, C.A.; Belfiore, A.; di Lauro, R.; Damante, G. Expression of thyroid-specific transcription factors TTF-1 and PAX-8 in human thyroid neoplasms. Cancer Res. 1994, 54, 4744–4749. [Google Scholar]
- Christophe-Hobertus, C.; Lefort, A.; Libert, F.; Christophe, D. Functional inactivation of thyroid transcription factor-1 in PCCl3 thyroid cells. Mol. Cell. Endocrinol. 2012, 358, 36–45. [Google Scholar] [CrossRef]
- Presta, I.; Arturi, F.; Ferretti, E.; Mattei, T.; Scarpelli, D.; Tosi, E.; Scipioni, A.; Celano, M.; Gulino, A.; Filetti, S.; et al. Recovery of NIS expression in thyroid cancer cells by overexpression of Pax 8 gene. BMC Cancer 2005, 5, 80–90. [Google Scholar] [CrossRef]
- Tonacchera, M.; Banco, M.E.; Montanelli, L.; di Cosmo, C.; Agretti, P.; de Marco, G.; Ferrarini, E.; Ordookhani, A.; Perri, A.; Chiovato, L.; et al. Genetic analysis of the PAX8 gene in children with congenital hypothyroidism and dysgenetic or eutopic thyroid glands identification of a novel sequence variant. Clin. Endocrinol. 2007, 67, 34–40. [Google Scholar] [CrossRef]
- Moeller, L.C.; Kimura, S.; Kusakabe, T.; Liao, X.H.; van Sande, J.; Refetoff, S. Hypothyroidism in thyroid transcription factor 1 haploinsufficiency is caused by reduced expression of the thyroid-stimulating hormone receptor. Mol. Endocrinol. 2003, 17, 2295–2302. [Google Scholar] [CrossRef]
- Jo, W.; Ishizu, K.; Fujieda, K.; Tajima, T. Congenital hypothyroidism caused by a PAX8 gene mutation manifested as sodium iodide symporter gene defect. J. Thyroid Res. 2010, 2010. [Google Scholar] [CrossRef]
- Schmitt, T.L.; Espinoza, C.R.; Loos, U. Transcriptional regulation of the human sodium/iodide symporter gene by Pax8 and TTF-1. Exp. Clin. Endocrinol. Diabetes 2001, 109, 27–31. [Google Scholar] [CrossRef]
- Taki, K.; Kogai, T.; Kanamoto, Y.; Hershman, J.M.; Brent, G.A. A thyroid-specific far-upstream enhancer in the human sodium/iodide symporter gene requires Pax-8 binding and cyclic adenosine 3′,5′-monophosphate response element-like sequence binding proteins for full activity and is differentially regulated in normal and thyroid cancer cells. Mol. Endocrinol. 2002, 16, 2266–2282. [Google Scholar] [CrossRef]
- Saito, T.; Endo, T.; Kawaguchi, A.; Ikeda, M.; Nakazato, M.; Kogai, T.; Onaya, T. Increased expression of the Na+/I− symporter in cultured human thyroid cells exposed to thyrotropin and in Graves’ thyroid tissue. J. Clin. Endocrinol. Metab. 1997, 82, 3331–3336. [Google Scholar] [CrossRef]
- Pasca di Magliano, M.; di Lauro, R.; Tannini, M. Pax8 has a key role in thyroid cell differentiation. Proc. Natl. Acad. Sci. USA 2000, 97, 13144–13149. [Google Scholar] [CrossRef]
- Mu, D.; Huang, R.; Ma, X.; Li, S.; Kuang, A. Radio iodine therapy of thyroid carcinoma following Pax-8 gene transfer. Gene Ther. 2012, 19, 435–442. [Google Scholar]
- Levy, O.; Dai, G.; Riedel, C.; Ginter, C.S.; Paul, E.M.; Lebowitz, A.N.; Carrasco, N. Characterization of the thyroid Na+/I− symporter with an anti-COOH terminus antibody. Proc. Natl. Acad. Sci. USA 1997, 94, 5568–5573. [Google Scholar]
- Van der Elst, J.P.; van der Heide, D.; Kastelijn, J.; Rousset, B.; Obregón, M.J. The expression of the sodiumiodide symporter is up-regulated in the thyroid of fetuses of iodine-deficient rats. Endocrinology 2001, 142, 3736–3741. [Google Scholar] [CrossRef]
- Gereben, B.; Zavacki, A.M.; Ribich, S.; Kim, B.W.; Huang, S.A.; Simonides, W.S.; Zeöld, A.; Bianco, A.C. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr. Rev. 2008, 29, 898–938. [Google Scholar] [CrossRef]
- Lavado-Autric, R.; Calvo, R.M.; de Mena, R.M.; de Escobar, G.M.; Obregon, M.J. Deiodinase activities in thyroids and tissues of iodine-deficient female rats. Endocrinology 2013, 154, 529–536. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wei, W.; Wang, Y.; Dong, J.; Wang, Y.; Min, H.; Song, B.; Shan, Z.; Teng, W.; Xi, Q.; Chen, J. Hypothyroxinemia Induced by Mild Iodine Deficiency Deregulats Thyroid Proteins during Gestation and Lactation in Dams. Int. J. Environ. Res. Public Health 2013, 10, 3233-3245. https://doi.org/10.3390/ijerph10083233
Wei W, Wang Y, Dong J, Wang Y, Min H, Song B, Shan Z, Teng W, Xi Q, Chen J. Hypothyroxinemia Induced by Mild Iodine Deficiency Deregulats Thyroid Proteins during Gestation and Lactation in Dams. International Journal of Environmental Research and Public Health. 2013; 10(8):3233-3245. https://doi.org/10.3390/ijerph10083233
Chicago/Turabian StyleWei, Wei, Yi Wang, Jing Dong, Yuan Wang, Hui Min, Binbin Song, Zhongyan Shan, Weiping Teng, Qi Xi, and Jie Chen. 2013. "Hypothyroxinemia Induced by Mild Iodine Deficiency Deregulats Thyroid Proteins during Gestation and Lactation in Dams" International Journal of Environmental Research and Public Health 10, no. 8: 3233-3245. https://doi.org/10.3390/ijerph10083233



