Effectiveness of a Multi-Component Smoking Cessation Support Programme (McSCSP) for Patients with Severe Mental Disorders: Study Design
Abstract
:1. Introduction
2. Methods
2.1. Study Design
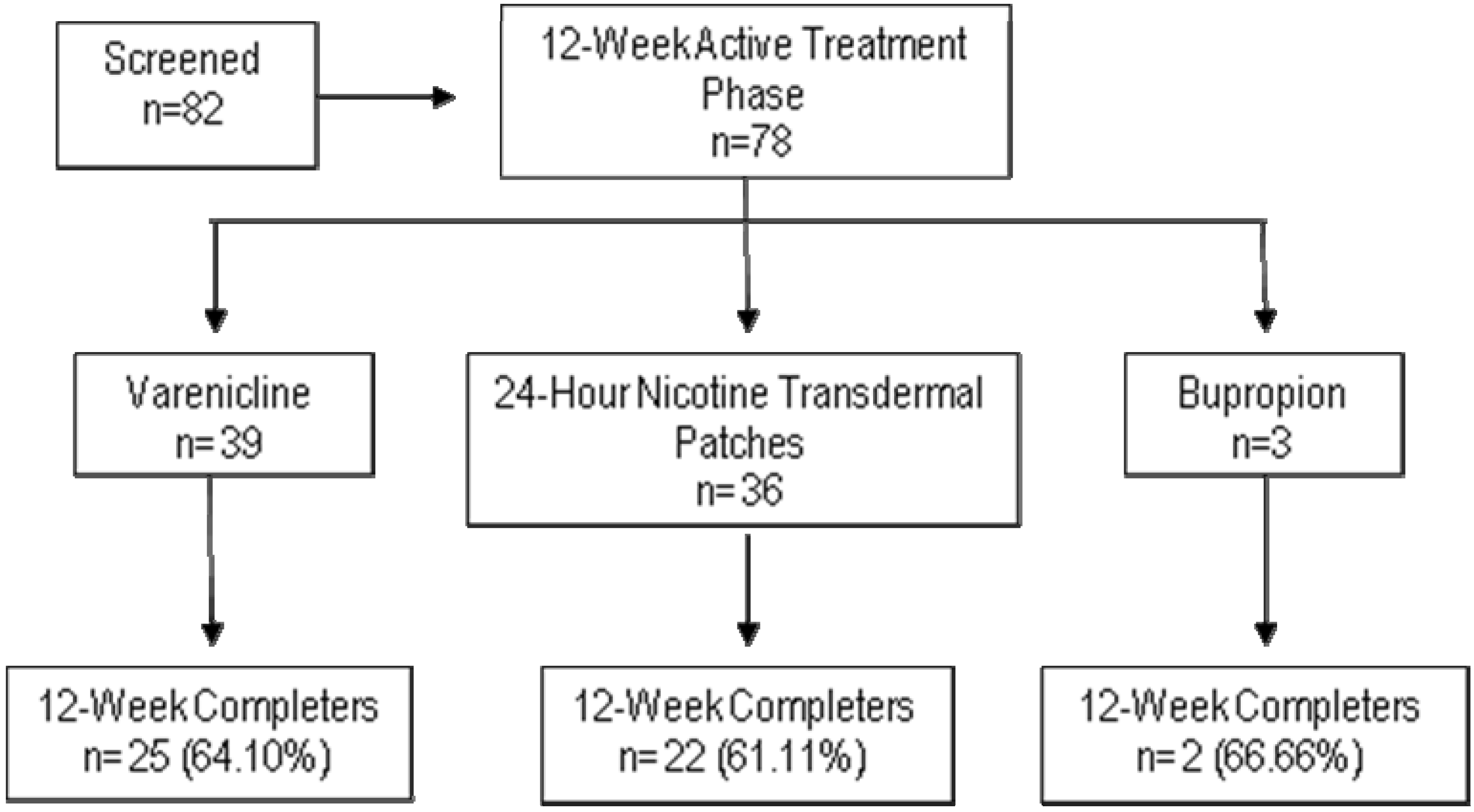
2.2. Subjects
2.3. Diagnostic Interview
2.4. Demographic and Clinical Data
2.5. Clinical Evaluations
| Area of Assessment | Psychometric Instruments/ Biological Parameters | Inclusion Criteria | Exclusion Criteria | |
|---|---|---|---|---|
| Smoking history | Pattern of tobacco use |
|
| |
| Nicotine dependence |
|
| ||
| Motivation to quit |
| |||
| Substance use | Caffeine |
| ||
| Other |
| |||
| Psychopathology | Schizophrenia or schizoaffective patients |
|
| |
| Patients with bipolar disorder |
|
| ||
| Both types of patients |
|
|
| |
| Adverse events |
| |||
| Biological evaluation | Anthropometrics |
| ||
| Vital signs |
| |||
| Laboratory tests |
|
| ||
2.5.1. Smoking History
2.5.2. Substance Use
2.5.3. Psychopathology
2.5.4. Adverse Events
2.5.5. Anthropometrics, Vital Signs, and Laboratory Tests
2.6. Assessments
| Study Phase | Motivation | Active Treatment | Follow-up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit number | MV1 | V0 | V1 | V2 | V3 | V4 | V5 | V6 | V7 | V8 | FU- V1 | FU- V2 |
| Week | w-12 to w-4 | w0 | w1 | w2 | w3 | w4 | w6 | w8 | w10 | w12 | w24 | w36 |
| Inclusion/Exclusion criteria | X | |||||||||||
| Informed consent | X | |||||||||||
| Demographic data | X | |||||||||||
| Clinical data | X | |||||||||||
| SCID-I: Schizophrenia, Schizoaffective and Bipolar sections | X | |||||||||||
| Smoking history | ||||||||||||
| Pattern of tobacco use | X | |||||||||||
| Cigarettes per day (CPD) | X | X | X | X | X | X | X | X | X | X | X | |
| Breath CO level | X | X | X | X | X | X | X | X | X | X | X | X |
| Fagerström Test for Nicotine Dependence (FTND) | X | X | X | X | X | X | X | X | X | X | X | X |
| X | X | X | X | X | X | X | X | X | X | X | X | |
| Glover-Nilsson Smoking Behavioral Questionnaire | X | X | ||||||||||
