Prevalence of Mycobacterium avium in Slaughter Pigs Based on Serological Monitoring Results and Bacteriological Validation
Abstract
:1. Introduction
2. Experimental Section
2.1. Collection of Samples
2.2. Applied ELISA and Herd Categorization
2.3. MA-ELISA Validation
2.3.1. Evaluation of Sensitivity under Field Conditions
2.3.2. Evaluation of Specificity under Field Conditions
2.3.3. Herd Sensitivity Calculations

2.3.4. Herd Specificity Calculations
2.3.5. Statistics
3. Results and Discussion
3.1. Results
3.1.1. Serological MA-Monitoring in the German and Dutch Slaughterhouse(s) and Herd Risk Categorization
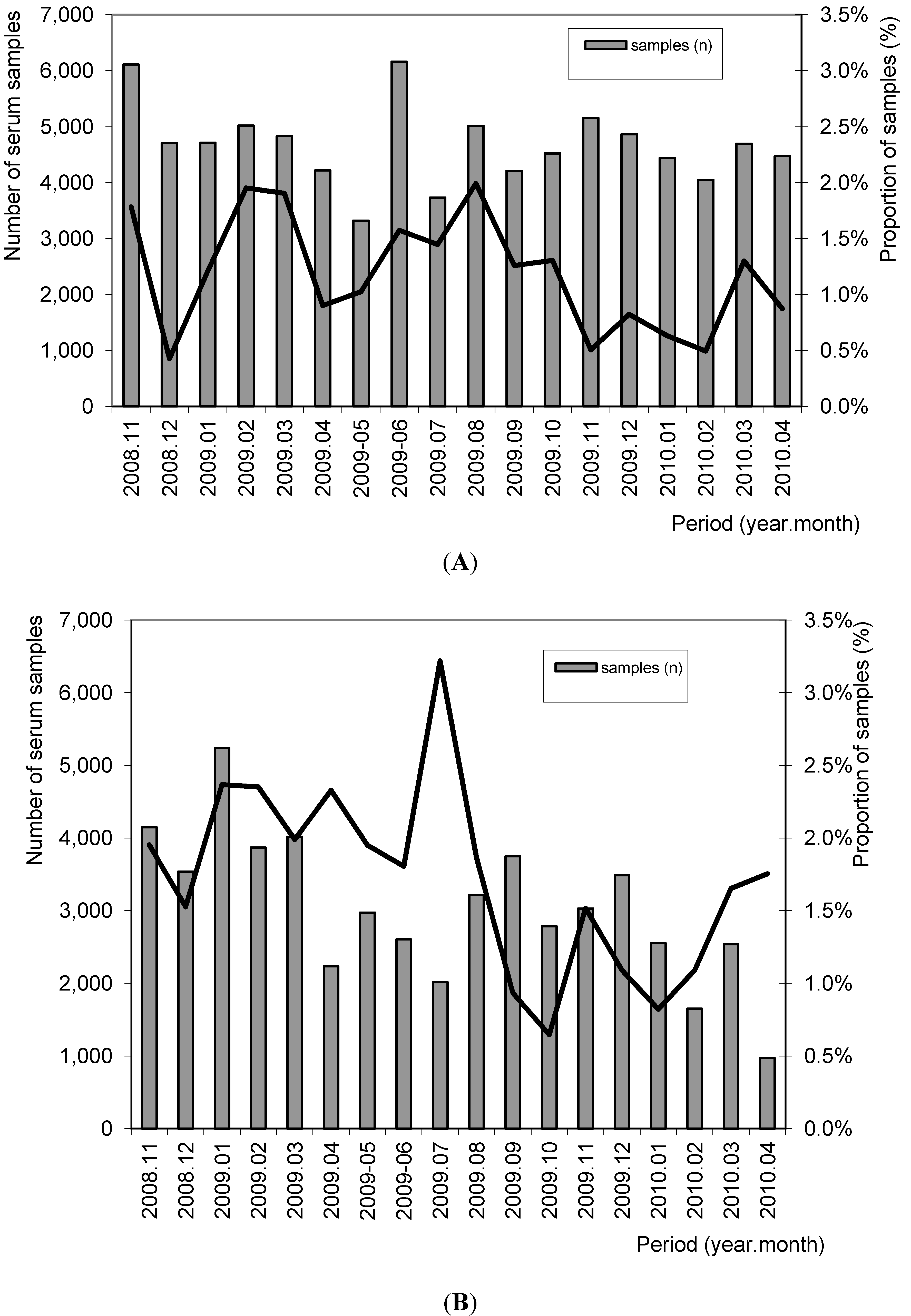
3.1.2. Evaluation of Sensitivity under Field Conditions
| Farm (Country) | Bacteriology 1 +ve/n (%) | Pathology 2 | Serology (PP > 20) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| +ve/n (%) | Se% | Sp% | +ve/n (%) | Se% (carcass) | Sp% | PPV% | NPV% | ||
| A 3 (NL) | 103/184 (56) | 57/184 (31) | 35.0 | 74.1 | 8/184 (4.3) | 4.9 | 96.3 | 62.5 | 44.3 |
| B3 (B) | 41/117 (35) | 9/117 (8) | 19.5 | 98.7 | 4/117 (3.4) | 2.4 | 96.1 | 25.0 | 64.6 |
| D 4 (GE) | 38/55 (66) | 16/58 (28) | 40.5 | 94.4 | 3/55 (5.5) | 2.7 | 88.9 | 33.3 | 30.7 |
| E 4(GE) | 6/19 (32) | 3/19 (16) | 66.7 | 92.3 | 2/19 (11) | 16.7 | 92.3 | 50.0 | 70.1 |
| TOTAL (%) | 188/375 (50) | 85/378 (23) | 32.6 | 87.2 | 17/375 (4.5) | 4.3 | 95.2 | 49.4 | 50.0 |
3.1.3. Evaluation of the Carcass Specificity of the MA-ELISA under Field Conditions
| Pig farm | Number of pigs sampled | Serology | Lnn. mandibulares | Lnn. mesenteriales | ||||
|---|---|---|---|---|---|---|---|---|
| Pathology 1 | Bacteriology 2 | Pathology | Bacteriology | |||||
| PP > 20 | −ve | +ve | +ve | −ve | +ve | +ve | ||
| 116 | 3 | 0 | 3 | 0 | 0 | 3 | 0 | 0 |
| 724 | 10 | 0 | 10 | 0 | 0 | 10 | 0 | 0 |
| 736 | 10 | 0 | 10 | 0 | 0 | 8 | 2 | 0 |
| 875 | 33 | 0 | 31 | 2 | 0 | 33 | 0 | 0 |
| 907 | 41 | 0 | 38 | 3 | 0 | 39 | 2 | 0 |
| 826 | 39 | 0 | 36 | 1 | 0 | 31 | 0 | 0 |
| 014 | 33 | 0 | 32 | 0 | 0 | 24 | 2 | 0 |
| 088 | 71 | 0 | 70 | 1 | 0 | 45 | 0 | 0 |
| Total (%) | 239 | 0 | 230 | 7 (3.0%) | 0 | 193 | 6 (3.1%) | 0 |
| Sp | 100% | 97% | 96.9% | |||||
3.1.4. Herd Sensitivity Calculations
3.1.5. Herd Specificity Calculations