| X | X | |||||||||||
| Richmond test | ||||||||||||
| University of Rhode Island Change Assessment (URICA) scale | ||||||||||||
| Substance use | ||||||||||||
| Daily caffeine consumption | X | X | X | X | X | X | X | X | X | X | X | X |
| Drug Use, Table from the Addiction Severity Index 6th version (ASI6) | X | X | X | X | X | X | X | X | X | X | X | X |
| Psychopathology | ||||||||||||
| Positive and Negative Syndrome Scale (PANSS) | X | X | X | X | X | X | X | X | X | X | X | X |
| X | X | X | X | X | X | X | X | X | X | X | X | |
| Hamilton Depression Rating Scale (HDRS) | X | X | X | X | X | X | X | X | X | X | X | X |
| X | X | X | X | X | X | X | X | X | X | X | X | |
| Young Mania Rating Scale (YMRS) | X | X | X | X | X | X | X | X | X | X | X | X |
| Columbia Suicide Severity Rating Scale (CSSRS) | X | X | X | X | X | X | X | X | X | X | X | X |
| X | X | X | X | X | X | X | X | X | X | |||
| Clinical Global Impression (CGI) | ||||||||||||
| Severity | ||||||||||||
| Change | ||||||||||||
| Adverse events | ||||||||||||
| UKU Side Effects Rating Scale (UKU) | X | X | X | X | X | X | X | X | X | X | X | X |
| Biological evaluation | ||||||||||||
| Anthropometrics and vital signs | X | X | X | X | X | X | X | X | X | X | X | X |
| Laboratory tests | X | X | X | X | X | |||||||
2.7. Statistical Plan
2.8. Treatment
2.8.1. Pharmacological Treatment
2.8.2. Psychological Interventions
3. Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Shafey, O.; Eriksen, M.; Ross, H.; Mackay, J. The Tobacco Atlas, 3rd ed.; American Cancer Society: Atlanta, GA, USA, 2010. [Google Scholar]
- Lancet, E. Smoke alarm: Mental illness and tobacco. Lancet 2013, 381, 1071. [Google Scholar] [CrossRef]
- Lising-Enriquez, K.; George, T.P. Treatment of comorbid tobacco use in people with serious mental illness. J. Psychiatr. Neurosci. 2009, 34, 1–2. [Google Scholar]