| Within-herd bacteriological prevalence of M. avium | MA-ELISA carcass sensitivity | ||||
|---|---|---|---|---|---|
| 2.4 | 5% | 10% | 16.7% | 20% | |
| probability ≥ 1 out of 36 positive blood serum samples | |||||
| 30% | 23% | 42% | 67% | 84% | 89% |
| 40% | 29% | 52% | 77% | 92% | 95% |
| 50% | 35% | 60% | 84% | 96% | 98% |
| 60% | 41% | 67% | 89% | 98% | 99% |
| 70% | 46% | 73% | 93% | 99% | 100% |
| probability ≥ 2 out of 36 positive blood serum samples | |||||
| 30% | 3% | 10% | 29% | 54% | 64% |
| 40% | 5% | 16% | 42% | 70% | 79% |
| 50% | 7% | 23% | 54% | 81% | 89% |
| 60% | 9% | 29% | 64% | 89% | 94% |
| 70% | 12% | 36% | 73% | 93% | 97% |
3.2. Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ashford, D.A.; Whitney, E.; Raghunathan, P.; Cosivi, O. Epidemiology of selected mycobacteria that infect humans and other animals. Rev. Sci. Tech. 2001, 20, 325–337. [Google Scholar]
- Falkinham, J.O. Epidemiology of infection by nontuberculous mycobacteria. Clin. Microbiol. Rev. 1996, 9, 177–215. [Google Scholar]
- Bermudez, L.E.; Petrofsky, M.; Kolonosi, P. An animal model of Mycobacterium avium complex disseminated infection after colonization of the intestinal tract. J. Infect. Dis. 1992, 165, 75–79. [Google Scholar] [CrossRef]
- Eriksson, M.; Bennet, R.; Danielsson, N. Non-tuberculous mycobacterial lymphadenitis in healthy children: Another “lifestyle disease”? Acta Paedriatica 2001, 90, 1340–1342. [Google Scholar] [CrossRef]
- Nylen, O.; Berg-Kelly, K.; Andersson, B. Cervical lymph node infections with non-tuberculous mycobacteria in preschool children: Interferon gamma deficiency as a possible cause of clinical infection. Acta Paedriatica 2000, 89, 1322–1325. [Google Scholar] [CrossRef]
- Komijn, R.E.; de Haas, P.E.; Schneider, M.M.; Eger, T.; Nieuwenhuijs, J.H.; van den Hoek, R.J.; Bakker, D.; van Zijderveld, F.G.; van Soolingen, D. Prevalence of Mycobacterium avium in slaughter pigs in The Netherlands and comparison of IS1245 restriction fragment length polymorphism patterns of porcine and human isolates. J. Clin. Microbiol. 1999, 37, 1254–1259. [Google Scholar]
- Martin, G.; Schimmel, D. Die Mycobacterium avium—Infektion des Geflügels-(k)eine Gefahr für die menschliche Gesundheit? (Article in German). Deutsche Tierärztliche Wochenschrift 2000, 107, 53–58. [Google Scholar]