- Tidey, J.W.; Rohsenow, D.J.; Kaplan, G.B.; Swift, R.M.; Ahnallen, C.G. Separate and combined effects of very low nicotine cigarettes and nicotine replacement in smokers with schizophrenia and controls. Nicotine Tob. Res. 2013, 15, 121–129. [Google Scholar] [CrossRef]
- Dickerson, F.; Stallings, C.R.; Origoni, A.E.; Vaughan, C.; Khushalani, S.; Schroeder, J.; Yolken, R.H. Cigarette smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999–2011. Psychiatr. Services 2013, 64, 44–50. [Google Scholar] [CrossRef]
- Bobes, J.; Arango, C.; Garcia-Garcia, M.; Rejas, J. Healthy lifestyle habits and 10-year cardiovascular risk in schizophrenia spectrum disorders: An analysis of the impact of smoking tobacco in the clamors schizophrenia cohort. Schizophr. Res. 2010, 119, 101–109. [Google Scholar] [CrossRef]
- Garcia-Portilla, M.P.; Saiz, P.A.; Benabarre, A.; Florez, G.; Bascaran, M.T.; Diaz, E.M.; Bousono, M.; Bobes, J. Impact of substance use on the physical health of patients with bipolar disorder. Acta Psychiatr. Scand. 2010, 121, 437–445. [Google Scholar]
- Gonzalez-Pinto, A.; Gutierrez, M.; Ezcurra, J.; Aizpuru, F.; Mosquera, F.; Lopez, P.; de Leon, J. Tobacco smoking and bipolar disorder. J. Clin. Psychiat. 1998, 59, 225–228. [Google Scholar] [CrossRef]
- Encuesta Nacional de Salud (National Health Survey); Ministerio de Sanidad: Madrid, Spain, 2006.
- Ziedonis, D.; Hitsman, B.; Beckham, J.C.; Zvolensky, M.; Adler, L.E.; Audrain-McGovern, J.; Breslau, N.; Brown, R.A.; George, T.P.; Williams, J.; et al. Tobacco use and cessation in psychiatric disorders: National institute of mental health report. Nicotine Tob. Res. 2008, 10, 1691–1715. [Google Scholar] [CrossRef]
- Ripoll, R.M. Lifestyle Medicine: The importance of considering all the causes of disease. Rev. Psiquiat. Salud Ment. 2012, 5, 48–52. [Google Scholar] [CrossRef]
- Colton, C.W.; Manderscheid, R.W. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev. Chron. Dis. 2006, 3. Available online: http://www.cdc.gov/pcd/issues/2006/apr/05_0180.htm (accessed on 23 December 2013).
- Ruiz, J.S.; Garcia, J.B.; Ruiloba, J.V.; Ubago, J.G.; Gonzalez, M.P.J.-P. Consensus on physical health of patients with schizophrenia from the spanish societies of psychiatry and biological psychiatry. Acta. Esp. Psiquiat. 2008, 36, 251–264. [Google Scholar]
- Bobes, J.; Ruiz, J.S.; Montes, J.M.; Mostaza, J.; Rico-Villademoros, F.; Vieta, E. Spanish consensus on physical health of patients with bipolar disorder. Rev. Psiquiatr. Salud Ment. 2008, 1, 26–37. [Google Scholar] [CrossRef]
- Chapman, S.; Ragg, M.; McGeechan, K. Citation bias in reported smoking prevalence in people with schizophrenia. Aust. N. Z. J. Psychiat. 2009, 43, 277–282. [Google Scholar] [CrossRef]
- De Leon, J.; Diaz, F.J. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr. Res. 2005, 76, 135–157. [Google Scholar] [CrossRef]
- Goff, D.C.; Cather, C.; Evins, A.E.; Henderson, D.C.; Freudenreich, O.; Copeland, P.M.; Bierer, M.; Duckworth, K.; Sacks, F.M. Medical morbidity and mortality in schizophrenia: Guidelines for psychiatrists. J. Clin. Psychiat. 2005, 66, 183–194. [Google Scholar] [CrossRef]
- Aguilar, M.C.; Gurpegui, M.; Diaz, F.J.; de Leon, J. Nicotine dependence and symptoms in schizophrenia: Naturalistic study of complex interactions. Br. J. Psychiat. 2005, 186, 215–221. [Google Scholar] [CrossRef]
- de Leon, J.; Diaz, F.J.; Aguilar, M.C.; Jurado, D.; Gurpegui, M. Does smoking reduce akathisia? Testing a narrow version of the self-medication hypothesis. Schizophr. Res. 2006, 86, 256–268. [Google Scholar] [CrossRef]
- Ziedonis, D.M.; Kosten, T.R.; Glazer, W.M.; Frances, R.J. Nicotine dependence and schizophrenia. Hosp. Community Psychiat. 1994, 45, 204–206. [Google Scholar]
- Ostacher, M.J.; Nierenberg, A.A.; Perlis, R.H.; Eidelman, P.; Borrelli, D.J.; Tran, T.B.; Ericson, G.M.; Weiss, R.D.; Sachs, G.S. The relationship between smoking and suicidal behavior, comorbidity, and course of illness in bipolar disorder. J. Clin. Psychiat. 2006, 67, 1907–1911. [Google Scholar] [CrossRef]
- Vanable, P.A.; Carey, M.P.; Carey, K.B.; Maisto, S.A. Smoking among psychiatric outpatients: Relationship to substance use, diagnosis, and illness severity. Psychol. Addict. Behav. 2003, 17, 259–265. [Google Scholar] [CrossRef]
- Goldstein, B.I.; Birmaher, B.; Axelson, D.A.; Goldstein, T.R.; Esposito-Smythers, C.; Strober, M.A.; Hunt, J.; Leonard, H.; Gill, M.K.; Iyengar, S.; et al. Significance of cigarette smoking among youths with bipolar disorder. Amer. J. Addiction 2008, 17, 364–371. [Google Scholar]
- Ostacher, M.J.; Lebeau, R.T.; Perlis, R.H.; Nierenberg, A.A.; Lund, H.G.; Moshier, S.J.; Sachs, G.S.; Simon, N.M. Cigarette smoking is associated with suicidality in bipolar disorder. Bipolar Disord. 2009, 11, 766–771. [Google Scholar] [CrossRef]
- Altamura, A.C.; Bassetti, R.; Bignotti, S.; Pioli, R.; Mundo, E. Clinical variables related to suicide attempts in schizophrenic patients: A retrospective study. Schizophr. Res. 2003, 60, 47–55. [Google Scholar]
- de Leon, J. Smoking and vulnerability for schizophrenia. Schizophr. Bull. 1996, 22, 405–409. [Google Scholar] [CrossRef]
- Sacco, K.A.; Termine, A.; Seyal, A.; Dudas, M.M.; Vessicchio, J.C.; Krishnan-Sarin, S.; Jatlow, P.I.; Wexler, B.E.; George, T.P. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: Involvement of nicotinic receptor mechanisms. Arch. Gen. Psychiat. 2005, 62, 649–659. [Google Scholar] [CrossRef]
- Leonard, S.; Adams, C.E. Smoking cessation and schizophrenia. Amer. J. Psychiat. 2006, 163, 1877. [Google Scholar] [CrossRef]