- Möbius, P.; Lentzsch, P.; Moser, J.; Naumann, L.; Martin, G.; Kohler, H. Comparative macrorestriction and RFLP analysis of Mycobacterium avium subsp. avium and Mycobacterium avium subsp. hominissuis isolates from man, pig, and cattle. Vet. Microbiol. 2006, 117, 284–291. [Google Scholar] [CrossRef]
- Thoen, C.O. Tuberculosis. In Diseases of Swine, 9th ed.; Straw B.E. Zimmermann, J.J., D’Allaire, S., Taylor, D.J., Eds.; Blackwell Publishing: Iowa, IA, USA, 2006; pp. 807–822. [Google Scholar]
- Matlova, L.; Dvorska, L.; Ayele, W.Y.; Bartos, M.; Amemori, T.; Pavlik, J. Distribution of Mycobacterium avium complex isolates in tissue samples of pigs fed peat naturally contaminated with mycobacteria as a supplement. J. Clin. Microbiol. 2005, 43, 1261–1268. [Google Scholar] [CrossRef]
- Trckova, M.; Hudcova, H.; Faldyna, M.; Zraly, Z.; Dvorska, L.; Beran, V.; Pavlik, J. Peat as a feed supplement for animals: A review. Vet. Med. 2005, 50, 361–377. [Google Scholar]
- Komijn, R.E.; Wisselink, H.J.; Rijsman, V.M.; Stockhofe-Zurwieden, N.; Bakker, D.; van Zijderveld, F.G.; Eger, T.; Wagenaar, J.A.; Putirulan, F.F.; Urlings, B.A. Granulomatous lesions in lymph nodes of slaughter pigs bacteriologically negative for Mycobacterium avium subsp. avium and positive for Rhodococcus equi. Vet. Microbiol. 2007, 120, 352–357. [Google Scholar] [CrossRef]
- Wisselink, H.J.; van Solt-Smits, C.B.; Oorburg, D.; van Soolingen, D.; Overduin, P.; Maneschijn-Bonsing, J.; Stockhofe-Zurwieden, N.; Buys-Bergen, H.; Engel, B.; Urlings, B.A.; et al. Serodiagnosis of Mycobacterium avium infections in pigs. Vet. Microbiol. 2010, 142, 401–407. [Google Scholar] [CrossRef]
- Hamilton, D.R.; Lyall, L.; Lester, S.; McOrist, S.; Hathaway, S.C.; Pointon, A.M. Risk-based evaluation of post-mortem inspection procedures for pigs in Australia. Vet. Record 2002, 151, 110–116. [Google Scholar] [CrossRef]
- SCVMPH. Opinion of the Scientific Committee on Veterinary Measures Relating to Public Health on Revision of Meat Inspection Procedures. 2000. Available online: http://ec.europa.eu/food/fs/sc/scv/out30_en.pdf (accessed on 24 February 2000).