- Baker, A.; Richmond, R.; Haile, M.; Lewin, T.J.; Carr, V.J.; Taylor, R.L.; Constable, P.M.; Jansons, S.; Wilhelm, K.; Moeller-Saxone, K. Characteristics of smokers with a psychotic disorder and implications for smoking interventions. Psychiat. Res. 2007, 150, 141–152. [Google Scholar] [CrossRef]
- Levander, S.; Eberhard, J.; Lindstrom, E. Nicotine use and its correlates in patients with psychosis. Acta Psychiat. Scand. Suppl. 2007, 27–32. [Google Scholar] [CrossRef]
- McEvoy, J.P.; Allen, T.B. The importance of nicotinic acetylcholine receptors in schizophrenia, bipolar disorder and tourette’s syndrome. Curr. Drug Targets CNS Neurol. Disord. 2002, 1, 433–442. [Google Scholar] [CrossRef]
- McKee, S.A.; Weinberger, A.H.; Harrison, E.L.; Coppola, S.; George, T.P. Effects of the nicotinic receptor antagonist mecamylamine on ad-lib smoking behavior, topography, and nicotine levels in smokers with and without schizophrenia: A preliminary study. Schizophr. Res. 2009, 115, 317–324. [Google Scholar] [CrossRef]
- Strand, J.E.; Nyback, H. Tobacco use in schizophrenia: A study of cotinine concentrations in the saliva of patients and controls. Eur. Psychiat. 2005, 20, 50–54. [Google Scholar] [CrossRef]
- Etter, M.; Mohr, S.; Garin, C.; Etter, J.F. Stages of change in smokers with schizophrenia or schizoaffective disorder and in the general population. Schizophr. Bull. 2004, 30, 459–468. [Google Scholar] [CrossRef]
- Weinberger, A.H.; Sacco, K.A.; Creeden, C.L.; Vessicchio, J.C.; Jatlow, P.I.; George, T.P. Effects of acute abstinence, reinstatement, and mecamylamine on biochemical and behavioral measures of cigarette smoking in schizophrenia. Schizophr. Res. 2007, 91, 217–225. [Google Scholar] [CrossRef]
- Williams, J.M.; Ziedonis, D. Addressing tobacco among individuals with a mental illness or an addiction. Addict. Behav. 2004, 29, 1067–1083. [Google Scholar] [CrossRef]
- Siru, R.; Hulse, G.K.; Tait, R.J. Assessing motivation to quit smoking in people with mental illness: A review. Addiction 2009, 104, 719–733. [Google Scholar] [CrossRef]
- Ashton, M.; Miller, C.L.; Bowden, J.A.; Bertossa, S. People with mental illness can tackle tobacco. Aust. N. Z. J. Psychiat. 2010, 44, 1021–1028. [Google Scholar]
- Evins, A.E.; Cather, C.; Rigotti, N.A.; Freudenreich, O.; Henderson, D.C.; Olm-Shipman, C.M.; Goff, D.C. Two-year follow-up of a smoking cessation trial in patients with schizophrenia: Increased rates of smoking cessation and reduction. J. Clin. Psychiat. 2004, 65, 307–311. [Google Scholar] [CrossRef]
- Baker, A.; Richmond, R.; Haile, M.; Lewin, T.J.; Carr, V.J.; Taylor, R.L.; Jansons, S.; Wilhelm, K. A randomized controlled trial of a smoking cessation intervention among people with a psychotic disorder. Amer. J. Psychiat. 2006, 163, 1934–1942. [Google Scholar] [CrossRef]
- Dalack, G.W.; Becks, L.; Hill, E.; Pomerleau, O.F.; Meador-Woodruff, J.H. Nicotine withdrawal and psychiatric symptoms in cigarette smokers with schizophrenia. Neuropsychopharmacology 1999, 21, 195–202. [Google Scholar] [CrossRef]
- Evins, A.E.; Deckersbach, T.; Cather, C.; Freudenreich, O.; Culhane, M.A.; Henderson, D.C.; Green, M.F.; Schoenfeld, D.A.; Rigotti, N.A.; Goff, D.C. Independent effects of tobacco abstinence and bupropion on cognitive function in schizophrenia. J. Clin. Psychiat. 2005, 66, 1184–1190. [Google Scholar] [CrossRef]
- George, T.P.; Vessicchio, J.C.; Sacco, K.A.; Weinberger, A.H.; Dudas, M.M.; Allen, T.M.; Creeden, C.L.; Potenza, M.N.; Feingold, A.; Jatlow, P.I. A placebo-controlled trial of bupropion combined with nicotine patch for smoking cessation in schizophrenia. Biol. Psychiat. 2008, 63, 1092–1096. [Google Scholar] [CrossRef]
- Rüther, T.; Bobes, J.; De Hert, M.; Svensson, T.; Mann, K.; Batra, A.; Gorwood, P.; Möller, H.-J. EPA—Position statement on smoking and strategies for smoking cessation in people with mental illness. Eur. Psychiat. 2013, 3. [Google Scholar] [CrossRef]
- First, M.; Spitzer, R.; Gibbon, M.; Williams, J. Entrevista Clínica Estructurada para los Trastornos del Eje I del DSM-IV—Versión Clinica (SCID-I); Masson: Barcelona, Spain, 2001. [Google Scholar]
- Puente, D.; Cabezas, C.; Rodriguez-Blanco, T.; Fernandez-Alonso, C.; Cebrian, T.; Torrecilla, M.; Clemente, L.; Martin, C. The role of gender in a smoking cessation intervention: A cluster randomized clinical trial. BMC Public Health 2011, 11, 369. [Google Scholar] [CrossRef]
- Masters, N.; Tutt, C. Smoking Pack Years Calculator. Available online: http://smokingpackyears.com/calculate (accessed on 23 December 2013).
- Heatherton, T.F.; Kozlowski, L.T.; Frecker, R.C.; Fagerstrom, K.O. The fagerstrom test for nicotine dependence: A revision of the fagerstrom tolerance questionnaire. Br. J. Addict. 1991, 86, 1119–1127. [Google Scholar] [CrossRef]
- Jarvis, M.J.; Tunstall-Pedoe, H.; Feyerabend, C.; Vesey, C.; Saloojee, Y. Comparison of tests used to distinguish smokers from nonsmokers. Amer. J. Public Health 1987, 77, 1435–1438. [Google Scholar] [CrossRef]
- Becona, E.; Vazquez, F.L. The fagerstrom test for nicotine dependence in a Spanish sample. Psychol. Rep. 1998, 83, 1455–1458. [Google Scholar]
- Nerin, I.; Crucelaegui, A.; Novella, P.; Beamonte, A.; Sobradiel, N.; Bernal, V.; Gargallo, P. Assessment of behavioral dependence with the glover-nilsson test in smoking cessation treatment. Arch. Bronconeumol. 2005, 41, 493–498. [Google Scholar] [CrossRef]
- Richmond, R.L.; Kehoe, L.A.; Webster, I.W. Multivariate models for predicting abstention following intervention to stop smoking by general practitioners. Addiction 1993, 88, 1127–1135. [Google Scholar] [CrossRef]
- Gomez-Pena, M.; Penelo, E.; Granero, R.; Fernandez-Aranda, F.; Alvarez-Moya, E.; Santamaria, J.J.; Moragas, L.; Aymami, M.N.; Bueno, B.; Gunnard, K.; et al. Motivation to change and pathological gambling: Analysis of the relationship with clinical and psychopathological variables. Br. J. Clin. Psychol. 2011, 50, 196–210. [Google Scholar] [CrossRef]
- Mesa, E.M.D.; Garcia-Portilla, P.; Saiz, P.A.; Bascaran, T.B.; Casares, M.J.; Fonseca, E.; Carreno, E.; Florez, G.; Guardia, J.; Ochoa, E.; et al. Psychometric performance of the 6th version of the addiction severity index in Spanish (ASI-6). Psicothema 2010, 22, 513–519. [Google Scholar]
- Lozano, R.P.; Garcia, Y.A.; Tafalla, D.B.; Albaladejo, M.F. Caffeine: A nutrient, a drug or a drug of abuse. Adicciones 2007, 19, 225–238. [Google Scholar]
- Peralta, V.; Cuesta, M.J. Psychometric properties of the positive and negative syndrome scale (PANSS) in schizophrenia. Psychiat. Res. 1994, 53, 31–40. [Google Scholar] [CrossRef]
- Bobes, J.; Bulbena, A.; Luque, A.; Dal-Re, R.; Ballesteros, J.; Ibarra, N. A comparative psychometric study of the Spanish versions with 6, 17, and 21 items of the Hamilton depression rating scale. Med. Clin. 2003, 120, 693–700. [Google Scholar]
- Colom, F.; Vieta, E.; Martinez-Aran, A.; Garcia-Garcia, M.; Reinares, M.; Torrent, C.; Goikolea, J.M.; Banus, S.; Salamero, M. Spanish version of a scale for the assessment of mania: Validity and reliability of the young mania rating scale. Med. Clin. 2002, 119, 366–371. [Google Scholar] [CrossRef]
- Guy, W. Early Clinical Drug Evaluation (Ecdeu) Assessment Manual; National Institute Mental Health: Rockville, MD, USA, 1976.