- Hiller, A.; Heres, L.; Schulze Althoff, G.; Urlings, B.; Klein, G. Implementierung einer risikoorientierten Fleischuntersuchung ohne Anschnitte beim Mastschwein in einem norddeutschen Schlachtunternehmen. Archiv. Für. Lebensmittelhygiene 2012, 63, 107–114. [Google Scholar]
- Hiller, A.; Wisselink, H.J.; van Solt-Smits, C.B.; Oorburg, D.; Schulze Althoff, G.; Klein, G. Confirmation of the Presence of Mycobacterium avium Infections in two Pig Herds which had a High Risk Profile for M. avium as Assessed by Serologically Monitoring. In Proceedings of the 21st International Pig Veterinary Society Congress, Vancouver, Canada, 18–21 July 2010; D’Allaire, S., Friendship, R., Eds.; IPVS 2010 Congress Secretariat: Vancouver, Canada, 2010; pp. 674, 980. [Google Scholar]
- Offermann, U.; Bodmer, T.; Audigé, L.; Jemmi, T. The prevalence of salmonella, yersinia and mycobacteria in slaughtered pigs in Switzerland. Schweiz. Archiv. Für. Tierheilkunde 1999, 141, 509–515. [Google Scholar]
- Van Ingen, J.; Wisselink, H.J.; van Solt-Smits, C.B.; Boeree, M.J.; van Soolingen, D. Isolation of mycobacteria other than Mycobacterium avium from porcine lymph nodes. Vet. Microbiol. 2010, 144, 250–253. [Google Scholar] [CrossRef]
- Dvorska, L.; Parmova, M.; Lavickova, M.; Bartl, J.; Vrbas, V.; Pavlik, J. Isolation of Rhodococcus equi and atypical mycobacteria from lymph nodes of pigs and cattle in herds with the occurrence of tuberculoid gross changes in the Czech Republic over the period 1996–1998. Vet. Med. 1999, 44, 321–330. [Google Scholar]
- Pate, M.; Zdovc, I.; Pirs, T.; Krt, B.; Ocepek, M. Isolation and characterisation of Mycobacterium avium and Rhodococcus equi from granulomatous lesions of swine lymph nodes in Slovenia. Acta Vet. Ungaricae 2004, 52, 143–150. [Google Scholar]
- Brown, J.; Neuman, M.A. Lesions of swine lymph nodes as a diagnostic test to determine mycobacterial infection. Appl. Environ. Microbiol. 1979, 37, 740–743. [Google Scholar]
- Fischer, S. Epidemiologische Untersuchungen zur Bedeutung von Mykobakterieninfektionen bei Schlachtschweinen unter besonderer Berücksichtigung des Mycobacterium avium-intracellulare-Komplexes; (Dissertation in German); Universität Leipzig: Leipzig, Deutschland, 1999. [Google Scholar]
- Meyer, S.; Großpietsch, R.; Oetjen, M.; Borgmann-Fuchs, D.; Fries, R. Prävalenz und Prädilektionsstellen von Mykobakterien des MAIC beim Schlachtschwein. (Article in German). 7 Fachtagung Fleisch- und Geflügelfleischhygiene für Angehörige der Veterinärverwaltung Berlin; Eigenverlag: Berlin, Germany, 2007; pp. 65–73. Available online: http://edocs.fu-berlin.de/docs/servlets/MCRFileNodeServlet/FUDOCS_derivate_000000001061/Uni-Bibliographie_27 2007.pdf?hosts= (accessed on 27 August 2013).
- Lücker, E.; Thorius-Ehler, S.; Zschöck, M.; Bülte, M. Zur Frage der Fleischhygienerechtlichen Beurteilung Tuberkulöser Veränderungen. (Article in German). In Proceedings of the 38 Arbeitstagung des Arbeitsgebietes Lebensmittelhygiene“ der Deutschen Veterinärmedizinischen Gesellschaft e.V., Garmisch-Partenkirchen, Germany, 1997; pp. 502–508.
- Dvorska, L.; Matlova, L.; Bartos, M.; Parmova, D.; Bartl, J.; Svastova, P.; Bull, T.J.; Pavlik, I. Study of Mycobacterium avium complex strains isolated from cattle in the Czech Republic between 1996 and 2000. Vet. Microbiol. 2004, 99, 239–250. [Google Scholar] [CrossRef]
- Peel, B.; Simmons, G.C. Factors in the spead of salmonella in meat works with special reference to contamination of knives. Aust. Vet. J. 1978, 54, 106–110. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hiller, A.; Oorburg, D.; Wisselink, H.J.; Solt-Smits, C.B.v.; Urlings, B.; Klein, G.; Althoff, G.S.; Heres, L. Prevalence of Mycobacterium avium in Slaughter Pigs Based on Serological Monitoring Results and Bacteriological Validation. Int. J. Environ. Res. Public Health 2013, 10, 4027-4038. https://doi.org/10.3390/ijerph10094027
Hiller A, Oorburg D, Wisselink HJ, Solt-Smits CBv, Urlings B, Klein G, Althoff GS, Heres L. Prevalence of Mycobacterium avium in Slaughter Pigs Based on Serological Monitoring Results and Bacteriological Validation. International Journal of Environmental Research and Public Health. 2013; 10(9):4027-4038. https://doi.org/10.3390/ijerph10094027
Chicago/Turabian StyleHiller, Anne, Derk Oorburg, Henk J. Wisselink, Conny B. van Solt-Smits, Bert Urlings, Günter Klein, Gereon Schulze Althoff, and Lourens Heres. 2013. "Prevalence of Mycobacterium avium in Slaughter Pigs Based on Serological Monitoring Results and Bacteriological Validation" International Journal of Environmental Research and Public Health 10, no. 9: 4027-4038. https://doi.org/10.3390/ijerph10094027