- Lingjaerde, O.; Ahlfors, U.G.; Bech, P.; Dencker, S.J.; Elgen, K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiat. Scand. Suppl. 1987, 334, 1–100. [Google Scholar]
- Fiore, M.; Jaen, C.R.; Baker, T.B.; Guideline, P. Treating tobacco use and dependence: 2008 update USA. Public health service clinical practice guideline executive summary. Respir. Care 2008, 53, 1217–1222. [Google Scholar]
- AEMPS. Guía de Prescripción Terapéutica. Available online: http://www.aemps.gob.es/medicamentosUsoHumano/portada/home.htm (accessed on 23 December 2013).
- Miller, W.R.; Rollnick, S. Motivational Interviewing: Preparing People for Change, 2nd ed.; Guilford Press: New York, NY, USA, 2002. [Google Scholar]
- Prochaska, J.O.; DiClemente, C.C. Stages and processes of self-change of smoking: Toward an integrative model of change. J. Consult. Clin. Psychol. 1983, 51, 390–395. [Google Scholar]
- Lawn, S. Australians with mental illness who smoke. Br J Psychiat. 2001, 178. [Google Scholar] [CrossRef]
- Riemsma, R.P.; Pattenden, J.; Bridle, C.; Sowden, A.J.; Mather, L.; Watt, I.S.; Walker, A. Systematic review of the effectiveness of stage based interventions to promote smoking cessation. BMJ 2003, 326, 1175–1177. [Google Scholar]
- West, R. Time for a change: Putting the transtheoretical (stages of change) model to rest. Addiction 2005, 100, 1036–1039. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Garcia-Portilla, M.P.; Garcia-Alvarez, L.; Saiz, P.A.; Diaz-Mesa, E.; Galvan, G.; Sarramea, F.; Garcia-Blanco, J.; Elizagarate, E.; Bobes, J. Effectiveness of a Multi-Component Smoking Cessation Support Programme (McSCSP) for Patients with Severe Mental Disorders: Study Design. Int. J. Environ. Res. Public Health 2014, 11, 373-389. https://doi.org/10.3390/ijerph110100373
Garcia-Portilla MP, Garcia-Alvarez L, Saiz PA, Diaz-Mesa E, Galvan G, Sarramea F, Garcia-Blanco J, Elizagarate E, Bobes J. Effectiveness of a Multi-Component Smoking Cessation Support Programme (McSCSP) for Patients with Severe Mental Disorders: Study Design. International Journal of Environmental Research and Public Health. 2014; 11(1):373-389. https://doi.org/10.3390/ijerph110100373
Chicago/Turabian StyleGarcia-Portilla, Maria Paz, Leticia Garcia-Alvarez, Pilar Alejandra Saiz, Eva Diaz-Mesa, Gonzalo Galvan, Fernando Sarramea, Josefa Garcia-Blanco, Edorta Elizagarate, and Julio Bobes. 2014. "Effectiveness of a Multi-Component Smoking Cessation Support Programme (McSCSP) for Patients with Severe Mental Disorders: Study Design" International Journal of Environmental Research and Public Health 11, no. 1: 373-389. https://doi.org/10.3390/ijerph110100373




